Abstract
Objective
Insomnia and Major Depressive Disorders (MDD) often co-occur, and such comorbidity has been associated with poorer outcomes for both conditions. However, individual differences in depressive symptom trajectories during and after treatment are poorly understood in comorbid insomnia and depression. This study explored the heterogeneity in long-term depression change trajectories, and examined their correlates, particularly insomnia-related characteristics.
Method
148 adults (age M±SD=46.6±12.6, 73.0% female) with insomnia and MDD received antidepressant pharmacotherapy, and were randomized to 7-session Cognitive Behavioral Therapy for Insomnia or control conditions over 16 weeks with 2-year follow-up. Depression and insomnia severity were assessed at baseline, bi-weekly during treatment, and every 4 months thereafter. Sleep effort and beliefs about sleep were also assessed.
Results
Growth mixture modeling revealed three trajectories: (1) Partial-Responders (68.9%) had moderate symptom reduction during early treatment (p-value<.001) and maintained mild depression during follow-ups. (2) Initial-Responders (17.6%) had marked symptom reduction during treatment (p-values<.001) and low depression severity at post-treatment, but increased severity over follow-up (p-value<.001). (3) Optimal-Responders (13.5%) achieved most gains during early treatment (p-value<.001), continued to improve (p-value<.01) and maintained minimal depression during follow-ups. The classes did not differ significantly on baseline measures or treatment received, but differed on insomnia-related measures after treatment began (p-values<.05): Optimal-Responders consistently endorsed the lowest insomnia severity, sleep effort, and unhelpful beliefs about sleep.
Conclusions
Three depression symptom trajectories were observed among patients with comorbid insomnia and MDD. These trajectories were associated with insomnia-related constructs after commencing treatment. Early changes in insomnia characteristics may predict long-term depression outcomes.
Keywords: depression, insomnia, antidepressant, sleep, change trajectories
Introduction
Insomnia and Depression
Insomnia and depressive symptomatology share an intimate bi-directional relationship. Symptoms of insomnia are reported by 80% – 90% of individuals with major depressive disorder (MDD; McCall, Reboussin, & Cohen, 2000; Novick et al., 2005). Poorer sleep and symptoms of insomnia have been associated with greater depressive symptom severity (Bei, Ong, Rajaratnam, & Manber, 2015), slower and lower rates of remission from depression (Dew et al., 1997; Franzen & Buysse, 2008), and higher risk for depression relapse (Buysse et al., 2008; Franzen & Buysse, 2008; Perlis, Giles, Buysse, Tu, & Kupfer, 1997). Therefore, sleep complaints are not only symptoms of depression, but may also play an aetiological role in the development and maintenance of depression. Further, sleep disturbance may be resistant to depression treatment. Data from the STAR*D trial suggest that 72% of patients who remitted from depression complained of residual sleep disturbance following treatment (Nierenberg et al., 2010). Insomnia symptoms therefore represent an important target for improving depression outcomes.
Cognitive behavioral therapy for insomnia (CBT-I) is an effective, first-line treatment for insomnia (Qaseem et al., 2016; Wilson et al., 2010). Treatment of Insomnia and Depression (TRIAD) is a randomized control trial (RCT) that examined whether CBT-I enhanced antidepressant treatment in patients with comorbid MDD and insomnia. While insomnia was significantly improved by the addition of CBT-I compared to the control condition, there was no differential improvement in remission from depression (Manber et al., 2016). Heterogeneity in treatment responses may explain this unexpected result, as well as prior trial results that did find differential effect on remission from depression following concurrent insomnia and depression treatment (Manber et al., 2008).
Depression Treatment Response Trajectories
Most RCTs of depression treatment have examined average participant responses, largely overlooking individual differences in depression change trajectories. Only a handful of studies examined the heterogeneity in response to antidepressant treatment, all of which focused on change trajectories during the treatment period.
One study used a sample of 2515 (n = 652 on placebo) MDD patients in an RCT that included duloxetine, or a selective serotonin reuptake inhibitor. In this study, the majority (> 80%) of those receiving antidepressants were classified as responders, with gradual improvement of depressive symptoms over eight weeks of observation; the remaining were classified as non-responders, with little improvement over time (Gueorguieva, Mallinckrodt, & Krystal, 2011). These results are consistent with findings in a smaller sample (N = 49) of MDD patients receiving fluoxetine or venlafaxine, also over eight weeks: 85% showed gradual improvements in depressive symptoms, whilst the remaining either did not show improvements, or improved initially but worsened later (B. Muthén, Brown, Leuchter, & Hunter, 2008).
Other studies identified somewhat different trajectories. In a study of 807 adults with MDD receiving citalopram or nortriptyline over 12 weeks, 75% showed gradual improvement with modest gains, whilst the remainder showed rapid improvement during early treatment and more substantial overall gains (Uher et al., 2010). Similar trajectories were identified in older adults treated with 12 weeks of nortriptyline or paroxetine (Gildengers et al., 2005). A more recent study on 453 older adults receiving venlafaxine over 12 weeks identified rapid- (i.e., fast improvement during early treatment), gradual-, and non-response trajectories (Smagula et al., 2015).
Collectively, these studies point to three common trajectories during the acute 8–12 weeks’ antidepressant treatment: (a) non-responders, (b) responders with gradual improvement throughout treatment, and (c) responders with rapid symptom reduction during early treatment. However, none of these studies examined long-term follow-up. Likewise, none of these studies examined depression symptom trajectories in patients with comorbid MDD and insomnia, nor whether insomnia and its related constructs predict depression trajectories.
Current Study
In the current study, we applied growth mixture modeling (GMM; Nylund, Asparouhov, & Muthén, 2007) on data drawn from the TRIAD study (Manber et al., 2016). This approach searches for heterogeneity in longitudinal data, and identifies classes of individuals with distinct change trajectories. Growth mixture modeling was used by all aforementioned depression trajectories studies except for Smagula et al. (2015). The aims of the present study are: (1) to identify distinct patterns of change in depressive symptom severity over 28 months (a 16-week treatment and a 2-year follow-up) among individuals with comorbid MDD and insomnia, and (2) to explore potential predictors of change trajectories, with a focus on insomnia and insomnia-related constructs.
Insomnia-related constructs selected for this study included: (a) tendency to experience insomnia in response to stress, selected because stress has been shown to contribute to both depression (Hammen, 2005) and insomnia (Morin, Rodrigue, & Ivers, 2003); (b) sleep effort, conceptualized as attempts to gain control over sleep, selected because it reflects cognitive inflexibility, which is found in both depression (Teasdale, Segal, & Williams, 1995) and insomnia (Ong, Ulmer, & Manber, 2012); and (c) unhelpful beliefs about sleep, selected because negative thoughts and beliefs have been linked to vulnerability to both depression (Beck, 1987) and sleep complaints (Bei, Wiley, Allen, & Trinder, 2015).
Although not directly related to insomnia, we also examined chronotype, an individual’s diurnal preference for rest and activity, as a correlate of symptom change trajectories. Eveningness has been associated with greater depressive symptomatology (Gaspar-Barba et al., 2009; Ong, Huang, Kuo, & Manber, 2007; Smagula et al., 2015), and less reduction in depressive symptoms among insomnia patients after CBT-I (Bei, Ong, et al., 2015). Other correlates examined included treatment conditions, the number of sessions attended, demographics, history and timelines of depression and insomnia, and baseline symptom levels.
