Abstract
Background
Whole-body 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is the standard of care for lymphoma. Simultaneous PET/MRI (magnetic resonance imaging) is a promising new modality that combines the metabolic information of PET with superior soft-tissue resolution and functional imaging capabilities of MRI while decreasing radiation dose. There is limited information on the clinical performance of PET/MRI in the pediatric setting.
Objective
This study evaluated the feasibility, dosimetry, and qualitative and quantitative diagnostic performance of simultaneous whole-body FDG-PET/MRI in children with lymphoma compared to PET/CT.
Materials and methods
Children with lymphoma undergoing standard of care FDG-PET/CT were prospectively recruited for PET/MRI performed immediately after the PET/CT. Images were evaluated for quality, lesion detection and anatomical localization of FDG uptake. Maximum and mean standardized uptake values (SUVmax/mean) of normal organs and SUVmax of the most FDG-avid lesions were measured for PET/MRI and PET/CT. Estimation of radiation exposure was calculated using specific age-related factors.
Results
Nine PET/MRI scans were performed in eight patients (mean age: 15.3 years). The mean time interval between PET/CT and PET/MRI was 51±10 min. Both the PET/CT and PET/MRI exams had good image quality and alignment with complete (9/9) concordance in response assessment. The SUVs from PET/MRI and PET/CT were highly correlated for normal organs (SUVmean r2: 0.88, P<0.0001) and very highly for FDG-avid lesions (SUVmax r2: 0.94, P=0.0002). PET/MRI demonstrated an average percent radiation exposure reduction of 39 % ± 13% compared with PET/CT.
Conclusion
Simultaneous whole-body PET/MRI is clinically feasible in pediatric lymphoma. PET/MRI performance is comparable to PET/CT for lesion detection and SUV measurements. Replacement of PET/CT with PET/MRI can significantly decrease radiation dose from diagnostic imaging in children.
Keywords: Children, Lymphoma, Positron emission tomography/Computed tomography, Positron emission tomography/Magnetic resonance imaging, Magnetic resonance imaging
Introduction
[F-18]2-fluoro-2-deoxyglucose positron emission tomography CT (F-18 FDG PET/CT) is an established imaging modality for staging and response evaluation of pediatric lymphoma patients [1, 2]. Several studies have demonstrated higher diagnostic imaging accuracy of FDG-PET/CT compared to conventional anatomical imaging for evaluation of pediatric lymphoma [3]. Advances in therapy have significantly improved the outcome of pediatric lymphoma with current 5-year survival of pediatric Hodgkin lymphoma and non-Hodgkin lymphoma estimated at 97% and 85%, respectively [4, 5]. As a result, there has been a shift in treatment paradigm, with emphasis on reducing iatrogenic toxicity and late side effects of chemotherapy. This can be achieved by modifying therapy based on early response assessment, and decreasing exposure to diagnostic and therapeutic ionizing radiation. Children with lymphoma and neuroblastoma have been shown to have the highest cumulative effective dose from diagnostic radiation, among all pediatric cancer patients [6]. The cornerstone of imaging safety is the As Low As Reasonably Achievable (ALARA) principle, and there is general consensus that reducing radiation exposure is desirable, particularly in children and young adults. Since the majority of children with lymphoma have good long-term survival, reducing the radiation dose from diagnostic imaging is highly desirable because it may decrease the risk of developing radiation-related malignancies later in life. Whole-body MR has shown potential in the evaluation of childrren with lymphoma and solid tumors [7–9]. It provides better soft-tissue contrast than CT and allows for incorporation of functional imaging information, which can add information about tumor behavior, improving diagnosis and treatment monitoring [10, 11]. However, differentiation between benign and malignant lymph nodes remains challenging even with the combination of diffusion-weighted imaging and anatomical criteria [12–15].
Several adult oncology studies have shown that clinical integrated PET/MRI is technically feasible, and has good image quality and excellent diagnostic accuracy for whole-body staging compared to conventional PET/CT [16–19]. However, these results cannot be directly extrapolated to pediatric oncology given the physiological differences in children (such as red vs. fat marrow, brown fat distribution) and differences in tumor biology. The technical performance and diagnostic accuracy of PET/MRI imaging needs to be independently assessed in children and, to date, data on the clinical performance of PET/MRI in pediatric oncology is limited [20, 21].
In this study, we evaluated the feasibility, dosimetry, and the diagnostic and quantitative accuracy of simultaneous whole-body FDG-PET/MRI in children with known lymphoma. The accuracy of PET/MRI for lesion detection, response assessment and standardized uptake values (SUV) measurement was evaluated using clinical PET/CT as the reference standard.
Materials and methods
This is an institutional review board approved, Health Insurance Portability and Accountability Act (HIPAA) compliant study. Children undergoing a clinical FDG-PET/CT were prospectively recruited for a research whole-body PET/MRI between October 2012 and May 2013. Written informed consent was obtained from all study participants and their legal guardians. The inclusion criteria were age 8 to 18 years, histologically proven lymphoma and clinically indicated standard of care FDG-PET/CT. The exclusion criteria were contraindication to MR or the need for sedation.
