Abstract
Background
Kidney transplantation is the preferred treatment for end-stage renal disease (ESRD), while peritoneal dialysis affords certain benefits over hemodialysis. Distributions and determinants of first ESRD treatment modality have not been compared across glomerulonephritis (GN) subtypes.
Methods
We identified all adult (18–75 years) patients with ESRD attributed to any of six GN subtypes [focal segmental glomerulosclerosis (FSGS), IgA nephropathy (IgAN), membranous nephropathy (MN), membranoproliferative GN (MPGN), lupus nephritis (LN) and vasculitis] who were first registered in the US Renal Data System (USRDS) between 1996 and 2011. We used multinomial logistic regression—adjusting for temporal, geographic, demographic, socioeconomic and comorbid factors—to determine odds ratios (ORs) with 95% confidence intervals (CIs) for transplantation versus hemodialysis, and for peritoneal dialysis versus hemodialysis, comparing other GN subtypes to IgAN.
Results
Among the 75 278 patients studied, patients with comparator GN subtypes were significantly less likely than those with IgAN to receive either transplantation or peritoneal dialysis. After adjusting for potentially confounding covariates, patients with comparator primary GN subtypes (FSGS, MN, MPGN) were at least as likely to receive transplantation [FSGS OR 0.98 (95% CI 0.93–1.15), MN OR 1.19 (95% CI 1.01–1.39), MPGN OR 1.08 (95% CI 0.93–1.26)] or peritoneal dialysis [FSGS OR 1.05 (95% CI 0.98–1.12), MN OR 1.30 (95% CI 1.18–1.43), MPGN OR 0.95 (95% CI 0.85–1.06)] as patients with IgAN. Conversely, patients with the secondary GN subtypes LN and vasculitis remained significantly less likely to receive either modality [transplantation OR 0.49 (95% CI 0.43–0.56) for LN and 0.27 (95% CI 0.22–0.34) for vasculitis, peritoneal dialysis OR 0.76 (95% CI 0.70–0.82) for LN and 0.54 (95% CI 0.48–0.60) for vasculitis].
Conclusions
Significant differences in ESRD treatment practice patterns are apparent among GN subtypes. To ensure equitable care for all patients, regardless of GN subtype, reasons for observed disparities should be elucidated and—if appropriate—eliminated.
Keywords: clinical epidemiology, dialysis modality, end-stage renal disease, glomerulonephritis, kidney transplantation
INTRODUCTION
Collectively, glomerulonephritides (GN) constitute the third leading cause of end-stage renal disease (ESRD) in the USA [1]. Once ESRD develops, three active treatment options are available: hemodialysis, peritoneal dialysis and kidney transplantation. Among these, kidney transplantation is considered the optimal approach: compared with hemodialysis or peritoneal dialysis, it prolongs survival [2, 3], reduces morbidity [4] and improves quality of life [5], particularly as a first ESRD treatment (i.e. preemptive kidney transplantation) [6–8]. Patients with ESRD attributed to GN also derive a survival benefit from kidney transplantation, which is at least comparable to the magnitude of benefit observed in other causes of ESRD [2, 9–11]. Among dialysis modalities, survival with peritoneal dialysis and hemodialysis is similar overall [12], including in patients with ESRD due to GN [13–15]. However, peritoneal dialysis offers certain advantages over hemodialysis, including reduced early mortality [16], better patient satisfaction [17, 18] and a lower infection risk [19]. Peritoneal dialysis is also a better value [20], costing Medicare an average of $16 315 less per patient per year than hemodialysis [1].
Investigators examining access to ESRD treatments have identified important demographic and socioeconomic factors influencing ESRD treatment modality distributions. Patient age [21–25], sex [21], race [21–23, 25, 26], Hispanic ethnicity [27, 28], insurance payer [7, 29], employment status [23, 25] and neighborhood poverty [22, 30] have all been found to independently associate with access to kidney transplantation and/or peritoneal dialysis. Little is known, however, regarding determinants of initial ESRD treatment modality in patients with GN, nor have differences across GN subtypes been determined. We recently identified strong associations between GN subtype and patient survival after ESRD therapy initiation in the USA [31]. We further observed that ESRD treatment modality differences partially explained survival disparities. However, we did not systematically compare modality distributions across GN subtypes, nor examine determinants of ESRD treatment modality using rigorous statistical methods. Elucidating such information might identify nonuniform practice patterns warranting further consideration by physicians, researchers and policymakers aiming to achieve equitable care for all patients, regardless of their underlying cause of ESRD.
In this study, we sought to compare initial ESRD treatment modality distributions across GN subtypes in the treated US ESRD population. After accounting for temporal, geographic, demographic, socioeconomic and comorbidity imbalances between groups, we hypothesized that GN subtype would independently associate with initial ESRD therapy. Specifically, we anticipated that patients with the secondary GN subtypes lupus nephritis (LN) and vasculitis (who may experience a more rapid progression to ESRD or a higher systemic illness burden at ESRD onset) would be less likely to receive either a kidney transplant or peritoneal dialysis as a first ESRD treatment modality than patients with renal-limited primary GN subtypes.
MATERIALS AND METHODS
Study population
We identified all adult (18–75 years) patients who initiated ESRD treatment and were registered in the US Renal Data System (USRDS) between 1 January 1996 and 31 December 2011 with ESRD attributed to one of four primary GN subtypes [focal segmental glomerulosclerosis (FSGS), IgA nephropathy (IgAN), membranous nephropathy (MN) and membranoproliferative GN (MPGN)] or two secondary GN subtypes (LN and vasculitis). Missing or uncertain cause of ESRD or a defined cause other than one of these six GN subtypes were the only exclusion criteria.