Methods
The TRIAD study (ClinicalTrials.gov identifier NCT00767624) was a multi-site randomized control trial conducted at Duke University (Durham, North Carolina), Stanford University (Palo Alto, California), and the University of Pittsburgh (Pittsburgh, Pennsylvania). Relevant methodologies are summarized below, with further details published elsewhere (Manber et al., 2016).
Participants
Participants were recruited through community advertisements between March 2009 and August 2013. Eligible participants were (a) 18–75 years of age, (b) fluent in English, (c) met DSM-IV-TR MDD criteria based on the Structured Clinical Interview for DSM-IV (SCID; First, L, Miriam, & Williams, 2002), (d) scored > 15 on the 17-item Hamilton Rating Scale for Depression (Hamilton, 1967), (e) met DSM-IV-TR criteria for insomnia (primary insomnia or insomnia due to another mental disorder) based on the Duke Structured Interview for Sleep Disorders (Edinger et al., 2009), and (f) scored > 10 on the Insomnia Severity Index (Morin, Belleville, Bélanger, & Ivers, 2011). Participants were excluded if they were involved in another active treatment, had been recently treated for depression or insomnia, or had uncontrolled or unstable medical conditions. Additional details about exclusion criteria can be found in Manber et al. (2016).
Procedures
All three sites followed identical study protocols, which were approved by each University’s institutional review board. Participants were screened for study eligibility after providing written consent. Eligible participants were randomized centrally (1:1 in random blocks of two and four, stratified by study site) to either CBT-I or control conditions. In both treatment conditions, antidepressant medication management was conducted along with seven 45-minute individual therapy sessions over 16 weeks. The first four sessions were conducted weekly, the next two sessions biweekly, and the seventh session a month after the sixth. Treatment manuals, centralized training, and group supervision for both conditions ensured that all participants in the same condition received the same treatment content at the same time intervals. See Manber et al. (2016) for additional details.
Pharmacotherapy followed principles used in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (Trivedi et al., 2006), which in the present study provided structured flexibility in the choice of the first medication to be tried and one switch to another medication. Antidepressant medications were limited to escitalopram, sertraline, and desvenlafaxine succinate. Study psychiatrists did not address sleep issues and were masked to treatment condition.
Therapy sessions for the CBT-I condition included sleep education (Session 1), sleep restriction (Session 2 and thereafter), stimulus control (Session 2 and thereafter), strategies for reducing somatic and sleep-related cognitive arousal (Session 2 and thereafter), cognitive restructuring of sleep-related thoughts, and relapse prevention (Session 3 and thereafter; Bootzin & Epstein, 2011; Manber & Carney, 2015; Spielman, Saskin, & Thorpy, 1987). Sessions for the control condition included sleep education (Session 1) and a quasi-desensitization procedure (Session 2 and thereafter). The quasi desensitization procedure is a means of eliminating the “conditioned arousal” which prolongs nocturnal awakenings; it involves desensitization exercises whereby distressing sleep related situations (identified by the patient; e.g. worrying about not sleeping or noticing the bed partner is asleep) are paired with neutral images (e.g. preparing a meal or driving to the local store). Distressing sleep related situations were ordered into a hierarchy of least likely to most likely to lead to a prolonged awakening and were addressed (two per session) in that order. This control condition was previously used as a credible insomnia control therapy (Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001; Manber et al., 2008). The same sleep education was conducted in the control and CBT-I conditions; there was no other overlap between treatment conditions. Sleep therapists did not discuss depression or other issues unrelated to sleep behaviors. After the treatment phase, participants were transitioned to community care and were followed-up for assessments over two years.
Measurements
Depression symptom severity
The 17-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1967), was administered by blind raters masked to treatment conditions. Higher scores indicate greater depressive symptom severity. This interviewer-administered semi-structured interview is one of the most widely used instruments in depression treatment studies (Miller, Bishop, Norman, & Maddever, 1985), with Cronbach’s ranging 0.72 – 0.87 across all time points in this study. In this study, the three sleep-related items from the HRSD were removed, so that depressive symptom trajectories were examined without overlapping items about sleep.
Insomnia symptom severity
The 7-item Insomnia Severity Index (ISI; Morin et al., 2011) provides an index of the global severity of insomnia, including perceived daytime consequences and distress from sleep difficulties. Higher scores on the ISI indicate greater insomnia severity, and scores > 7 indicate clinically significant insomnia symptoms (Morin et al., 2011). In this study, Cronbach’s for the ISI was 0.87–0.91.
Insomnia-related constructs
The 9-item Ford Insomnia Response to Stress Test (FIRST; = 0.86 in this study) assessed the likelihood of experiencing sleep disturbances in the context of stressful events (Drake, Richardson, Roehrs, Scofield, & Roth, 2004); higher scores indicate higher likelihood of experiencing sleep disturbance, with recent evidence that scores > 15 indicate elevated risk for insomnia (Kalmbach, Pillai, Arnedt, & Drake, 2016). Sleep effort was assessed using the 7-item Glasgow Sleep Effort Scale (GSES; Broomfield & Espie, 2005; ranged 0.75 – 0.83 in this study); higher scores indicate greater sleep effort. Beliefs and attitudes about sleep was assessed using the 16-item Dysfunctional Beliefs and Attitudes about Sleep (DBAS; Morin, Vallières, & Ivers, 2007; ranged 0.84 – 0.91 in this study), which assesses misattributions/amplification of the consequences of insomnia, worry about sleep, unrealistic sleep expectations, and beliefs about sleep medication. Higher scores on the DBAS indicates stronger endorsement of unhelpful beliefs, and scores > 3.8 were shown to be associated with clinically significant insomnia (Carney et al., 2010). Both actigraphy and sleep diary were collected during baseline, and their results are included in the Supplement.
Chronotype
The 13-item Composite Scale of Morningness (Smith, Reilly, & Midkiff, 1989) assessed preferred timing for various activities and ease of rising in the morning, with higher scores indicating more morning preference.
The FIRST and chronotype measures were administered at baseline (BL) only. The HRSD and ISI were collected at BL, biweekly during the acute treatment phase, and every four months thereafter until the end of 2-year follow-up (FU; 15 repeated assessments in total). The DBAS and GSES were collected at BL, Week 8 (treatment mid-point; MID), and Week 16 (post-treatment; POST).
Statistical Analyses
Identifying latent trajectory classes
Five separate latent growth models of the overall change in HRSD from BL to FU were tested and compared. The models had following trajectory configurations: Model A included one linear slope from BL to FU, hypothesizing an overall linear change. Model B included a linear and a quadratic slope from BL to FU, hypothesizing an overall quadratic change. Model C included two linear slopes, one from BL to POST, and the other from POST to FU to allow for the possibility of different rates of change between treatment and FU periods. Similarly, Model D included two linear slopes, one from BL to MID, and the other from MID to FU. Model E included three linear slopes, one from BL to MID, one from MID to POST, and the third from POST to FU. The transition points of piecewise growth curves (i.e., MID and POST) were selected based on empirical nature of the data. Sensitivity analysis showed that model fit was comparable when the first transition point was placed at Week 4, 6, or 8 (no convergence at Week 2). Conceptually, placing the first and second transition point at Week 8 (MID) and POST allowed us to examine changes during early-, late-, and post-treatment periods. All models integrated linear time (two weeks as the unit), so that unstandardized estimates of the slope can be interpreted as changes in raw HRSD score every two weeks.