PET/CT imaging parameters
FDG-PET/CT examinations were performed with a Siemens mCT PET/CT scanner or Siemens Biograph 40 HD PET/CT systems (Siemens Healthcare, Erlangen, Germany). The PET/CT examinations followed the standard low-dose non-enhanced CT protocol used for clinical care at our institution. All patients fasted for at least 4 h before imaging and their glucose levels were verified to be less than 200 mg/dl (mean: 98.6 mg/dl±5.26). Intravenous injection of standard of care, weight-adjusted doses of FDG were administrated following our institutional standard of care pediatric dosage of 0.07 mCi/pound (0.15 mCi/kg), with an average dosage of 18F-FDG of 8.7±1.4 mCi (328±52 MBq) with a range of 6 mCi to 9.9 mCi (220 MBq to 366.3 MBq). The PET/CT acquisitions were started 57±6 min (range: 50-71 min) after the administration of tracer with a mean delay between PET/CT and PET/MRI of 51±10 (range: 43-74 min). The average duration of the PET/CT scan was approximately 25 min. The scan protocol adheres to the ALARA guideline for exposure to ionizing radiation. A spiral CT scan for attenuation correction and anatomical localization of PET findings was obtained from the skull base through the upper thighs. Standardized, weight-adjusted protocols, including optimized dose modulation (CARE Dose; Siemens Healthcare, Erlangen, Germany) were used. Images were reconstructed with and without attenuation correction with a 3D-Ordered-Subset Expectation Maximization (3D-OSEM) algorithm with HD (TrueX) or time of flight (TOF). Scatter, decay and dead time corrections were used, as implemented by the equipment manufacturer.
PET/MRI imaging parameters
PET/MRI examinations were performed on a simultaneous PET/MRI system, 3.0-T Siemens Biograph mMR (Siemens Health Care, Erlangen, Germany). PET/MRI was initiated 109±10 min after 18F-FDG injection (range: 94-126 min) using the activity from the clinical FDG-PET/CT study. The average duration of the PET/MRI scan was approximately 60 min. The acquisition was performed on multiple-station mode for whole-body imaging and consisted of standard of care MR sequences with simultaneous PET acquisition for 2-5 min per bed position adjusted as needed based on subject height, weight and injected dose. The MR sequences include whole-body Dixon, T2 half-Fourier acquisition single-shot turbo spin-echo (HASTE), short tau inversion recovery (STIR) and diffusion-weighted imaging (DWI) (Table 1). Dual-echo VIBE (volume interpolated breathhold examination) Dixon sequence was acquired for attenuation correction and used for the MR-based segmentation map [22]. The PET/MRI images were acquired from skull vertex to mid-thigh level without administration of an MR imaging contrast agent (Fig. 1).
Table 1.
Whole body MR imaging sequence parameters
| Sequence | Axial Dixon | Axial HASTE | Axial DWI | Axial STIR |
|---|---|---|---|---|
| Parameters | ||||
| Acquisition time (mins) | 0.33 | 3 | 3 | 2 |
| Echo time (TE) (ms) | 1.23, 2.46 | 116 | 85 | 89–97 |
| Repetition time(TR) (ms) | 3.6 | 3000 | 10000 | 2910–3690 |
| Bandwidth (Hz/pixel) | 965 | 710 | 1628 | 260 |
| Matrix size | 192×79 | 320×240 | 128×96 | 320×163×218 |
| Slice thickness | 3.1 | 5 | 5 | 5–6 |
| Flip angle (degree) | 10 | 160 | 90 | 140 |
| Inversion time (TI) (ms) | 220 | |||
| B values (mm2/s) | 50,400,800 |
DWI diffusion weighted imaging, HASTE half-Fourier acquired single-shot turbo spin echo, STIR short-tau inversion recovery
Fig. 1.

Representative images of the whole-body PET/MRI protocol in a 15-year-old boy with relapsed Hodgkin lymphoma (case 2). a U-map is used for MR-based attenuation correction of the PET data and is generated from the following four Dixon sequences: (b) water-weighted, (c) opposed-phase, (d) fat-weighted and (e) in-phase images. f PET maximum intensity projection (MIP), (g) PET/MRI fusion and (h) DWI demonstrate multiple malignant lymph nodes that are FDG-avid and restrict diffusion
Image interpretation
The PET/MRI were evaluated qualitatively and semiquantitatively in consensus by two radiologists, one with subspecialty training in nuclear medicine (J.M. with 5 years of experience) and the other with subspecialty training in pediatric radiology (G.K. with 11 years of experience). After a minimum time interval of 30 days, the PET/CT images were evaluated by the same readers in consensus. The readers knew that the patients had biopsy-proven lymphoma, were aware of the indication of the clinical PET scan and had access to the prior PET/CT study that was performed 42-183 days before the PET/MRI scan. This provides for better image interpretation, follows standard clinical practices and is necessary for assessing interval response. They were blinded to the results of all other clinical information, including the clinical PET/CT report. A commercially available software package (MIM Encore version 6.4.1; MIM Software, Inc., Cleveland, OH) was used for imaging viewing, fusion and SUV measurements of the PET/MRI and PET/CT images.
Subjective assessments of image quality and PET alignment quality were assessed following the definitions presented in Table 2. For interpretive purposes, each scan was divided into four regions (head and neck, thorax, abdomen/pelvis and extremities) to determine the number of malignant lesion(s) per region (0, 1, 2, 3 and 3+) and the confidence level associated with the malignant process (confidence level: 1=low, 2=moderate and 3=high). An overall assessment was assigned to each scan, which was defined as complete metabolic response, partial metabolic response, stable disease and progression of disease compared to the immediate prior study [23, 24].