Data sources
The USRDS is a national registry that collects demographic, clinical and treatment-related information regarding virtually all patients receiving ESRD treatment in the USA. Baseline data are largely derived from Medical Evidence Reports (MERs) submitted by nephrologists, by federal mandate, within 45 days of a patient starting a new ESRD treatment. For this study, data regarding GN subtype, date and modality of first ESRD treatment, demographic characteristics, insurance payer, employment status, residential zip code, comorbidities and baseline laboratory values were extracted from USRDS Patients and MedEvid files. To better characterize socioeconomic status, residential zip codes (or dialysis facility zip code if residential zip code was missing) were matched to year 2000 US census data to obtain neighborhood-level socioeconomic variables.
Exposures, outcomes and covariates
Primary exposure
GN subtype was defined as the cause of ESRD cited in patients' first MERs. The sensitivity of this measure for detection of GN subtype is poor overall, but specificity is excellent, with a reported false-positive rate of <2% [32].
Outcomes
The first ESRD treatment modality (transplantation, peritoneal dialysis or hemodialysis) was the primary study outcome. Transplantation was defined as deceased or living donor kidney transplantation performed on the same day as a patient's first ESRD treatment.
Covariates
Variables that might potentially confound exposure–outcome associations were considered a priori and grouped into clinically meaningful categories: temporal (year of first ESRD treatment); geographic [region of residence in the USA (Northeast, Midwest, South or West)]; demographic [age, sex, race (white, black, Asian, other), Hispanic ethnicity (yes/no)]; patient-level socioeconomic [insurance payer (Medicare, Medicaid, Veterans Administration, employer group, other) and employment status (employed/unemployed)]; neighborhood-level socioeconomic status [percent below poverty line, percent with less than a high school education, percent unemployment, median household income, median rent]; and clinical, including comorbidities (diabetes, heart failure, coronary heart disease, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, current smoking, cancer, peripheral vascular disease and inability to ambulate), body mass index (BMI, kg/m2) and laboratory values reported at ESRD treatment initiation [albumin, hemoglobin, serum creatinine and estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula [33]].
Statistical analyses
We used cross tabulation and distribution plots to examine unadjusted differences in baseline characteristics across GN subtypes. Categorical variables were summarized as frequencies and proportions. Continuous variables were summarized as medians and interquartile ranges or as means and standard deviations, as appropriate.
Relative log odds of transplantation versus hemodialysis and peritoneal dialysis versus hemodialysis were compared between GN subtypes using multinominal logistic regression. IgAN was the exposure reference group; dummy variables were assigned to comparator GN subtypes. To account for confounding, we sequentially added groups of covariates to regression models: first temporal and geographic (Model 1), then demographic (Model 2), then patient-level and neighborhood-level socioeconomic (Model 3) and finally clinical, including comorbidities, laboratory variables and BMI (Model 4). Due to high collinearity between the variables serum creatinine and eGFR, only the former was retained in statistical models. Squared terms were included for continuous variables (age, BMI, albumin, hemoglobin and creatinine) based on the statistical significance of the squared term in logistic models.
Missing data
The proportion of variables with missing data ranged from <0.1% (age, sex and race) to 23.0% (serum albumin). A total of 32.2% of patients had at least one variable missing. We assumed data to be missing at random and used a fully conditional specification approach [34, 35] to obtain 30 imputed data sets [36]. The imputation model included all the variables in Model 4. Log odds ratios (ORs) from models applied to each imputation data set were combined using the rules described by Little and Rubin [37] and exponentiated to obtain estimated ORs and corresponding 95% confidence intervals (CIs). As a sensitivity analysis, we also report complete case analysis results, which included only patients with complete outcome and covariate data (67.8% of the total sample).
Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and StataMP version 13 (StataCorp, College Station, TX, USA). An internal review board of the Stanford University School of Medicine approved the study.
RESULTS
Patient characteristics
The final study population comprised 75 278 patients with ESRD due to GN: 30 604 (40.7%) with FSGS, 15 805 (21.0%) with LN, 12 178 (16.2%) with IgAN, 6084 (8.1%) with vasculitis, 6001 (8.0%) with MN and 4606 (6.1%) with MPGN, Table 1. The mean age was 47.0 ± 15.5 years. The study population was 53% male, 61% white and 12% Hispanic. Demographic characteristics differed considerably across GN subtypes: mean age ranged from 40 to 57 years in LN and vasculitis, respectively; male sex ranged from 18 to 68% in LN and IgAN, respectively; and black race ranged from 7 to 50% in IgAN and LN, respectively. Most comorbidities were least common in IgAN and LN. Patients with LN lived in the most socioeconomically disadvantaged neighborhoods, relied most upon Medicaid insurance and had a relatively high prevalence of unemployment.
Table 1.