The model with the best fit to the data was used as the basis for identifying latent trajectory classes using GMM. One to five-class solutions were systematically tested, and the solution with the best overall fit was chosen as the final model. The final model was also required to have a minimum sample size of 10 in each class, so correlates of class membership could be meaningfully examined. Probabilities for each class membership in the final model were inspected, and participants with uncertain classification were excluded from further analyses. Uncertain classification was operationalized as having < .5 probability of being in the most likely class, or if the probabilities of the most and second most likely classes differed by < .2.
Examining correlates of class membership
Associations between final class membership and categorical correlates were examined using Fisher's exact test. When correlates were continuous, one-way analyses of variance was used, with Tukey’s honest significant difference test for post hoc pairwise tests adjusting for multiple comparisons. Cohen’s d was also calculated for each pair to quantify effect sizes. We also used descriptive statistics and visual plots to further explore individual participant trajectories, trajectories of the percentage of participants with MDD within each class, and changes in specific depressive symptoms; see the Supplement.
Overall statistical methods
Model fit was assessed using a range of fit indices (see Table 1). Missing data was handled using full information maximum likelihood with a robust estimator in all latent growth and growth mixture models, and using pairwise deletion when examining correlates of class membership. Data were analyzed in R 3.3.1 (Core Team, 2016) and Mplus 7.31 (L. K. Muthén & Muthén, 1998–2016) via MplusAutomation 0.6-4 (Hallquist & Wiley, 2016). Statistical significance was determined by two-tailed p < .05. Effect sizes were quantified using Cohen’s d, with values above 0.2, 0.5, and 0.8 suggesting small, moderate, and large effect sizes, respectively (Cohen, 1988).
Table 1.
Fit Indices and Class Membership Counts for One- to Four-Class Growth Mixture Models Tested
| One-class | Two-class | Three-class | Four-class | |
|---|---|---|---|---|
| Parameters | 20 | 41 | 62 | 83 |
| LL | −4168.30 | −4058.88 | −4005.41 | −3965.61 |
| AIC | 8376.60 | 8199.76 | 8134.83 | 8097.22 |
| AICc | 8383.21 | 8232.25 | 8226.73 | 8315.10 |
| BIC | 8436.54 | 8322.65 | 8320.66 | 8345.99 |
| aBIC | 8373.25 | 8192.90 | 8124.45 | 8083.33 |
| Entropy | 1.00 | 0.80 | 0.78 | 0.82 |
|
| ||||
| Count (%) in each class | ||||
| Class 1 | 148 (100.0) | 118 (79.7) | 102 (68.9) | 98 (66.2) |
| Class 2 | -- | 30 (20.3) | 26 (17.6) | 27 (18.2) |
| Class 3 | -- | -- | 20 (13.5) | 6 (4.1) |
| Class 4 | -- | -- | -- | 17 (11.5) |
Note. LL = log likelihood, AIC = Akaike information criterion, AICc = AIC with sample size corrections, BIC = Bayesian information criterion, aBIC = sample size adjusted BIC. A 5-class solution was also tested, but the model did not converge.
Results
Sample Characteristics
A total of 150 participants were randomized (see CONSORT figure in Manber et al., 2016). This study included 148 participants after excluding one participant who developed psychotic symptoms before starting treatments, and another who developed bipolar symptoms at Week 4. Included participants had an average age of 46.56 (SD = 12.64), 73.0% were females, 71% were Caucasian, 44.9% were employed, and 38.8% were married or in a stable relationship. Across all 15 time points during the nearly 2.5 years’ data collection period, participants provided data on the HRSD for an average of 9.3 (62.0%) time points, with the rate of data completion being higher during the initial 16-weeks treatment period (72.8%) than it was during the subsequent 2-year follow-up (45.8%).
Identifying Latent Trajectory Classes
To determine the overall HRSD change trajectory, fit indices for Model A–E were compared (see Figure S1 in the Supplement). Model E provided the best overall fit to the data, and was therefore used for further GMM.
Model fit indices and class membership counts for one to four-class solutions are summarized in Table 1 and Figure S2. A five-class solution was also tested but did not converge. Three- and four-class solutions showed comparable fit, and both were superior to one- or two-class solutions. We chose the three-class solution for the final model, as the four-class solution contained a membership class with only six individuals.
Characterizing Trajectories for Each Class
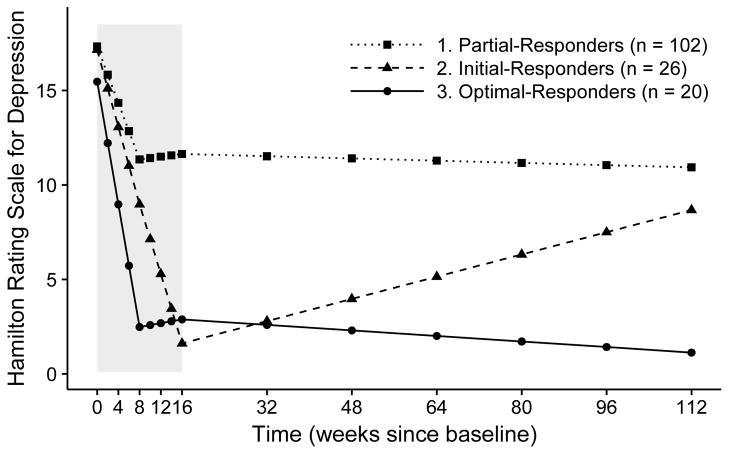
Latent change trajectories for each class in the final model are displayed graphically in Figure 1 and numerically in Table 2. Raw HRSD scores over time by class membership are plotted in Figure S3 of the Supplement. At baseline, the three classes did not differ significantly on HRSD (p > .05; all means around 17), although Class 3 (Optimal-Responders) reported somewhat lower depressive symptoms compared with other two classes (d = 0.38 and 0.49 compared with Class 1 and 2 respectively).
Figure 1.
Estimated change trajectories for depressive symptoms for the three classes emerged from growth mixture models. Scores on the Hamilton Rating Scale of Depression do not include the sleep-related items to avoid overlapping items with insomnia. Shaded area indicates treatment period.
Table 2.
Estimated Mean (Standard Error) for Growth Factors in the Final Growth Mixture Model
| Partial-Responders | Initial-Responders | Optimal-Responders | |
|---|---|---|---|
| Intercept a | 11.36*** (0.69) | 8.97*** (1.53) | 2.48*** (0.55) |
| Slope BL to MID | −1.50*** (0.16) | −2.05 *** (0.33) | −3.25*** (0.18) |
| Slope MID to POST | 0.07 (0.19) | −1.84*** (0.42) | 0.10 (0.17) |
| Slope POST to FU | −0.02 (0.03) | 0.15*** (0.03) | −0.04** (0.01) |
Note. Unstandardized estimates are shown.