Table 2.
Image interpretation. Definitions of subjective assessment of image quality (a) and PET alignment used for image interpretation (b)
| a Images Quality | |
|---|---|
| 1 | Poor quality, not diagnostically acceptable for interpretation |
| 2 | Suboptimal quality, worse than routine images with excessive image noise |
| 3 | Acceptable quality, diagnostic interpretation possible but noise more than routine images |
| 4 | Good quality, noise similar to routine images |
| 5 | Excellent quality, no image noise |
| b Quality of positron emission tomography (PET)/CT alignment | |
|---|---|
| 1 | Poor alignment, complete lack of alignment |
| 2 | Suboptimal alignment, differs by more than 25 mm |
| 3 | Acceptable alignment, differs >5 but <25 mm |
| 4 | Good alignment, differs <5 mm |
| 5 | Excellent alignment, no misalignment |
Maximum and mean standardized uptake values (SUVmax and SUVmean) of normal organs were obtained from MR attenuation-corrected (DIXON-based segmented μmap) PET images and compared with values obtained from CT-based attenuation correction PET/CT. The SUVs of normal organs were measured using a 1.5-cm region of interest (ROI) within the respective organs, these being lung (right upper lobe), mediastinal blood pool (right atrium), bone (L4 vertebral body) and muscle (psoas muscle) by a third radiologist with dual training in pediatric radiology and nuclear medicine (M.R.P. with 10 years and 3 years of experience, respectively). Additionally, SUVmax measurements were made in the most FDG-avid lesions in each of the four defined body regions using a gradient-based segmentation method to delineate tumor contours (PET edge mode tool) [25].
Estimation of radiation exposure
The total effective dose from the FDG injection and CT scan was evaluated for each patient using specific age-related factors. The effective dose from the FDG injection was obtained by multiplying the International Commission on Radiological Protection (ICRP) publication 106 [26] age-related effective dose by the injected activity with the following age breakdown: 18-year-old used 0.019 mSv/MBq, 17- to 15-year-old used 0.024 mSv/MBq, 14- to 12-year-old used 0.024 mSv/MBq and 11- to 7-year-old used 0.037 mSv/MBq. The CT whole-body effective doses were calculated using the dose-length product (DLP) with pediatric correction factors [27]. An average dose length product to ED conversion factor of 0.017 mSv/mGy.cm [28] was employed along with pediatric correction factors of 1.0 for 18-year-old, 1.1 for 17- to 15-year-old, 1.15 for 14- to 12-year-old and 1.2 for 11- to 7-year-old [27]. The percent dose reduction from eliminating the CT component of PET/CT, performed for attenuation correction and localization, was calculated as the ratio of the effective dose from the CT divide by the total effective dose from the PET/CT for each scan run. The average and standard deviation for the percent dose reduction using PET/MRI was obtained from the set of scan runs (n=9).
Data analysis
Pearson correlation coefficients (r2) were calculated to quantify the correlation between SUV values on attenuation correction PET/CT and PET/MRI images. The SUV values are expressed as means ± standard deviations. The r2 were interpreted as following: 0.9 to 1.0, very high correlation; 0.7 to 0.90, high positive correlation; 0.50 to 0.70, moderate correlation; 0.30 to 0.50, low positive correlation, and 0.00 to 0.30, negligible correlation [28]. Image quality and alignment were expressed in absolute numbers.
Results
Nine PET-MRI studies were performed on 8 patients (6 boys and 2 girls) with a mean age of 15.3 years (range: 12-17.5 years). The lymphoma histologies were as follows: 4 Hodgkin lymphoma, 2 diffuse large B-cell lymphoma and 2 Burkitt lymphoma. The patient characteristics are given in Table 3.
Table 3.
Study patients’ characteristics. There are eight patients and nine scans since case 4 received two scans.
| Case No. | Age (yrs) | Sex | Diagnosis | Primary tumor site(s) | PET/CT indication |
|---|---|---|---|---|---|
| 1 | 17.5 | Male | Hodgkin lymphoma (classical type) | Mediastinal mass | Treatment monitoring |
| 2 | 15.6 | Male | Hodgkin lymphoma (classical type) | Neck and mediastinal lymph nodes | Restaging after completion of chemotherapy |
| 3 | 17.0 | Male | Non-Hodgkin lymphoma (diffuse large B-cell) | Mediastinal mass | Restaging after completion of chemotherapy |
| 4(a) | 14.8 | Female | Hodgkin lymphoma (classical type) | Mediastinal mass, axillary and cervical lymph nodes | Restaging after completion of chemotherapy |
| 4(b) | 15.3 | Female | Hodgkin lymphoma (classical type) | Mediastinal mass, axillary and cervical lymph nodes | Suspected recurrence |
| 5 | 12.0 | Male | Non-Hodgkin lymphoma (diffuse large B-cell) | Mediastinal mass | Restaging after completion of chemotherapy |
| 6 | 15.0 | Male | Non-Hodgkin lymphoma (Burkitt) | Small bowel | Treatment monitoring |
| 7 | 15.3 | Male | Non-Hodgkin lymphoma (Burkitt) | Ascending colon | Restaging after completion of chemotherapy |
| 8 | 15.5 | Female | Hodgkin lymphoma (classical type) | Mediastinal mass | Treatment monitoring |
Image quality
The image quality was evaluated independently for MRI sequences and PET images as well as together for image alignment quality between the two imaging modalities. All nine PET/CT exams were rated as having image quality and image alignment scores of good. The image quality scores of PET/MRI studies was as follows: excellent, n=1; good, n=7, and acceptable, n=1. The alignment scores for PET/MRI studies were as follows: good, n=7, and acceptable, n=2. The quality of the PET/MRI umap was good in five patients and acceptable in four patients. The cause of acceptable quality on the PET/MRI umap was the presence of attenuation artifacts from the child’s dental braces (n=2) projecting over the frontal lobes and face (Fig. 2), artifacts in the chest related to the child’s port catheter (n=1), and lungs on the Dixon sequences affecting the umap (n=1). An additional artifact was observed in one child at the lung base related to breathing motion (Fig. 3).