Patient characteristics at initiation of ESRD therapy (transplantation, PD or HD), according to GN subtype, 1996–2011 (n = 75 278)
| Primary GN subtypes |
Secondary GN subtypes |
|||||
|---|---|---|---|---|---|---|
| FSGS | IgAN | MN | MPGN | LN | Vasculitis | |
| [n = 30 604 (40.7%)] | [n = 12 178 (16.2%)] | [n = 6001 (8.0%)] | [n = 4606 (6.1%)] | [n = 15 805 (21.0%)] | [n = 6084 (8.1%)] | |
| Age (years), mean (SD) | 48.1 (15.3) | 44.1 (14.2) | 54.1 (13.9) | 49.6 (15.1) | 39.8 (13.9) | 57.3 (14.1) |
| Male sex | 18 801 (61.4) | 8235 (67.6) | 4002 (66.7) | 2798 (60.7) | 2851 (18.0) | 3291 (54.1) |
| Race | ||||||
| White | 17 173 (56.1) | 9144 (75.1) | 3999 (66.7) | 3391 (73.7) | 6735 (42.6) | 5299 (87.1) |
| Black | 11 984 (39.2) | 823 (6.8) | 1735 (28.9) | 888 (19.3) | 7909 (50.1) | 533 (8.8) |
| Asian | 1011 (3.3) | 1851 (15.2) | 174 (2.9) | 216 (4.7) | 848 (5.4) | 130 (2.1) |
| Other | 432 (1.4) | 360 (3.0) | 91 (1.5) | 107 (2.3) | 309 (2.0) | 120 (2.0) |
| Hispanic ethnicity | 2658 (9.1) | 1527 (13.0) | 594 (10.3) | 484 (11.0) | 2482 (16.3) | 600 (10.1) |
| Insurance payer | ||||||
| Medicaid | 5940 (19.6) | 1644 (13.6) | 1078 (18.1) | 994 (21.8) | 5123 (32.6) | 809 (13.3) |
| Medicare | 8216 (27.1) | 1968 (16.3) | 2193 (36.9) | 1347 (29.5) | 3913 (24.9) | 2602 (42.9) |
| Veterans | 528 (1.7) | 178 (1.5) | 132 (2.2) | 118 (2.6) | 104 (0.7) | 77 (1.3) |
| Employer group | 12 874 (42.4) | 6547 (54.2) | 2243 (37.7) | 1740 (38.1) | 5516 (35.1) | 2230 (36.8) |
| Other | 5954 (19.6) | 2232 (18.5) | 1463 (24.6) | 954 (20.9) | 2491 (15.8) | 1745 (28.8) |
| Employed | 8912 (29.4) | 5176 (42.8) | 1432 (24.1) | 1103 (24.2) | 3115 (19.8) | 1071 (17.7) |
| Neighborhood characteristics | ||||||
| Percent below poverty line, mean (SD) | 14.0 (9.6) | 11.7 (8.6) | 13.5 (9.3) | 13.1 (9.2) | 15.5 (10.1) | 12.0 (8.4) |
| Percent less than high school, mean (SD) | 21.5 (11.6) | 19.5 (11.8) | 21.4 (11.6) | 20.6 (11.4) | 23.5 (12.7) | 19.9 (11.1) |
| Percent unemployment, mean (SD) | 6.7 (4.4) | 5.8 (3.9) | 6.4 (4.1) | 6.3 (4.2) | 7.3 (4.6) | 5.8 (3.7) |
| Median household income, mean (SD) | 41 840 (15 774) | 45 741 (16 801) | 42 067 (15 777) | 42 542 (15 465) | 41 085 (15 724) | 43 348 (15 631) |
| Median rent, mean (SD) | 520 (253) | 562 (291) | 514 (256) | 524 (260) | 537 (249) | 515 (273) |
| Comorbidities | ||||||
| Diabetes | 3979 (13.1) | 1067 (8.8) | 881 (14.8) | 674 (14.8) | 1390 (8.8) | 963 (15.9) |
| Heart failure | 3783 (12.5) | 847 (7.0) | 1011 (17.0) | 744 (16.3) | 2283 (14.5) | 919 (15.2) |
| Coronary heart disease | 3075 (10.1) | 674 (5.6) | 778 (13.1) | 406 (8.9) | 929 (5.9) | 713 (11.8) |
| CVA/TIA | 1063 (3.5) | 256 (2.1) | 303 (5.1) | 175 (3.8) | 803 (5.1) | 298 (4.9) |
| Hypertension | 23 958(78.9) | 9638 (79.8) | 4667 (78.4) | 3557 (78.0) | 11 872 (75.5) | 4023 (66.3) |
| COPD | 1562 (5.1) | 289 (2.4) | 395 (6.6) | 296 (6.5) | 355 (2.3) | 570 (9.4) |
| Current smoker | 2429 (8.0) | 597 (4.9) | 496 (8.3) | 432 (9.5) | 675 (4.3) | 320 (5.3) |
| Cancer | 1220 (4.0) | 281 (2.3) | 330 (5.5) | 231 (5.1) | 237 (1.5) | 297 (4.9) |
| PVD | 1284 (4.2) | 298 (2.5) | 344 (5.8) | 197 (4.3) | 516 (3.3) | 415 (6.8) |
| Nonambulant | 373 (1.2) | 88 (0.7) | 116 (1.9) | 84 (1.8) | 351 (2.2) | 163 (2.7) |
| BMI (kg/m2), mean (SD) | 29.2 (8.0) | 27.8 (6.9) | 28.0 (7.0) | 26.6 (6.6) | 26.4 (7.2) | 26.9 (6.6) |
| BMI, missing | 1451 (4.7) | 555 (4.6) | 298 (5.0) | 233 (5.1) | 643 (4.1) | 257 (4.2) |
| Laboratory variables | ||||||
| Albumin (g/dL), mean (SD) | 3.3 (0.8) | 3.5 (0.7) | 2.9 (0.9) | 3.0 (0.8) | 2.9 (0.8) | 3.0 (0.7) |
| Albumin missing | 7074 (23.1) | 2743 (22.5) | 1321 (22.0) | 1031 (22.4) | 3745 (23.7) | 1422 (23.4) |
| Hemoglobin (g/dL), mean (SD) | 10.1 (1.9) | 10.1 (1.9) | 9.9 (1.8) | 9.8 (1.8) | 9.4 (1.8) | 9.6 (1.7) |
| Hemoglobin missing | 3563 (11.6) | 1406 (11.5) | 696 (11.6) | 547 (11.9) | 1601 (10.1) | 649 (10.7) |
| eGFR (mL/min/1.73 m2) mean (SD) | 8.5 (4.1) | 8.3 (4.0) | 9.0 (4.5) | 9.4 (4.6) | 9.3 (4.6) | 8.4 (4.2) |
| eGFR missing | 825 (2.7) | 259 (2.1) | 163 (2.7) | 138 (3.0) | 390 (2.5) | 108 (1.8) |
| Creatinine (mg/dL), mean (SD) | 8.6 (4.3) | 8.5 (4.0) | 8.0 (3.9) | 7.6 (3.7) | 7.4 (3.5) | 7.8 (3.7) |
| Creatinine, missing | 649 (2.1) | 182 (1.5) | 106 (1.8) | 83 (1.8) | 218 (1.4) | 75 (1.2) |
All values represent n (%) except where otherwise stated.