Intercept was centered at MID; BL = baseline, MID = mid-treatment, POST = immediately post-treatment, FU = 2-year follow-up.
p<.01,
p<.001.
Class 1 (Partial-Responders; 68.9% of the sample). The trajectory for this class showed a significant (p < .001) reduction at the rate of 1.5 points every two weeks on the HRSD during the first half of the treatment, with little additional reduction from MID (M±SD = 12.24±5.12) to POST (M±SD = 11.41±5.33), or from POST to FU (M±SD = 9.66±5.85; both slopes non-significant). The reduction in HRSD scores was suboptimal, as 59.3% and 40.6% of participants in this class scored >10 on the HRSD at POST and FU respectively.
Class 2 (Initial-Responders; 17.6%). This class showed a significant reduction on the HRSD during early treatment at 2.05 points every two weeks (p < .001; M±SD = 8.71±6.84 for MID). It continued to improve significantly at 1.84 point every two weeks (p < .001) during the second half of the treatment. Depressive symptoms were minimal (HRSD M±SD = 1.50±1.30) at the end of the treatment, reflecting full remission. Therapeutic gains were, however, only partially maintained over FU: there was a small but significant increase in HRSD (0.15 point every two weeks) after termination of the treatment. At the end of the FU, depressive symptom severity of this class (M±SD = 7.38±6.49) was lower than (d = 0.38), but not significantly different from, that of Partial-Responders (p > .05).
Class 3 (Optimal-Responders; 13.5%). This class achieved most of the gains during early-treatment (M±SD = 2.41±2.00 for MID), with HRSD reducing at 3.25 point every two weeks, the most rapid among all three classes. Depressive symptoms remained low with no significant changes during the second half of the treatment (M±SD = 2.75±2.67 for POST). After treatment, this class showed small but statistically significant further improvements at 0.04 point every two weeks. Therapeutic gains were maintained at the end of FU, with participants in this class reporting minimal symptoms (HRSD M±SD = 1.33±1.15).
To further characterize the three trajectory classes, as well as to demonstrate the convergent validity of the classification, we plotted the proportion of participants meeting MDD criteria based on the SCID depression module, which was administered at the same time points as HRSD. Trajectories of MDD proportions over time for the three classes are consistent with those identified using HRSD (Figure S4).
To further characterize changes in specific depressive symptoms, distributions of individual item scores of the HRSD from BL and POST are shown in Figure S5 and S6. Symptom profiles appear similar among classes during BL. After intervention, notable residual symptoms in Partial-Responders include depressed mood, guilt, insomnia (especially sleep maintenance difficulties), anxiety, somatic symptoms (especially diminished libido), and functional impairment.
Correlates of Class Membership
Eight participants were considered to have uncertain classification based on the aforementioned criteria, and the remaining 140 were included in this section of the analyses. Descriptive statistics for each class and between-class comparisons are shown in Table 3 (categorical variables) and Table 4 (continuous variables).
Table 3.
Descriptive Statistics for Categorical Variables Among the Three Classes
| N = 140† | Partial-Responders (n = 97) | Initial-Responders (n = 25) | Optimal-Responders (n = 18) | χ2 (df), p |
|---|---|---|---|---|
| Group | ||||
|
| ||||
| Control: 71 (50.7) | 47 (48.5) | 13 (52.0) | 11 (61.1) | 0.99 (2), 0.61 |
| CBT-I: 69 (49.3) | 50 (51.5) | 12 (48.0) | 7 (38.9) | |
|
| ||||
| Sex | ||||
|
| ||||
| Male: 37 | 24 (24.7) | 6 (24.0) | 7 (38.9) | 1.65 (2), 0.44 |
| Female: 103 | 73 (75.3) | 19 (76.0) | 11 (61.1) | |
|
| ||||
| Race | ||||
|
| ||||
| White: 103 | 68 (70.1) | 20 (80.0) | 15 (83.3) | 2.01 (2), 0.37 |
| Non-white: 37 | 29 (29.9) | 5 (20.0) | 3 (16.7) | |
|
| ||||
| Partnered | ||||
|
| ||||
| Yes: 51 | 33 (34.4) | 10 (40.0) | 8 (44.4) | 0.81 (2), 0.67 |
| No: 88 | 63 (65.6) | 15 (60.0) | 10 (55.6) | |
|
| ||||
| Employed | ||||
|
| ||||
| Yes: 65 | 42 (43.8) | 13 (52.0) | 10 (55.6) | 1.18 (2), 0.55 |
| No: 75 | 54 (56.2) | 12 (48.0) | 8 (44.4) | |
|
| ||||
| Insomnia timing | ||||
|
| ||||
| Before depression: 61 | 41 (46.6) | 12 (50.0) | 8 (50.0) | 5.83 (6),0.44 |
| Same time: 24 | 13 (14.7) | 6 (25.0) | 5 (31.2) | |
| After depression: 43 | 34 (38.6) | 6 (25.0) | 3 (18.8) | |
|
| ||||
| Past insomnia episodes | ||||
|
| ||||
| None: 102 | 69 (71.1) | 19 (76.0) | 14 (82.4) | 1.04 (2), .60 |
| 1 or more: 37 | 28 (28.9) | 6 (24.0) | 3 (17.6) | |
|
| ||||
| Past depression episodes | ||||
|
| ||||
| None: 33 | 20 (21.5) | 9 (36.0) | 4 (25.0) | 2.58 (4), .63 |
| 1 or 2: 43 | 30 (32.3) | 7 (28.0) | 6 (37.5) | |
| 3 or more: 58 | 43 (46.2) | 9 (36.0) | 6 (37.5) | |
|
| ||||
| History of a chronic depressive episode | ||||
|
| ||||
| Absence: 53 | 29 (30.9) | 15 (60.0) | 9 (52.9) | 8.65 (2), .013 |
| Presence: 83 | 65 (69.1) | 10 (40.0) | 8 (47.1) | |
|
| ||||
| Remission at the end of treatment | ||||
|
| ||||
| Yes: 56 | 22 (22.7) | 17 (68.0) | 17 (94.4) | 42.52 (2), <.001 |
| No: 84 | 75 (77.3) | 8 (32.0) | 1 (5.6) | |
Note. n (%) are presented. Overall differences were tested using two-tailed Fisher's exact test.
Participants were excluded from analyses if their probability of being in the most likely class was < .5 (n = 6), or if the probabilities of the most and second most likely classes differ by < .2 (n = 2); sample sizes for “Partnered” and “Insomnia timeline” are 139 and 138 respective due to 1 and 2 individuals from Class 1 had missing data on these two variables. “Partnered”: “yes” = married or having live-in partners; “no”: single, divorced, separated, or widowed.
Table 4.