Fig. 2.
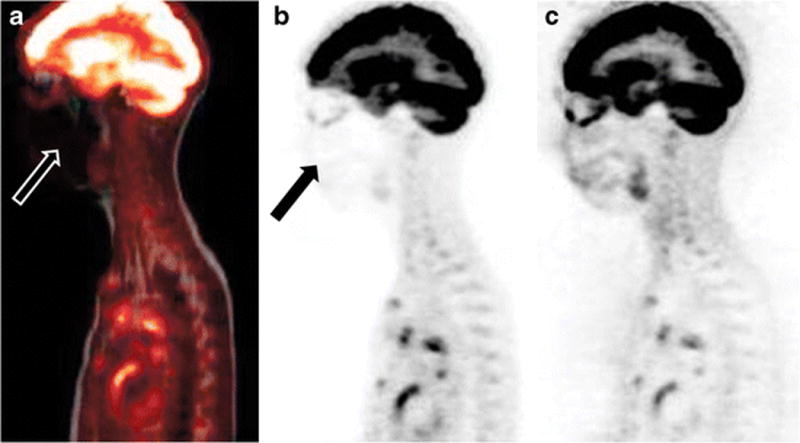
PET/MRI artifact in a 15-year-old boy (case 6) with braces. There is significant degradation of image quality and loss of signal on the fused PET/MRI image (a) and the MR-based attenuation corrected PET image (b) in the region of the braces. The non-attenuated PET image (c) demonstrates normal FDG activity in the region of the head and neck
Fig. 3.

Images (a–c) demonstrate a breathing artifact in a 15-year-old boy with non-Hodgkin lymphoma (case 6): (a) PET images from PET/MRI, (b) fusion PET/MRI and (c) PET/CT. The breathing artifact simulates a lung base nodule (arrows in a and b). Lung window CT (c) demonstrates no abnormalities
Quantitative analysis
SUVmean of normal organs derived from PET/MRI and PET/CT studies showed a high correlation (r2=0.88, P <0.0001). Correlation coefficient for SUVmean was highest for bone (r2=0.90), liver (r2 =0.86) and muscle (r2=0.78) and moderate for lung (r2 =0.65) and blood pool (r2 =0.61) (Fig. 4). One of the children presented with bone marrow stimulation, associated with high SUVs. If this child is excluded from the analysis, the correlation coefficient for bone is 0.73.
Fig. 4.

Correlation plots of PET/CT and PET/MRI for all selected normal organs (a) and FDG-avid lesions (b) with the corresponding correlation coefficients (r2)
SUVmax in 16 lesions was measured on the PET/MRI images and PET/CT images. The PET/MRI SUVmax values ranged from 2.2 to 21.4, and the PET/CT SUVmax values ranged from 2.1 to 19.6. There was very high correlation of SUVmax of FDG-avid lesions as measured on PET/MRI and PET/CT (r2 = 0.94, P< 0.0002) (Fig. 4). The mean SUVmax for FDG-avid lesions was 1.6 % higher for PET/MRI than PET/CT.
Qualitative analysis
Table 4 summarizes the number of malignant lesions identified per case and the response assessment at PET/MRI and PET/CT. There was complete (9/9) concordance between PET/CT and PET/MRI regarding response assessment. A few discrepancies were observed between PET/CT and PET/MRI in terms of number of lesions identified. In one child, PET/CT detected two foci of FDG uptake in residual anterior mediastinal mass while PET/MRI showed only one area of focal uptake in the residual anterior mediastinal mass (case 1). In case 2, PET/MRI detected two lesions with increased FDG uptake in the neck while PET/CT detected one lesion in this region. Finally, case 3 had a renal lesion with markedly increased FDG uptake that was obscured by excreted activity in the urine and not detected on PET/CT but was readily identified on PET/MRI on the diffusion-weighted sequences due to restricted diffusion (Fig. 5). PET/CT imaging follow-ups were available for all patients with the time interval between the research PET/MRI and clinical follow-up PET/CT of 3 to 20 months. The clinical and imaging follow-up confirmed the PET/CT and PET/MRI findings with the results reported in Table 4.
Table 4.