ESRD, end-stage renal disease; PD, peritoneal dialysis; HD, hemodialysis; GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; LN, lupus nephritis; CVA, cerebrovascular accident; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Treatment modality outcomes
A majority of patients (79.7%) received hemodialysis as their first ESRD treatment modality. By comparison, only 13.8% received peritoneal dialysis and 6.4% received a pre-emptive kidney transplant (Table 2).
Table 2.
Proportion of patients receiving transplantation, PD and HD as a first ESRD treatment, 1996–2011, according to GN subtype (n = 75 278)
| Primary GN subtypes |
Secondary GN subtypes |
|||||
|---|---|---|---|---|---|---|
| FSGS | IgAN | MN | MPGN | LN | Vasculitis | |
| [n = 30 604 (40.7%)] | [n = 12 178 (16.2%)] | [n = 6001 (8.0%)] | [n = 4606 (6.1%)] | [n = 15 805 (21.0%)] | [n = 6084 (8.1%)] | |
| Kidney transplantation | 2061 (6.7) | 1523 (12.5) | 300 (5.0) | 322 (7.0) | 496 (3.1) | 121 (2.0) |
| Living donor | 1583 (76.8) | 1268 (83.3) | 228 (76.0) | 234 (72.7) | 385 (77.6) | 97 (80.2) |
| Deceased donor | 464 (22.5) | 247 (16.2) | 72 (24.0) | 82 (25.5) | 107 (21.6) | 24 (19.8) |
| Unknown donor | 14 (0.68) | 8 (0.53) | 0 (0.0) | 6 (1.9) | 4 (0.8) | 0 (0.0) |
| Peritoneal dialysis | 4638 (15.3) | 2107 (17.5) | 828 (13.9) | 553 (12.2) | 1720 (11.0) | 456 (7.5) |
| CCPD/IPD | 3064 (65.9) | 1400 (66.3) | 564 (68.0) | 360 (65.0) | 1190 (69.0) | 315 (69.1) |
| CAPD | 1585 (34.1) | 712 (33.7) | 266 (32.0) | 194 (35.0) | 534 (31.0) | 141 (30.9) |
| Hemodialysis | 23 566 (77.9) | 8390 (69.8) | 4808 (81.0) | 3669 (80.7) | 13 478 (85.9) | 5477 (90.5) |
| Missing modality | 339 (1.1) | 158 (1.3) | 65 (1.1) | 62 (1.3) | 111 (0.7) | 30 (0.5) |
All values represent n (%).
PD, peritoneal dialysis; HD, hemodialysis; ESRD, end-stage renal disease; GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; LN, lupus nephritis; CCPD, continuous cycling PD; IPD, intermittent PD; CAPD, continuous ambulatory PD.
In Model 1, which adjusted for region of residence and year of ERSD only, patients with all other primary and secondary GN subtypes were less likely to receive either a transplant or peritoneal dialysis than patients with IgAN (Table 3). Sequentially accounting for demographic, socioeconomic and clinical differences among subtypes (Models 2, 3 and 4, respectively) substantially attenuated ORs for both transplantation and peritoneal dialysis when comparing all other primary GN subtypes (FSGS, MN and MPGN) to IgAN (Table 3 and Supplementary data, Figure S1). In the fully adjusted model (Model 4), transplantation [OR 1.19 (95% CI 1.01–1.39)] and peritoneal dialysis [OR 1.30 (95% CI 1.18–1.43)] were more likely in MN than in IgAN and were equally as likely in the other primary GN subtypes (FSGS and MPGN) as in IgAN (Table 3 and Figure 1).
Table 3.
ORs with 95% CIs for transplantation versus hemodialysis and for PD versus hemodialysis, according to GN subtype
| Primary GN subtypes |
Secondary GN subtypes |
|||||
|---|---|---|---|---|---|---|
| FSGS | IgAN | MN | MPGN | LN | Vasculitis | |
| [n = 30 604 (40.7%)] | [n = 12 178 (16.2%)] | [n = 6001 (8.0%)] | [n = 4606 (6.1%)] | [n = 15 805 (21.0%)] | [n = 6084 (8.1%)] | |
| TX versus HD | ||||||
| Model 1 | 0.50 (0.46–0.54) | Ref | 0.36 (0.31–0.41) | 0.50 (0.44–0.57) | 0.22 (0.19–0.24) | 0.12 (0.10–0.15) |
| Model 2 | 0.85 (0.79–0.92) | Ref | 0.61 (0.54–0.70) | 0.68 (0.59–0.77) | 0.35 (0.31–0.39) | 0.18 (0.15–0.22) |
| Model 3 | 0.95 (0.88–1.03) | Ref | 0.73 (0.63–0.83) | 0.84 (0.73–0.96) | 0.41 (0.37–0.46) | 0.21 (0.17–0.25) |
| Model 4 | 0.98 (0.93–1.15) | Ref | 1.19 (1.01–1.39) | 1.08 (0.93–1.26) | 0.49 (0.43–0.56) | 0.27 (0.22–0.34) |
| PD versus HD | ||||||
| Model 1 | 0.79 (0.74–0.83) | Ref | 0.68 (0.63–0.75) | 0.60 (0.54–0.66) | 0.50 (0.47–0.54) | 0.33 (0.30–0.37) |
| Model 2 | 0.95 (0.90–1.01) | Ref | 0.85 (0.78–0.93) | 0.66 (0.59–0.73) | 0.53 (0.49–0.57) | 0.37 (0.33–0.42) |
| Model 3 | 1.02 (0.96–1.08) | Ref | 0.93 (0.85–1.02) | 0.74 (0.67–0.82) | 0.60 (0.55–0.64) | 0.40 (0.36–0.45) |
| Model 4 | 1.05 (0.98–1.12) | Ref | 1.30 (1.18–1.43) | 0.95 (0.85–1.06) | 0.76 (0.70–0.82) | 0.54 (0.48–0.60) |
Multiple imputation analysis (n = 75 278).