Descriptive Statistics for Continuous Variables Among the Three Classes
| 1. Partial-Responders | 2. Initial-Responders | 3. Optimal-Responders | Overall F (df) | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| n = 97 | n = 25 | n = 18 | 1 vs 2 | 1 vs 3 | 2 vs 3 | ||
| Age | 46.54 (12.57) | 46.84 (12.82) | 49.17 (12.43) | 0.33 (2, 137) | −0.02 | −0.21 | −0.18 |
| Age: 1st insomnia episode | 31.76 (15.60) | 31.60 (16.22) | 32.27 (12.40) | 0.01 (2, 130) | 0.01 | −0.03 | −0.04 |
| Age: 1st depression episode | 25.92 (14.89) | 26.64 (16.03) | 28.24 (11.75) | 0.18 (2, 135) | −0.05 | −0.16 | −0.11 |
| Chronotype | 31.09 (8.12) | 31.08 (8.02) | 32.12 (6.11) | 0.12 (2, 129) | 0.00 | −0.13 | −0.14 |
| FIRST | 25.02 (6.08) | 26.15 (5.98) | 22.83 (5.34) | 1.60 (2, 117) | −0.19 | 0.37 | 0.58 |
| Number of sessions attended | 5.32 (2.30) | 6.29 (1.33) | 6.24 (1.68) | 2.17 (3, 123)† | −0.46 | −0.41 | 0.04 |
|
| |||||||
| GSES | |||||||
|
| |||||||
| BL | 8.27 (3.03) | 8.67 (3.64) | 7.44 (2.94) | 0.80 (2, 127) | −0.12 | 0.27 | 0.36 |
| MID | 6.48 (3.32) | 5.72 (3.89) | 4.06 (2.24) | 3.78 (2, 89)* | 0.22 | 0.78* | 0.53 |
| POST | 5.52 (3.30) | 3.91 (2.89) | 2.47 (1.33) | 7.85 (2, 97)*** | 0.50† | 1.02*** | 0.61 |
|
| |||||||
| DBAS | |||||||
|
| |||||||
| BL | 6.08 (1.38) | 6.68 (1.69) | 5.68 (1.70) | 2.55 (2, 127)† | −0.42 | 0.28 | 0.59† |
| MID | 5.24 (1.89) | 5.16 (1.90) | 3.79 (1.52) | 4.42 (2, 88)* | 0.04 | 0.80* | 0.80† |
| POST | 4.49 (1.79) | 3.83 (2.01) | 3.12 (1.64) | 3.94 (2, 95)* | 0.36 | 0.78* | 0.38 |
|
| |||||||
| ISI | |||||||
|
| |||||||
| BL | 19.07 (4.01) | 19.44 (3.76) | 18.00 (4.91) | 0.70 (2, 137) | −0.09 | 0.26 | 0.34 |
| ISI slope a | −1.86 (0.96) | −2.13 (1.05) | −2.55 (1.10) | 3.97 (2, 137)* | 0.28 | 0.70* | 0.39 |
| MID | 13.05 (5.39) | 9.94 (5.45) | 8.11 (5.09) | 6.74 (2, 89)** | 0.58† | 0.93** | 0.35 |
| POST | 10.47 (5.67) | 5.30 (5.79) | 4.71 (3.89) | 11.94 (2, 97)*** | 0.91*** | 1.08*** | 0.12 |
| FU | 12.16 (6.51) | 8.54 (7.61) | 4.25 (5.46) | 6.55 (2, 54)** | 0.53 | 1.26** | 0.64 |
|
| |||||||
| HRSD | |||||||
|
| |||||||
| BL | 17.55 (3.58) | 17.60 (2.55) | 16.22 (3.15) | 1.23 (2, 137) | −0.02 | 0.38 | 0.49 |
| MID, % Responders | 12.24 (5.12), 27.8% | 8.71 (6.84), 58.8% | 2.41 (2.00), 100% | 24.48 (2, 85)*** | 0.64* | 2.14*** | 1.25** |
| POST, % Responders | 11.41 (5.33), 30.5% | 1.50 (1.30), 100% | 2.75 (2.67), 87.5% | 53.70 (2, 94)*** | 2.14*** | 1.76*** | −0.63 |
| FU, % Responders | 9.66 (5.85), 53.1% | 7.38 (6.49), 69.2% | 1.33 (1.15), 100% | 10.32 (2, 54)*** | 0.38 | 1.64*** | 1.27* |
Note. Mean (standard deviation) are shown for descriptive statistics. FIRST = Ford’s Insomnia Response to Stress Test, GSES = Glasgow Sleep Effort Scale, DBAS = Dysfunctional Beliefs and Attitude about Sleep Scale, ISI = Insomnia Severity Index, HRSD = Hamilton Rating Scale of Depression (less sleep-related items); % of responders (defined as 50% or more reduction in HRSD scores) are also listed. BL = baseline, MID = mid-treatment, POST = immediately post-treatment, FU = 2-year follow-up.
“ISI slope” (i.e., initial treatment response) is the slope of ISI during the first six weeks of the treatment as identified by Manber et al. (2016). Cohen’s d-values above 0.2, 0.5 and 0.8 suggesting small, moderate and large effect sizes, respectively. Statistical significance was based on Tukey’s honest significant difference test for post hoc pairwise tests adjusting for multiple comparisons.
p < .1,
p < .05,
p < .01,
p < .001.
Demographics and chronotype
There were no significant differences among the classes in sex, race, relationship status, and employment status (all p-values > .35). Age also did not differ significantly (p > .05), although Optimal-Responders were almost three years older than Partial-Responders (d = .21). The three classes did not differ significantly on chronotype (p = .89).
Treatment arms and session attendance
The classes did not differ significantly on whether they were allocated to CBT-I or control condition (p = .61). Although not statistically significant, Partial-Responders on average attended one less session (out of the total seven) than other two classes (close to moderate effect size).
Insomnia history and timeline
The classes did not differ significantly on age of the first insomnia episode (p = .99). Overall, 26.7% of participants had one or more insomnia episodes, and the rate of past insomnia episodes was somewhat but not significantly higher in Partial-Responders (28.9%) compared to Optimal-Responders (17.6%).
In all classes, about half of participants reported their current insomnia episode starting before their current depression. Compared to Optimal-Responders (18.8%), Partial-Responders (38.6%) were over twice as likely to have an insomnia episode commencing after depression.