Detection of malignant lesions and response assessment by PET/MRI vs. PET/CT
| Case No. | Number and location of suspected malignant lesion(s) | Response assessment compared to immediate prior scan | Imaging follow-up number and location of suspected malignant lesion(s) | ||
|---|---|---|---|---|---|
| PET/CT | PET/MRI | PET/CT | PET/MRI | PET/CT | |
| 1 | 2, anterior mediastinum | 1, anterior mediastinum | Partial response | Partial response | 0, anterior mediastinum 1, posterior mediastinumn |
| 2 | 1, neck >3, chest |
2, neck > 3, chest |
Progressive disease | Progressive disease | >2, necks >3, chesti >3 bone lesionsn |
| 3 | 1, chest 1, retroperitoneal lymph node |
1, chest 1, retroperitoneal lymph node 1, kidney |
Progressive disease | Progressive disease | 0, chest 0, retroperitoneal lymph node 0, kidney |
| 4 (a) | 0 | 0 | Complete response | Complete response | 0 |
| 4 (b) | 0 | 0 | Complete response | Complete response | 0 |
| 5 | 1, anterior mediastinum | 1, anterior mediastinum | Stable disease | Stable disease | 1, anterior mediastinums |
| 6 | 0 | 0 | Complete response | Complete response | 0 |
| 7 | 0 | 0 | Complete response | Complete response | 0 |
| 8 | 0 | 0 | Complete response | Complete response | 0 |
new lesion detected on the PET/CT follow-up
increase in number of suspected lesion(s)
stable lesion since previous PET/CT and PET/MRI
Fig. 5.
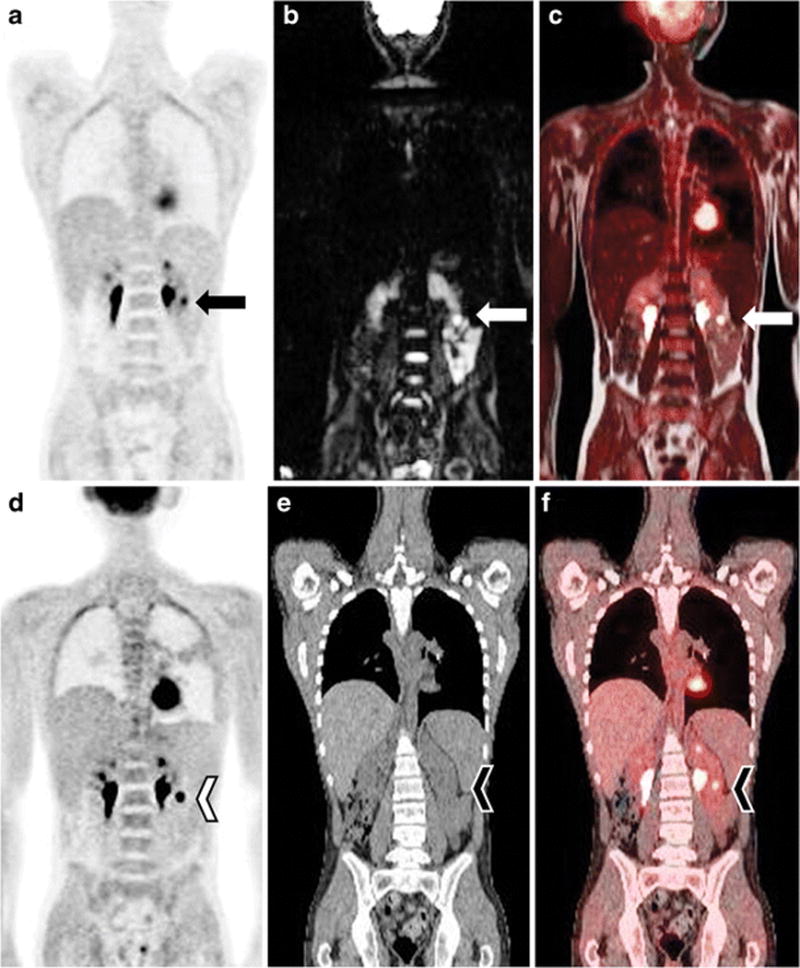
PET/MRI (a–c), and PET/CT (d–f) in a 17-year-old boy with large B-cell lymphoma with left renal infiltration (case 3). The presence of restricted diffusion on DWI (arrows in a–c) and the excellent PET/MRI coregistration allowed better identification of a renal lesion and differentiation from excreted FDG in the collecting system than PET/CT (arrowheads in d–f)
Radiation dose
The mean effective dose of whole-body PET/CT examinations was 13.8±3.9 mSv, with PET accounting for 8.0±0.9 mSv and CT accounting for 5.8±3.3 mSv. PET/MRI demonstrated an average radiation exposure reduction by 39%±13% compared with PET/CT. The average volume CT dose index (CTDIvol) was 3.07 mGy (range: 1.4-6.13 mGy).
Discussion
The purpose of our study was to evaluate the feasibility, qualitative and quantitative diagnostic performance of simultaneous whole-body FDG-PET/MRI in children with lymphoma compared to PET/CT.