HD, hemodialysis; PD, peritoneal dialysis; GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; LN, lupus nephritis; TX, transplantation.
Model 1: adjusted for year of first ESRD therapy and region of residence.
Model 2: adjusted for Model 1 variables plus demographic variables (age, sex, race, ethnicity).
Model 3: adjusted for Model 2 variables plus patient-level (employment status, insurance payer) and neighborhood-level (percent below poverty line, percent less than high school education, percent unemployment, median income, median rent) socioeconomic variables.
Model 4: adjusted for Model 3 variables plus comorbidities (diabetes, heart failure, coronary heart disease, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, current smoking, cancer, peripheral vascular disease, nonambulant status), body mass index (BMI) and laboratory variables (serum albumin, hemoglobin, serum creatinine).
FIGURE 1:
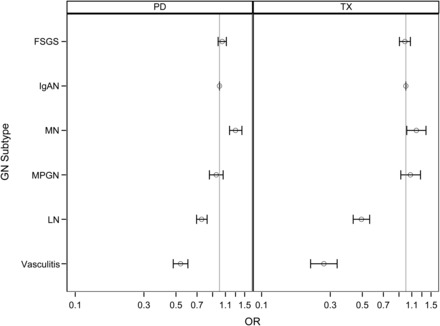
ORs with 95% CIs for peritoneal dialysis (PD) versus hemodialysis and kidney transplantation (TX) versus hemodialysis, comparing focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), membranoproliferative glomerulonephritis (MPGN), lupus nephritis (LN) and vasculitis to the reference group IgA nephropathy (IgAN). ORs were computed using multinomial regression, adjusting for year of ESRD, geographic region, sociodemographic factors, comorbidities and laboratory variables (Model 4). Missing data were imputed using multiple imputation techniques (n = 75 278).
For secondary GN subtypes (LN and vasculitis), the odds for transplantation and peritoneal dialysis remained significantly lower than in IgAN, even after adjusting for confounding. In the fully adjusted model (Model 4), ORs for preemptive kidney transplantation were 0.49 (95% CI 0.43–0.56) for LN and 0.27 (95% CI 0.22–0.34) for vasculitis, comparing with IgAN, (Table 3 and Figure 1). Odds ratios for peritoneal dialysis were 0.76 (95% CI 0.70–0.82) and 0.54 (95% CI 0.48–0.60), respectively.
Sensitivity analysis
Analyses of 51 071 patients with complete data for all covariates did not identify any meaningful differences in effect estimates or statistical inferences compared with analyses using multiply imputed data (Supplementary data, Table S1 and Figures S2 and S3).
DISCUSSION
We have demonstrated in a national cohort of 75 278 patients with ESRD attributed to GN that GN subtype independently associates with first ESRD treatment modality. After accounting for temporal, geographic, demographic, socioeconomic and comorbidity differences among GN subtypes, patients with two common secondary GN subtypes—LN and vasculitis—had approximately one-half and one-quarter the odds, respectively, of receiving a preemptive kidney transplant as patients with IgAN. Patients with LN or vasculitis were also significantly less likely to receive peritoneal dialysis. In contrast, patients with comparator primary GN subtypes (FSGS, MN and MPGN) were less likely to receive a kidney transplant or peritoneal dialysis than patients with IgAN in unadjusted analyses only. After adjustment for demographic, socioeconomic and clinical factors previously reported to influence ESRD modality distributions, these differences disappeared and did not appear to independently relate to GN subtype.
These imbalances in initial ESRD treatment modality suggest that approaches to ESRD treatment in patients with GN—rightly or wrongly—differ considerably depending upon GN subtype. We suspect that this finding previously escaped detection in research and public health reporting as patients with GN are conventionally grouped in to a single disease category when examining ESRD practice patterns and outcomes, ignoring potential heterogeneity among subtypes [38]. In a recent study challenging this paradigm, we identified strong and independent associations between GN subtype and mortality in a national ESRD patient cohort [39]. The present study advances upon these findings by focusing on a potentially important, and modifiable, explanation for survival imbalances.
There are several potentially plausible reasons for the reduced access to pre-emptive kidney transplantation we observed in LN and vasculitis. On the one hand, delaying kidney transplantation might be clinically appropriate or unavoidable in these patient groups. Patients with secondary GN subtypes might be more likely than patients with primary GN subtypes to be systemically unwell at the onset of ESRD, contraindicating early kidney transplantation. Rapidity of onset of ESRD may be another important discriminating factor. Progression to ESRD can be particularly rapid in vasculitis, necessitating emergent commencement of dialysis and precluding timely counseling, planning and preparation for kidney transplantation. For example, one study of 136 patients with ESRD due to ANCA vasculitis reported that ESRD was already established at the time of vasculitis diagnosis in 51% of patients [40]. In LN, however, rapid progression to ESRD occurs much less frequently [41], with a median time from LN diagnosis to ESRD of 36 months in one study [42]. This pace of progression should allow sufficient time to plan for pre-emptive kidney transplantation (and/or peritoneal dialysis).