Insomnia severity and insomnia-related constructs
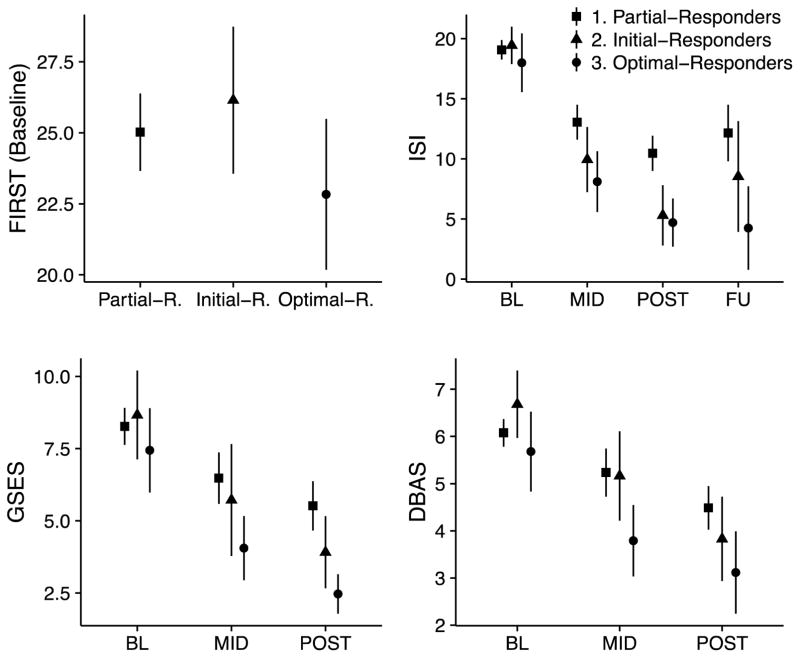
Scores on the ISI, FIRST, GSES, and DBAS for each class are illustrated in Figure 2, with additional details in Table 4. At BL, overall differences on ISI, FIRST, GSES, and DBAS were not statistically significant (p-values > .05). However, there were small to medium effect sizes comparing Optimal-Responders to the other two classes: Compared to Partial- or Initial-Responders, Optimal-Responders had somewhat lower symptoms of insomnia (d = 0.26 and 0.34), insomnia in response to stress (d = 0.37 and 0.58), sleep effort (d = 0.27 and 0.36), and dysfunctional beliefs about sleep (d = 0.28 and 0.59). Initial-Responders had the highest DBAS compared to other two classes (d = 0.42 and 0.59). The three classes also did not differ significantly on baseline actigraphy or sleep diary variables (all p-values > .24), with Optimal-Responders showing a trend for longer sleep onset latency compared to the other classes on both measures (Table S1).
Figure 2.
Means and 95% confidence intervals for the three classes on FIRST (Ford’s Insomnia to Stress Response Test) at baseline, ISI (Insomnia Severity Index), GSES (Glasgow Sleep Effort Scale), and DBAS (Dysfunctional Beliefs and Attitudes about Sleep Scale) at BL (baseline), MID (mid-treatment), and POST (immediately post-treatment); the ISI has an additional 2-year follow-up (FU).
All three classes showed reductions in ISI, GSES, and DBAS after treatment began but differed in the amount of reduction, resulting in significant differences in ISI, GSES, and DBAS among classes at all post-baseline measurements (p-values < .05).
On the ISI, Partial-Responders showed the highest insomnia severity compared to other two classes at MID, POST, and FU time points (d ranges 0.53 – 1.26). Difference in ISI between Initial- and Optimal-Responders was small at MID (d = 0.35), negligible at POST (d = 0.12), and moderate at FU (d = 0.64); Optimal-Responders showed lower insomnia severity at all post-baseline time points. Further, the initial insomnia treatment response (i.e., slope of the ISI from BL to Week 6 as identified in our previous publication; Manber et al., 2016) was significantly different among classes (p < .05), with Optimal-Responders having most rapid initial insomnia improvement, and Partial-Responders slowest.
The GSES showed similar patterns of differences. At both MID and POST, Optimal-Responders showed the lowest sleep effort (d ranges 0.53 – 1.02), followed by Initial-Responders, with Partial-Responders scoring the highest on GSES; between-class differences are particularly pronounced at POST, with medium to large effect sizes.
For DBAS, Partial-Responders and Initial-Responders had comparable scores at MID, and scored significantly higher than Optimal-Responders (d = 0.80 for both); at POST, Optimal-Responders continued to report the lowest DBAS (d = 0.78 and 0.36 compared to Partial- and Initial-Responders respectively), whilst Partial-Responders reported the highest DBAS (d = 0.36 compared to Initial-Responders).
Depression history
The classes did not differ significantly on age of the first depressive episode (p = .83). The rates of past depressive episodes were comparable among classes, but Initial-Responders were somewhat more likely to have no previous episode (36.0% vs. 21.5% and 25%), and Partial-Responders were more likely to have three or more past episodes (46.2% vs. 36.0% and 37.5%). The group’s propensity for having a past chronic depressive episode (a depressive episode that lasted at least two years) differed significantly between classes (p < .05): nearly 70% of Partial-Responders had a history of chronic depressive episode compared to 40.0% and 47.1% in the other two classes.
Discussion
Summary of Findings
The TRIAD study provided a unique opportunity to examine change trajectories in depression severity over a 28-month period, consisting of a 16-week treatment phase and a 2-year naturalistic follow-up. This study identified three distinct depressive symptom change trajectories in patients with comorbid major depressive and insomnia disorders (see Figure 1). The classes did not differ significantly on whether they received CBT-I or control intervention, and their differences on most baseline measures were negligible to small. However, they differed significantly on insomnia severity and insomnia-related constructs after treatment began. Optimal-Responders had the most rapid improvements, and consistently scored the healthiest on all insomnia-related measures. In addition, Partial-Responders were more likely to have experienced a chronic depressive episode in the past.
Depressive Symptom Change Trajectories
For the acute treatment period, depressive symptom change trajectories identified in this study echo trajectories identified by previous studies: one class with sub-optimal response (Partial-Responders) and two other classes with gradual (Initial-Responders) and rapid (Optimal-Responders) responses. Unlike previous studies that showed little improvements in the worst response class (Gueorguieva et al., 2011; B. Muthén et al., 2008), the class with the worst response in this study (i.e., Partial-Responders) achieved about 1/3 of reduction in symptom severity. It is possible that the option of switching medications during treatment enhanced treatment response in this study. Compared to studies that showed over 80% of individuals having at least 50% symptom reductions (e.g., Gueorguieva et al., 2011; B. Muthén et al., 2008), response rate is lower in this study at 55.7%, and remission rate is also somewhat low at 39.9%. It is possible that MDD comorbid with insomnia disorder, as opposed to less severe insomnia symptoms typically reported in other studies, may have contributed to less robust treatment response for the combined treatment arms.
Adding to the existing literature, this study showed that long-term depressive symptom change trajectories are also heterogeneous. At 2-year follow-up, most participants (Optimal- and Partial-Responders albeit higher symptoms in the latter) maintained therapeutic gains. However, nearly one in five (Initial-Responders), experienced worsening of symptoms during the follow-up. This is consistent with the notion that for a small percent of individuals, MDD is a chronic condition (Keller et al., 1992), especially MDD co-existing with insomnia (Buysse et al., 2008).
Within all three classes identified, we observed heterogeneity in individual trajectories (see Figure S3). This suggests that further individual differences may contribute to heterogeneity beyond class membership. It is possible that variances in each identified class represent the true heterogeneity. It is also possible that a larger sample size is required to identify more numerous and more homogeneous classes. As in all GMM analyses, the larger the sample size, the better “resolution” and “precision” in understanding the underlying heterogeneity. Further, intra-individual variability (i.e., instability over time) in HRSD scores may also contribute to heterogeneity of trajectories within each class.