In a cohort of prospectively recruited children who underwent whole-body simultaneous PET/MRI and PET/CT on the same day, we have shown that PET/MRI has similar diagnostic performance as PET/CT for response assessment with complete concordance noted in all nine scans. PET/MRI accurately identified all sites of disease noted on PET/CT. PET/MRI also accurately detected the number of pathological lesions in these disease sites in all cases except one, where PET/CT showed two foci of FDG uptake in the anterior mediastinum while PET/MRI showed only one SUV avid area in the residual anterior mediastinal mass. The reason for this discrepancy is unclear but may be due to redistribution of FDG between the PET/CT and PET/MRI acquisitions. In addition, the superior soft-tissue contrast of PET/MRI helped detect a pathological neck lymph node, and the DWI sequence allowed for the assessment of diffusion restriction assisting in the detection and characterization of a FDG-avid renal lesion that was missed on PET/CT due to being obscured by adjacent excreted activity in the renal collecting system. This case illustrates the complementary benefits of combining molecular information from PET data with high-resolution anatomical information provided by MR, and the ability to measure metabolic and biochemical parameters. More studies in larger patient populations are needed to determine if these potential advantages of MRI over CT lead to superior diagnostic accuracy with PET/MRI. One previous pediatric study showed improved detection of solid organ and bone lesions at PET/MRI compared to PET/CT. The prior study had a heterogeneous patient population with a variety of malignant tumors [21]. Currently, there are no standards for the MRI sequences used in PET/MRI scans. For aggressive lymphomas, which are almost universally FDG-avid, lesion detection is usually not an issue unless adjacent to a site of high physiological FDG accumulation (e.g., brain and genitourinary system). Thus, anatomical localization is likely sufficient in this particular clinical setting. Additionally, we acquired diffusion-weighted sequences to provide functional information that complement the anatomical information from the axial HASTE and STIR sequences. MRI may play a much larger role in other malignancies, which have variable and, in some cases, low FDG uptake. Based on our study and prior publications, we recommend that DWI should be a standard sequence in whole-body PET/MRI protocols in pediatric oncology.
Two children in our cohort had pulmonary lesions. PET/MRI accurately identified pulmonary involvement in both patients. Previous studies have shown the limitations of PET/MRI in detecting pulmonary metastasis. We believe lymphomatous involvement of lung is well depicted by PET/MRI as these masses are typically more than a few millimeters in diameter. However, children with solid tumors undergoing PET/MRI still need a diagnostic chest CT as both PET and MR have been shown to have limited sensitivity for detecting pulmonary nodules <5 mm in diameter [29].
In terms of PET quantification obtained with MR- and CT-based attenuation correction, our results show good correlation of the mean and maximum SUVs obtained for normal organs and FDG-avid lesions, implying reliable SUV quantification with PET/MRI. Prior adult studies have shown strong correlation between SUVs estimated by MR-based attenuation correction and CT-based attenuation correction [30, 31]. Due to physiological differences between children and adults, these results cannot be directly extrapolated to the pediatric population. To the best of our knowledge, only two prior publications have evaluated this issue in children [21, 32]. Our study adds to the body of evidence that PET/MRI provides reliable SUV quantitative assessment in children. The median SUVs for PET/MRI were lower than the respective values for PET/CT for all normal organs as has been observed in adults. This difference could be due to technical factors such as the performance characteristics of the different PET technologies and differences in attenuation correction methodology, as well as the interval between image acquisitions [33]. The Siemens Biograph mMR (Siemens Healthcare, Erlangen, Germany) PET/MRI with absolute sensitivity of 15 kcps/MBq [34] measured in air has better performance characteristics than the Siemens Biograph 40 HD (Siemens Healthcare, Erlangen, Germany) PET/CT with an absolute sensitivity of 8.1 kcps/MBq [35]. The two studies were performed sequentially with an average delay between PET/CT and PET/MRI of 51±10 min resulting in a longer post-injection acquisition time for all PET/MRI studies. The increased post-injection delay contributes to changes in biodistribution and washout of 18F-FDG from normal organs. The radiation decay accounted, in part, for longer scanning time per/bed position (2 min in PET/CT, 2.5-5 min in PET/MRI) and, thus, image statistics were similar in PET/CT and PET/MRI. However, PET/MRI benefited from a slightly more favorable biodistribution relative to PET/CT in our measurements. On the other hand, the mean SUVmax for FDG-avid lesions at PET/MRI was 1.6% higher than that estimated at PET/CT, and this difference can be again be explained by the mean delay between PET/CT and PET/MRI, which extends the uptake phase increasing trapping of the tracer within pathological lesions as previously reported [36].
In our cohort of older children, we have shown that eliminating the CT component of PET/CT would decrease the effective dose by an average of 39%. This is in contradistinction to the 73-80% radiation dose reduction reported in previous studies [20, 21]. This difference is likely because our clinical PET/CT is comprised of a low-dose CT performed for attenuation correction and localization only. We do not use this for diagnostic purposes and do not administer iodinated intravenous contrast for PET/CT. As the radiopharmaceutical contributes substantially to the overall radiation dosimetry, reduction of the administered amount of FDG potentially compensated by longer PET acquisition times could further reduce the overall patient radiation exposure. The extra time at each bed position can be utilized to obtain additional MR sequences given the simultaneous scanning capability of the PET/MRI scanner. Further reductions in radiation exposure are expected as PET/MRI technology advances. The development of PET detectors with better performance characteristics would allow for lower dose of injected radiotracers because of the better imaging sensitivity. Additionally, longer PET acquisition times along with multisequence MRI collection may further reduce the amount of activity administered.