On the other hand, reduced access to kidney transplantation in LN and vasculitis could be viewed as an example of inequitable care, i.e. these patient groups are being denied the best available ESRD treatment. Supporting this possibility, lower odds of transplantation in LN or vasculitis persisted even after adjusting for differences in laboratory surrogates of inflammation or malnutrition (hemoglobin, albumin and serum creatinine), in severity of renal dysfunction (as measured by serum creatinine) at initiation of ERSD treatment and in medical comorbidity burden. While residual confounding by illness acuity and severity undoubtedly persists, we suggest that it is unlikely to explain the magnitude of the differences in access to transplantation we observed. We wonder whether it is instead possible that the management of patients with these secondary GN subtypes focuses more on controlling systemic disease manifestations and less on current and future aspects of renal care.
We speculate that physician biases and preferences might also influence transplant-related decisions in LN and vasculitis. Uncertainty among physicians regarding the safety and optimal timing of kidney transplantation in vasculitis has previously been reported. In a survey of 32 nephrologists in Europe, 40% agreed that transplantation should be delayed for at least 12 months after vasculitis remission induction and 16% felt that transplantation should be postponed until ANCA antibodies become undetectable [43]. This tendency to delay kidney transplantation in vasculitis is poorly supported by data. Although a higher mortality in patients transplanted within a year of receiving vasculitis induction therapy has been reported [43], this finding has not been replicated. The presence of detectable ANCA antibody at the time of kidney transplantation has not been shown to negatively impact posttransplant outcomes [43, 44]. Patient and allograft survival rates after transplantation are comparable, if not superior, to those in other causes of ERSD [11, 45, 46]. Finally, delaying kidney transplantation by preceding it with a longer duration of dialysis is not protective against posttransplantation vasculitis relapse [44]. On balance, there is little reason to believe that patients with vasculitis should not enjoy the same benefits afforded by preemptive kidney transplantation as the general ESRD population, and clinical practice guidelines advising caution appear unsupported by data [47, 48]. Clearly, studies aiming to establish best practices with respect to the timing of kidney transplantation in vasculitis are urgently needed. We are hopeful that recent reports demonstrating superior patient and allograft outcomes following pre-emptive and early kidney transplantation in patients with ESRD due to LN [49–51] will not only promote increased uptake of these transplant strategies in LN, but will also encourage the conduct of similar studies in other GN subtypes, including vasculitis.
While the reduced likelihood of transplantation we identified in patients with LN and vasculitis is the most striking and concerning finding arising from this study, the reduced likelihood of peritoneal dialysis (versus hemodialysis) in these GN subtypes also merits discussion. Although peritoneal dialysis does not prolong overall patient survival over hemodialysis, it offers some important health benefits, including greater treatment-related satisfaction [17, 18], reduced healthcare costs [20] and possibly less early mortality [16]. Peritoneal dialysis compared with hemodialysis before transplantation is also associated with superior allograft outcomes [52, 53]. Peritoneal dialysis is also viable—and associated with less infectious complications than hemodialysis—in patients who require urgent, unplanned dialysis initiation, factors relevant to patients with rapidly progressive GN or to those receiving immunosuppressive therapy [19]. Accordingly, we posit that concerns over potentially (and unproven) increased risks for peritonitis among patients with LN or vasculitis should not preclude peritoneal dialysis as a treatment option in these patient groups, and that any potential risks are likely counterbalanced by high risks for vascular access infection with hemodialysis. Ultimately, the relative merits of peritoneal dialysis and hemodialysis should be weighed carefully for each individual patient commencing dialysis therapy. Our study did not attempt to address the relative efficacy of these dialysis approaches in patients with GN. However, our findings suggest that peritoneal dialysis might be a grossly underutilized modality in LN and vasculitis.
One final and somewhat unexpected finding arising from this study was the greater use of transplantation and peritoneal dialysis we observed in MN than in IgAN, after adjusting for differences in case mix. A priori, we hypothesized that patients with IgAN—which typically exhibits a relatively slow rate of progression to ESRD and a low requirement for immunosuppressive therapy—would be the group most likely to receive transplantation or peritoneal dialysis as an initial ESRD treatment modality. Membranous nephropathy, on the other hand, typically presents with nephrotic syndrome, is often treated with immunosuppressive therapy and is associated with earlier recurrence in the transplanted kidney, factors that might discourage physicians from recommending peritoneal dialysis or kidney transplantation in this patient group. Although we cannot confirm this hypothesis, we wonder whether the more florid presentation of MN with nephrotic syndrome, compared with nephritic syndrome in IgAN, might result in earlier presentation of otherwise similar patients to a nephrologist, which might expedite ESRD planning. Clearly, this specific finding has less face validity than the consistently lower odds for transplantation and peritoneal dialysis we observed in all unadjusted and adjusted analyses in either LN or vasculitis, and should be interpreted cautiously.
Our study has certain limitations. First, we cannot verify the accuracy of GN subtype designations submitted to the USRDS. A previous study demonstrated poor agreement between cause of ESRD diagnoses submitted to the USRDS in MERs and those identified by kidney biopsy (the gold standard) in 217 patients with ESRD due to GN, a finding largely explained by a high proportion of patients with missing and nonspecific diagnoses in MERs [32]. However, once a specific GN subtype was selected in a MER, specificity for GN subtype was excellent, with a <2% false-positive rate. Agreement also improved after 1995, following a revision to cause of ESRD diagnosis categories in the MER in that year. Accordingly, we restricted our study population to patients with a specific GN subtype diagnosis who were enrolled in the USRDS after 1995. Thus, the internal validity of our study is strengthened, although study findings may not be directly applicable to patients with GN who are misclassified in the USRDS or who lack a definitive histologic or serologic diagnosis. Second, although we adjusted for multiple variables known to associate with access to transplantation or peritoneal dialysis [7, 21–24, 26, 29, 30, 54], residual confounding by misclassified or unmeasured covariates might persist. We anticipate that misclassification of measured covariates is likely to be nondifferential across GN subtypes and to bias toward the null. With respect to unmeasured covariates, we lacked data on some relevant socioeconomic variables that might impact modality choice, such as personal educational attainment [7, 23, 55] and marital status [23, 54]; however, we expect to have captured some of the effect from these variables by adjusting for employment status, insurance payer and neighborhood-level socioeconomic characteristics. Third, we could not distinguish the relative contributions from appropriate and inappropriate treatment-related decisions to study findings. For example, we lacked information regarding the duration of GN prior to ESRD, a factor that might strongly influence modality choice. Neither could we directly measure systemic disease activity or immunosuppressive medication burden at or before ESRD, relying instead upon laboratory markers and comorbidities as surrogate measures of acute illness. Finally, our study focused on first ESRD treatment modality and did not examine modality transitions over time. We nevertheless posit that first ESRD modality is a particularly important clinical outcome, in light of evidence supporting preemptive kidney transplantation as the optimal transplant approach [7, 50, 56] and reports advocating for peritoneal dialysis as the preferred first-line dialysis strategy [57].