Predictors and Correlates of Depressive Symptom Change Trajectories
Insomnia history, severity, and insomnia-related constructs
As sleep-related items were removed from the HRSD in this study, associations between depression trajectories and insomnia-related constructs are not related to overlapping measurement items. The three classes did not differ on age of onset for insomnia, but Partial-Responders tended to be more likely to have had previous insomnia episode(s). This suggests that depression outcomes may be worse in those with recurrent or chronic insomnia. A novel finding is that Partial-Responders were twice as likely to have an insomnia episode commencing after depression compared to Optimal-Responders. It is possible that participants who first experienced depression perceived depression (rather than sleep) as the main concern, and did not respond as well in a study that focuses on insomnia. One may further speculate that perception of the primary condition may influence treatment outcomes via different treatment expectancy. This, however, is not likely the case: sensitivity analyses showed no significant differences between classes on baseline treatment expectancy related to CBT-I, desensitization (i.e., control condition), or the pharmacological treatment component (all p-values > .59).
Although Optimal-Responders had lower insomnia severity and healthier scores on all insomnia-related constructs administered at baseline compared with the other two trajectories, these differences were relatively small. There was, however, a robust finding in reductions in insomnia severity, sleep effort, and unhelpful beliefs about sleep after baseline (see Figure 2): On all three measures, Optimal-Responders had the largest reduction, whilst Partial-Responders had the smallest at all post-baseline time points, starting at treatment midpoint. Further, symptoms of insomnia, especially sleep maintenance difficulties, were among the notable residual symptoms among Partial-Responders after the intervention. In the same dataset, one of these three insomnia-related measures, insomnia severity after 6 weeks of treatment, mediated depression outcomes (Manber et al., 2016). Findings from the current analyses indicated that the other two measures, reflecting insomnia-associated cognitive processes (i.e., sleep effort and beliefs) are relevant to depression symptom change trajectories. Improvements towards lower insomnia severity and healthier sleep-related cognitive processes predict more optimal depression trajectories.
All classes showed improvements in insomnia and related constructs, and each had comparable proportions of individuals in CBT-I vs. control arms. This suggests that mechanisms other than CBT-I might also be involved in the improvements in insomnia and maladaptive sleep cognitions. Whilst CBT-I directly targets cognitive-behavioral factors, pharmacotherapy for depression may also do so indirectly, for example by improving depression severity, which in turn reduced maladaptive thought processes.
Treatment arms and session attendance
Depressive symptom trajectories are comparable between participants receiving CBT-I or control intervention in addition to pharmacotherapy. This is consistent with the primary findings from the TRIAD study, and suggests that in patients with MDD and comorbid insomnia, CBT-I may not significantly alter responses to antidepressant treatment, on either the overall reduction in symptom severity (Manber et al., 2016), or symptom change trajectories. It is possible that, with a robust response to an antidepressant pharmacotherapy algorithm (Trivedi et al., 2006) provided to both arms, the added indirect effects of CBT-I on depression severity was relatively small. As session attendance may reflect overall treatment compliance, the finding that Partial-Responders attended on average one less intervention session than the other two groups raises the possibility that treatment compliance may play a role in depressive symptom trajectories.
Depression history and baseline depression severity
The three classes did not differ on age of onset for depression (consistent with Gueorguieva et al., 2011). The finding that Partial-Responders were up to 1.75 times more likely to have had a chronic depressive episode is consistent with previous studies that linked chronicity of depressive episodes with being in a less treatment-responsive trajectory (Gueorguieva et al., 2011; Smagula et al., 2015; Uher et al., 2010). Also consistent with previous studies (Gildengers et al., 2005; Gueorguieva et al., 2011; Smagula et al., 2015), lower baseline depressive symptoms predicted better treatment response trajectory. Specifically, Optimal-Responders had lower depression severity at baseline compared to the other two classes (d = 0.38 and 0.49).
Other predictors and correlates
Consistent with previous studies (Gueorguieva et al., 2011; Smagula et al., 2015; Uher et al., 2010), baseline demographic factors sex, race, relationship, and employment status did not predict depressive symptom trajectories. There was a trend for older age to be associated with Optimal-Response class, which is consistent with findings in older (60 +; Smagula et al., 2015) but not middle-aged (Uher et al., 2010) adults. The study by Uher et al. (2010) had a somewhat younger sample than the current study (mean age 43 vs 47), and reported that younger age was associated with rapid response trajectory. Chronotype, or individuals’ morningness-eveningness preferences did not predict depressive symptom change trajectories in this study.
Limitations
Findings in this study need to be interpreted in light of a number of limitations. First our sample is unique in that all participants had dual diagnosis of MDD and insomnia disorder. It is therefore not clear if the results, particularly those pertaining to insomnia severity and sleep-related cognitions, are generalizable to other samples of patients with depression. Although the long follow-up is a significant strength of this study and the amount of missing data in this study is comparable to other trials for both the treatment (Gueorguieva et al., 2011) and follow-up periods (Licht-Strunk et al., 2009), the relatively large amount of missing data is a weakness. Due to a relatively small sample, we could not assess how missing data have affected the results. Although our approach was taken by the majority of similar studies with similarly large amount of missing data, we cannot preclude the possibility that missing data may have introduced bias. With a larger sample size, individual differences may be further explored beyond the three-class solution within this study. A limitation inherent in a naturalistic follow-up is the possibility that some patients might have received treatment for depression and/or insomnia after the study treatment ended. During the two-year follow-up period, 56.4% participants provided sufficient information to determine whether or not they have received depression treatment. Within this subsample, the proportion who received depression treatment did not differ significantly between classes (p = .49). However, the effects of continued treatment on depression trajectory requires further research with more comprehensive documentation.
Clinical Implications
Despite the above limitations, this is the first study to examine depression trajectories in comorbid MDD and insomnia, with several clinical implications. First, the heterogeneity in depression trajectories over both acute treatment phase and long-term follow-up highlights the importance of considering individual differences in depression treatment. In this study, most patients experienced reduction in depression symptom severity, but continued to have residual depressive symptoms (Partial-Responders). This finding highlights the need for ongoing care after the acute treatment phase. Patients with residual symptoms may benefit from additional treatment for depression and/or insomnia. Specific residual symptoms identified in this study could inform the planning and delivery of continued care. For the small subgroup (Initial-Responders) who experienced full remission during treatment but symptom worsening during follow-up, continued symptom monitoring of depressive symptoms and sleep are particularly important, so that steps to address early worsening can be initiated in order to reduce the risk of depression relapse.
The clinical challenges are to predict depression trajectory so actions can be taken to switch and/or augment treatment to optimize outcome. Although we did not find significant baseline predictors of response trajectories, we did find that a rapid initial reduction in insomnia severity, sleep effort, and maladaptive beliefs about sleep may indicate better depression outcomes, even for those not receiving therapy that specifically targets insomnia. Whilst rapid initial reduction in depressive symptoms itself was also associated with better depression outcomes, this study highlighted the predictive values of non-depression related factors. In treating individuals with comorbid MDD and insomnia, assessing and documenting changes in insomnia and insomnia-related cognition, in addition to depressive symptoms, may be valuable in providing insight into longer term prognosis.