PET/MRI artifacts were observed, related to abnormal attenuation correction leading to incomplete evaluation of the cervical lymph node chains, in one child. Breathing artifact simulating increased uptake in the right lung base was noted in another patient. Errors in attenuation correction within the lung can be caused by respiratory misalignment of PET and MR data due to the difference in the duration of the two examinations resulting in mismatch of PET emission and MR attenuation data. This type of tissue misclassification could be reduced by adopting MR-guided non-rigid motion correction techniques [37, 38] or respiratory gating [39].
A major limitation of our study is the small sample size. However, this data in conjunction with the literature in adult studies comparing PET/MRI to PET/CT for whole-body staging indicate that whole-body PET/MRI has a diagnostic performance comparable to PET/CT in the pediatric lymphoma population. Currently, our main indication for clinical whole-body PET/MRI scanning are pediatric oncological patients who need a whole-body scan in lieu of a PET/CT, especially if the child needs a whole-body PET scan and a diagnostic regional MRI. This allows for both studies to be performed as a “one-stop shop” under the same sedation. The primary limitation of this approach is the need for an additional chest CT in children with malignancies at risk for pulmonary metastasis. We typically perform this as a non-sedated, non-contrast chest CT as the mediastinum is well evaluated by PET/MRI. Given the inclusion criteria of this study, only non-sedated patients were enrolled with an age range of 12.0–17.5 years.
Another limitation of our study is that not all children had evidence of disease at the time of the research scan. However, several patients had multiple lesions with good correlation between the two methodologies. Despite our focus on pediatric patients with lymphoma, the clinical indication for the PET/CT was heterogeneous and included patients who were still undergoing therapy as well as children who had completed therapy. The lack of pathological correlation of malignant/suspicious findings is another limitation since it was not feasible in all the cases due to ethical concerns. Thus, our reference interpretations were based on PET/CT imaging follow-up, histological findings when available and clinical follow-up.
Our goal was to demonstrate that FDG-PET/MRI is feasible in children and offers substantial dose reduction without reducing diagnostic accuracy compared to PET/CT. There is extensive literature addressing the accuracy of FDG-PET and FDG-PET/CT for aggressive lymphomas in adults and to a lesser extent children [3, 40, 41] and, thus, FDG-PET/CT is a reasonable reference standard for our study.
Conclusion
Our results suggest that 18F-FDG-PET/MRI is a clinically feasible modality that has high correlation with PET/CT for both qualitative and quantitative diagnostic performance of response assessment in pediatric lymphoma and perhaps other pediatric malignancies. The superior soft-tissue contrast and functional imaging capabilities of MR can aid in lesion detection at PET/MRI, especially in solid organs. PET/MRI decreases exposure to ionizing radiation in this patient population.
Footnotes
Compliance with ethical standards
Conflicts of interest None
References
- 1.Kluge R, Kurch L, Montravers F, Mauz-Körholz C. FDG PET/CT in children and adolescents with lymphoma. Pediatr Radiol. 2013;43:406–417. doi: 10.1007/s00247-012-2559-z. [DOI] [PubMed] [Google Scholar]
- 2.London K, Cross S, Onikul E, et al. 18F-FDG PET/CT in paediatric lymphoma: comparison with conventional imaging. Eur J Nucl Med Mol Imaging. 2011;38:274–284. doi: 10.1007/s00259-010-1619-6. [DOI] [PubMed] [Google Scholar]
- 3.Cheng G, Servaes S, Zhuang H. Value of 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan versus diagnostic contrast computed tomography in initial staging of pediatric patients with lymphoma. Leuk Lymphoma. 2013;54:737–742. doi: 10.3109/10428194.2012.727416. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49. doi: 10.1111/j.1365-2141.2005.05735.x. [DOI] [PubMed] [Google Scholar]
- 5.Sherief LM, Elsafy UR, Abdelkhalek ER, et al. Hodgkin lymphoma in childhood: clinicopathological features and therapy outcome at 2 centers from a developing country. Medicine. 2015;94:e670. doi: 10.1097/MD.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed BA, Connolly BL, Shroff P, et al. Cumulative effective doses from radiologic procedures for pediatric oncology patients. Pediatrics. 2010;126:e851–858. doi: 10.1542/peds.2009-2675. [DOI] [PubMed] [Google Scholar]
- 7.Kwee TC, Vermoolen MA, Akkerman EA, et al. Whole-body MRI, including diffusion-weighted imaging, for staging lymphoma: comparison with CT in a prospective multicenter study. J Magn Re Imaging. 2014;40:26–36. doi: 10.1002/jmri.24356. [DOI] [PubMed] [Google Scholar]
- 8.Adams HJ, Kwee TC, Lokhorst HM, et al. Potential prognostic implications of whole-body bone marrow MRI in diffuse large B-cell lymphoma patients with a negative blind bone marrow biopsy. J Magn Res Imaging. 2014;39:1394–1400. doi: 10.1002/jmri.24318. [DOI] [PubMed] [Google Scholar]
- 9.Siegel MJ, Acharyya S, Hoffer FA, et al. Whole-body MR imaging for staging of malignant tumors in pediatric patients: results of the American College of Radiology Imaging Network 6660 Trial. Radiology. 2013;266:599–609. doi: 10.1148/radiol.12112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knopp MV, von Tengg-Kobligk H, Choyke PL. Functional magnetic resonance imaging in oncology for diagnosis and therapy monitoring. Mol Cancer Ther. 2003;2:419–426. [PubMed] [Google Scholar]