Despite these limitations, our study has numerous strengths. To our knowledge, practice patterns regarding dialysis and transplantation have not previously been compared across GN subtypes, and imbalances identified in this study have not previously been reported. As a population-based study, our findings largely apply to all US patients with known ESRD due to GN and can guide the design of comparative effectiveness research aiming to elucidate underlying reasons for, and consequences of, observed disparities. Misclassification of our primary exposure and outcome is likely to be infrequent and nondifferential, minimizing selection bias. We applied sophisticated statistical techniques to overcome shortfalls inherent to observational trial design, including multivariate regression to account for confounding and multiple imputation to handle missing data.
To conclude, we have demonstrated in a nationally representative ESRD population that initial ESRD treatment modality differs substantially across GN subtypes in ways that are fully explained by observed patient characteristics among primary GN subtypes but only partially so among the secondary GN subtypes LN and vasculitis. The underlying reasons for these disparities should be further investigated, and future studies comparing outcomes between ESRD treatment modalities in GN should consider the effect of GN subtype when designing study protocols and reporting study findings.
CONFLICT OF INTEREST STATEMENT
W.C.W. reports having served as an advisor or consultant, unrelated to the topic of this article, to ACUMEN, Amgen, Astra-Zeneca, Bayer, Keryx, Medtronic, Mitshubishi-Tanabe and Rockwell Pharma. R.A.L. reports having served as an advisor or consultant, unrelated to the topic of this article, to Genentech, Fibrogen and Questcor. M.M.O. and M.E.M.-R. have no financial disclosures to report.
Supplementary Material
ACKNOWLEDGEMENTS
The manuscript was reviewed and approved for publication by an officer of the National Institute of Diabetes and Digestive and Kidney Diseases. The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. M.M.O. was supported by a Ben J. Lipps Research Fellowship from the American Society of Nephrology (ASN) Foundation for Kidney Research. The Stanford nephrology fellowship program is supported by grant T32DK007357. W.C.W. receives salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
REFERENCES
- 1. United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 2. Rabbat CG, Thorpe KE, Russell JD et al. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol 2000; 11: 917–922 [DOI] [PubMed] [Google Scholar]
- 3. Wolfe RA, Ashby VB, Milford EL et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 4. Meier-Kriesche HU, Schold JD, Srinivas TR et al. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 2004; 4: 1662–1668 [DOI] [PubMed] [Google Scholar]
- 5. Cameron JI, Whiteside C, Katz J et al. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000; 35: 629–637 [DOI] [PubMed] [Google Scholar]
- 6. Goldfarb-Rumyantzev A, Hurdle JF, Scandling J et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrol Dial Transplant 2005; 20: 167–175 [DOI] [PubMed] [Google Scholar]
- 7. Kasiske BL, Snyder JJ, Matas AJ et al. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol 2002; 13: 1358–1364 [DOI] [PubMed] [Google Scholar]
- 8. Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002; 74: 1377–1381 [DOI] [PubMed] [Google Scholar]
- 9. Andresdottir MB, Hoitsma AJ, Assmann KJ et al. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol 2001; 56: 279–288 [PubMed] [Google Scholar]
- 10. Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 2005; 16: 1859–1865 [DOI] [PubMed] [Google Scholar]
- 11. Hruskova Z, Stel VS, Jayne D et al. Characteristics and outcomes of granulomatosis with polyangiitis (Wegener) and microscopic polyangiitis requiring renal replacement therapy: results from the European Renal Association-European Dialysis and Transplant Association Registry. Am J Kidney Dis 2015; 66: 613–620 [DOI] [PubMed] [Google Scholar]
- 12. Mehrotra R, Chiu YW, Kalantar-Zadeh K et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 13. Chang YS, Liu CJ, Wu TH et al. Survival analysis in systemic lupus erythematosus patients on maintenance dialysis: a nationwide population-based study in Taiwan. Rheumatology (Oxford) 2013; 52: 166–172 [DOI] [PubMed] [Google Scholar]
- 14. Contreras G, Pagan J, Chokshi R et al. Comparison of mortality of ESRD patients with lupus by initial dialysis modality. Clin J Am Soc Nephrol 2014; 9: 1949–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang SH, Chung BH, Choi SR et al. Comparison of clinical outcomes by different renal replacement therapy in patients with end-stage renal disease secondary to lupus nephritis. Korean J Intern Med 2011; 26: 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinhandl ED, Foley RN, Gilbertson DT et al. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010; 21: 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fadem SZ, Walker DR, Abbott G et al. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol 2011; 6: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubin HR, Fink NE, Plantinga LC et al. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 2004; 291: 697–703 [DOI] [PubMed] [Google Scholar]
- 19. Koch M, Kohnle M, Trapp R et al. Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrol Dial Transplant 2012; 27: 375–380 [DOI] [PubMed] [Google Scholar]
- 20. Berger A, Edelsberg J, Inglese GW et al. Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease. Am J Manag Care 2009; 15: 509–518 [PubMed] [Google Scholar]
- 21. Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 1998; 280: 1148–1152 [DOI] [PubMed] [Google Scholar]
- 22. Patzer RE, Amaral S, Wasse H et al. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 2009; 20: 1333–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol 2002; 13: 1279–1287 [DOI] [PubMed] [Google Scholar]