Future Directions
Findings from this study point to potentially fruitful areas for future research. For example, there may be further individual differences that exist beyond the three classes dentified in this study. If better characterized in a larger sample, these individual differences may further inform individualised treatment. There has been increasing research into the nature and correlates of intraindividual variability of both sleep (Bei, Wiley, Trinder, & Manber, 2016) and mood (End & Diener, 1999). This study revealed significant intraindividual variability of depressive symptoms over time, which warrants further research. More work is also needed to better understand partial responders - the largest class identified in this study. In particular, better understanding and addressing residual symptoms, as well as improving treatment compliance may help enhance treatment outcomes in patients with suboptimal responses. Finally, many other mechanistic links between sleep and depression were unexamined in this study. For example, depression is a 24-hour disorder, and depression treatment is associated with increases in daytime activities and reduction in nighttime activity (Burton et al., 2013). It is also possible that bed restriction, a component of CBT-I, may improve depression through indirectly increasing behavioural activation. Therefore, the rest-activity ratio and daytime activities are examples of many factors that require further research.
Supplementary Material
Public Health Significance.
This study identified three distinct depression trajectories in patients with comorbid major depression and insomnia disorders during treatment and 2-year follow-up. Those with the largest and most sustained improvements in depression consistently scored the lowest on post-baseline insomnia and insomnia-related cognitions. Early changes in insomnia symptoms and insomnia-related characteristics may be useful for predicting longer-term depression outcomes.
Acknowledgments
Support: This was not an industry supported study. There was no off-label or investigational use in this study.
This research was supported by three linked grants from the National Institute of Mental Health, Grant numbers MH078924, MH078961, and MH079256.
Footnotes
The authors have declared all conflict of interest.
References
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Bei B, Ong JC, Rajaratnam SMW, Manber R. Chronotype and Improved Sleep Efficiency Independently Predict Depressive Symptom Reduction after Group Cognitive Behavioral Therapy for Insomnia. Journal of Clinical Sleep Medicine. 2015;11(9):1021–1027. doi: 10.5664/jcsm.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Allen NB, Trinder J. A cognitive vulnerability model on sleep and mood in adolescents under naturalistically restricted and extended sleep opportunities. Sleep. 2015;38(3):453–461. doi: 10.5665/sleep.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews. 2016;28:108–124. doi: 10.1016/j.smrv.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Epstein DR. Understanding and treating insomnia. Annual Review of Clinical Psychology. 2011;7:435–458. doi: 10.1146/annurev.clinpsy.3.022806.091516. [DOI] [PubMed] [Google Scholar]
- Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. Journal of Sleep Research. 2005;14(4):401–407. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- Burton C, McKinstry B, Tătar AS, Serrano-Blanco A, Pagliari C, Wolters M. Activity monitoring in patients with depression: a systematic review. Journal of affective disorders. 2013;145(1):21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Edinger JD, Morin CM, Manber R, Rybarczyk B, Stepanski EJ, … Lack L. Examining maladaptive beliefs about sleep across insomnia patient groups. Journal of psychosomatic research. 2010;68(1):57–65. doi: 10.1016/j.jpsychores.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- Core Team, R. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Retrieved from http://www.R-project.org. [Google Scholar]
- Dew MA, Reynolds CF, 3rd, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Archives of General Psychiatry. 1997;54(11):1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive Behavioral Therapy for Treatment of Chronic Primary Insomnia: A Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A, … Radtke RA. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Sleep. 2009;32:A265–A265. [Google Scholar]
- Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. Journal of Personality and Social Psychology. 1999;76(4):662–676. [Google Scholar]
- First MB, LSR, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues in Clinical Neuroscience. 2008;10(4):473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. Depressive symptomatology is influenced by chronotypes. Journal of Affective Disorders. 2009;119(1–3):100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Houck PR, Mulsant BH, Dew MA, Aizenstein HJ, Jones BL, … Reynolds CF., 3rd Trajectories of treatment response in late-life depression: psychosocial and clinical correlates. Journal of Clinical Psychopharmacology. 2005;25(4 Suppl 1):S8–13. doi: 10.1097/01.jcp.0000161498.81137.12. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Mallinckrodt C, Krystal JH. Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Archives of General Psychiatry. 2011;68(12):1227–1237. doi: 10.1001/archgenpsychiatry.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M, Wiley JF. MplusAutomation: Automating Mplus Model Estimation and Interpretation. R package. 2016 Retrieved from http://cran.r-project.org/package=MplusAutomation.
- Hamilton M. Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Arnedt JT, Drake CL. Identifying at-risk individuals for insomnia using the ford insomnia response to stress test. Sleep. 2016;39(2):449–456. doi: 10.5665/sleep.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Archives of General Psychiatry. 1992;49(10):809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Licht-Strunk E, Van Marwijk HWJ, Hoekstra T, Twisk JWR, De Haan M, Beekman ATF. Outcome of depression in later life in primary care: longitudinal cohort study with three years’ follow-up. BMJ. 2009;338:a3079. doi: 10.1136/bmj.a3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, … Thase ME. Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: A randomized controlled trial. The Journal of Clinical Psychiatry. 2016;77(10):e1316–e1323. doi: 10.4088/JCP.15m10244. [DOI] [PubMed] [Google Scholar]
- Manber R, Carney CE. Treatment Plans and Interventions for Insomnia: A Case Formulation Approach. Guilford Publications; 2015. [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall WV, Reboussin BA, Cohen W. Subjective measurement of insomnia and quality of life in depressed inpatients. Journal of Sleep Research. 2000;9(1):43–48. doi: 10.1046/j.1365-2869.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Research. 1985;14(2):131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine. 2003;65(2):259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Brown H, Leuchter A, Hunter A. Causality and Psychopathology: Finding the Determinants of Disorders and Their Cures. Washington, DC: American Psychiatric Publishing; 2008. General approaches to analysis of course: applying growth mixture modeling to randomized trials of depression medication; pp. 159–178. [Google Scholar]
- Muthén LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables : User’s Guide. 1998–2016. [Google Scholar]
- Muthén, Muthén, Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, … Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychological Medicine. 2010;40(1):41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, Nierenberg AA … STAR*D investigators. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. The Journal of Clinical Psychiatry. 2005;66(8):1002–1011. doi: 10.4088/jcp.v66n0807. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 2007;3(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: a metacognitive model of insomnia. Behaviour Research and Therapy. 2012;50(11):651–660. doi: 10.1016/j.brat.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of Affective Disorders. 1997;42(2–3):209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- Smagula SF, Boudreau RM, Stone K, Reynolds CF, III, Bromberger JT, Ancoli-Israel S, … Cauley JA. Latent activity rhythm disturbance sub-groups and longitudinal change in depression symptoms among older men. Chronobiology international. 2015;32(10):1427–1437. doi: 10.3109/07420528.2015.1102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Butters MA, Anderson SJ, Lenze EJ, Dew MA, Mulsant BH, … Reynolds CF., 3rd Antidepressant Response Trajectories and Associated Clinical Prognostic Factors Among Older Adults. JAMA Psychiatry. 2015;72(10):1021–1028. doi: 10.1001/jamapsychiatry.2015.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. The Journal of Applied Psychology. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JM. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behaviour Research and Therapy. 1995;33(1):25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L … STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American Journal of Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Uher R, Muthén B, Souery D, Mors O, Jaracz J, Placentino A, … McGuffin P. Trajectories of change in depression severity during treatment with antidepressants. Psychological Medicine. 2010;40(8):1367–1377. doi: 10.1017/S0033291709991528. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, … Wade AG. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L … STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American Journal of Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.