- 11.Histed SN, Lindenberg ML, Mena E, et al. Review of functional/anatomical imaging in oncology. Nucl Med Commun. 2012;33:349–361. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwee TC, Takahara T, Ochiai R, et al. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET: the state of the art and potential applications. J NuclMed. 2010;51:1549–1558. doi: 10.2967/jnumed.109.073908. [DOI] [PubMed] [Google Scholar]
- 13.Kwee TC, Takahara T, Luijten PR, et al. ADC measurements of lymph nodes: inter- and intra-observer reproducibility study and an overview of the literature. EurJ Radiol. 2010;75:215–220. doi: 10.1016/j.ejrad.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Prenzel KL, Monig SP, Sinning JM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–467. doi: 10.1378/chest.123.2.463. [DOI] [PubMed] [Google Scholar]
- 15.Fueger BJ, Yeom K, Czernin J, et al. Comparison of CT, PET, and PET/CT for staging of patients with indolent non-Hodgkin’s lymphoma. Mol Imaging Biol. 2009;11:269–274. doi: 10.1007/s11307-009-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchbender C, Heusner TA, Lauenstein TC, et al. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med. 2012;53:1244–1252. doi: 10.2967/jnumed.112.109306. [DOI] [PubMed] [Google Scholar]
- 17.Littooij AS, Kwee TC, Barber I, et al. Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol. 2014;24:1153–1165. doi: 10.1007/s00330-014-3114-0. [DOI] [PubMed] [Google Scholar]
- 18.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 19.Tian J, Fu L, Yin D, et al. Does the novel integrated PET/MRI offer the same diagnostic performance as PET/CT for oncological indications? PloS One. 2014;9:e90844. doi: 10.1371/journal.pone.0090844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch FW, Sattler B, Sorge I, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43:860–875. doi: 10.1007/s00247-012-2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schafer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273:220–231. doi: 10.1148/radiol.14131732. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Moller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–526. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 23.Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 25.Shah B, Srivastava N, Hirsch AE, et al. Intra-reader reliability of FDG PET volumetric tumor parameters: effects of primary tumor size and segmentation methods. Ann Nucl Med. 2012;26:707–714. doi: 10.1007/s12149-012-0630-3. [DOI] [PubMed] [Google Scholar]
- 26.ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. Ann ICRP. 2008;38:1–197. doi: 10.1016/j.icrp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Theocharopoulos N, Damilakis J, Perisinakis K, et al. Estimation of effective doses to adult and pediatric patients from multislice computed tomography: A method based on energy imparted. Med Phys. 2006;33:3846–3856. doi: 10.1118/1.2349694. [DOI] [PubMed] [Google Scholar]
- 28.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 29.Biederer J, Hintze C, Fabel M. MRI of pulmonary nodules: technique and diagnostic value. Cancer Imaging. 2008;8:125–130. doi: 10.1102/1470-7330.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heusch P, Buchbender C, Beiderwellen K, et al. Standardized uptake values for [(1)(8)F] FDG in normal organ tissues: comparison of whole-body PET/CT and PET/MRI. Eur J Radiol. 2013;82:870–876. doi: 10.1016/j.ejrad.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Heacock L, Weissbrot J, Raad R, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015;204:842–848. doi: 10.2214/AJR.14.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons K, Seghers V, Sorensen JI, et al. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in a tertiary pediatric hospital: A prospective study. AJR Am J Roentgenol. 2015;205:1094–1101. doi: 10.2214/AJR.15.14304. [DOI] [PubMed] [Google Scholar]
- 33.Westerterp M, Pruim J, Oyen W, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392–404. doi: 10.1007/s00259-006-0224-1. [DOI] [PubMed] [Google Scholar]
- 34.Delso G, Furst S, Jakoby B, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 35.Jakoby BW, Bercier Y, Watson CC, et al. Performance characteristics of a new LSO PET/CT scanner with extended axial field-of-view and PSF reconstruction. IEEE Trans Nucl Sci. 2009;56:633–639. [Google Scholar]
- 36.Chin BB, Green ED, Turkington TG, et al. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2009;11:118–122. doi: 10.1007/s11307-008-0177-9. [DOI] [PubMed] [Google Scholar]
- 37.Keller SH, Holm S, Hansen AE, et al. Image artifacts from MR-based attenuation correction in clinical, whole-body PET/MRI. MAGMA. 2013;26:173–181. doi: 10.1007/s10334-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 38.Wurslin C, Schmidt H, Martirosian P, et al. Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system. J Nucl Med. 2013;54:464–471. doi: 10.2967/jnumed.112.105296. [DOI] [PubMed] [Google Scholar]
- 39.Nehmeh SA, Erdi YE, Ling CC, et al. Effect of respiratory gating on reducing lung motion artifacts in PET imaging of lung cancer. Med Phys. 2002;29:366–371. doi: 10.1118/1.1448824. [DOI] [PubMed] [Google Scholar]
- 40.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 41.Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]