- 24. Jager KJ, Korevaar JC, Dekker FW et al. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 2004; 43: 891–899 [DOI] [PubMed] [Google Scholar]
- 25. Miskulin DC, Meyer KB, Athienites NV et al. Comorbidity and other factors associated with modality selection in incident dialysis patients: the CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis 2002; 39: 324–336 [DOI] [PubMed] [Google Scholar]
- 26. Ayanian JZ, Cleary PD, Weissman JS et al. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 1999; 341: 1661–1669 [DOI] [PubMed] [Google Scholar]
- 27. Arce CM, Goldstein BA, Mitani AA et al. Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clin J Am Soc Nephrol 2013; 8: 2149–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devlin A, Waikar SS, Solomon DH et al. Variation in initial kidney replacement therapy for end-stage renal disease due to lupus nephritis in the United States. Arthritis Care Res 2011; 63: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johansen KL, Zhang R, Huang Y et al. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol 2012; 7: 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall YN, O'Hare AM, Young BA et al. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. Am J Transplant 2008; 8: 2402–2409 [DOI] [PubMed] [Google Scholar]
- 31. O'Shaughnessy MM, Montez-Rath ME, Lafayette RA et al. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol 2015; 10: 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Layton JB, Hogan SL, Jennette CE et al. Discrepancy between medical evidence form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 2010; 5: 2046–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 34. Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999; 18: 681–694 [DOI] [PubMed] [Google Scholar]
- 35. Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM et al. Fully conditional specification in multivariate imputation. J Stat Comput Simul 2006; 76: 1049–1064 [Google Scholar]
- 36. Montez-Rath ME, Winkelmayer WC, Desai M. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 2014; 9: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Little R, Rubin DB. Statistical Analysis with Missing Data, 2nd edn Hoboken, NJ: John Wiley & Sons, 2002 [Google Scholar]
- 38. United States Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 39. O'Shaughnessy MM, Montez-Rath ME, Lafayette RA et al. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol 2015; 10: 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lionaki S, Hogan SL, Jennette CE et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int 2009; 76: 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nossent HC, Swaak TJ, Berden JH. Systemic lupus erythematosus: analysis of disease activity in 55 patients with end-stage renal failure treated with hemodialysis or continuous ambulatory peritoneal dialysis. Dutch Working Party on SLE. Am J Med 1990; 89: 169–174 [DOI] [PubMed] [Google Scholar]
- 42. Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med 1992; 152: 2082–2088 [PubMed] [Google Scholar]
- 43. Little MA, Hassan B, Jacques S et al. Renal transplantation in systemic vasculitis: when is it safe? Nephrol Dial Transplant 2009; 24: 3219–3225 [DOI] [PubMed] [Google Scholar]
- 44. Nachman PH, Segelmark M, Westman K et al. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int 1999; 56: 1544–1550 [DOI] [PubMed] [Google Scholar]
- 45. Geetha D, Eirin A, True K et al. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a multicenter experience. Transplantation 2011; 91: 1370–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen J, Gill J, Shangguan M et al. Outcomes of renal transplantation in recipients with Wegener's granulomatosis. Clin Transplant 2011; 25: 380–387 [DOI] [PubMed] [Google Scholar]
- 47. Kidney Disease: Improving Global Outcomes Glomerulonephritis Workgroup. KDIGO clinical practice guideline for glomerulonephritis. Chapter 13: Pauci-immune focal and segmental necrotizing glomerulonephritis. Kidney Int Suppl 2012; 2: 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knoll G, Cockfield S, Blydt-Hansen T et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ 2005; 173: 1181–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chung MC, Yu TM, Shu KH et al. Influence of pretransplantation dialysis time and lupus activity on outcome of kidney transplantation in systemic lupus erythematosus. Transplant Proc 2014; 46: 336–338 [DOI] [PubMed] [Google Scholar]
- 50. Naveed A, Nilubol C, Melancon JK et al. Preemptive kidney transplantation in systemic lupus erythematosus. Transplant Proc 2011; 43: 3713–3714 [DOI] [PubMed] [Google Scholar]
- 51. Plantinga LC, Patzer RE, Drenkard C et al. Association of time to kidney transplantation with graft failure among U.S. patients with end-stage renal disease due to lupus nephritis. Arthritis Care Res 2015; 67: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang H, Chelamcharla M, Baird BC et al. Factors affecting kidney-transplant outcome in recipients with lupus nephritis. Clin Transplant 2008; 22: 263–272 [DOI] [PubMed] [Google Scholar]
- 53. Bleyer AJ, Burkart JM, Russell GB et al. Dialysis modality and delayed graft function after cadaveric renal transplantation. J Am Soc Nephrol 1999; 10: 154–159 [DOI] [PubMed] [Google Scholar]
- 54. Chanouzas D, Ng KP, Fallouh B et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 2012; 27: 1542–1547 [DOI] [PubMed] [Google Scholar]
- 55. Schaeffner ES, Mehta J, Winkelmayer WC. Educational level as a determinant of access to and outcomes after kidney transplantation in the United States. Am J Kidney Dis 2008; 51: 811–818 [DOI] [PubMed] [Google Scholar]
- 56. Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344: 726–731 [DOI] [PubMed] [Google Scholar]
- 57. Ghaffari A, Kalantar-Zadeh K, Lee J et al. PD First: peritoneal dialysis as the default transition to dialysis therapy. Semin Dial 2013; 26: 706–713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


