Abstract
Objectives
HIV-2-infected individuals usually initiate antiretroviral therapy (ART) at an advanced age compared with HIV-1 patients, with a potential impact on treatment outcomes. This study aimed to investigate the effect of sex and age on mortality and loss to follow-up (LTFU) among HIV-2-infected individuals initiating ART.
Methods
Analyses were conducted using the database of the IeDEA–HIV-2 West Africa collaboration. LTFU was considered if the interval between the last visit and the closing date for this analysis was >180 days. Probability of death and LTFU were estimated with Kaplan-Meier methods. A Cox regression model was used to identify factors associated with death and LTFU over the first 24 months on ART.
Results
A total of 1,825 HIV-2-infected individuals including 60% women were considered for this analysis. The median age, baseline CD4 and follow-up duration were 45 years (IQR[38-52]), 185 cells/mm3 (IQR[95–297]) and 28.8 months (IQR[9.8–58.9]), respectively. Over the first 24 months, the mortality rate was 5.2 per100 pyo (95%CI[4.4-6.1]) and 469 (25.7%) were LTFU. Male sex (HR=1.9; 95%CI[1.4;2.8]), baseline CD4<100 cell/mm3 (HR=4.4 95%CI[1.7;11.1]; ref≥350 cell/mm3), haemoglobin 7.5–10g/dL (HR=2.4 95%CI[1.3;4.4]; ref≥12 g/dL); and body mass index <18 kg/m2 (HR=2.1 95%CI[1.3;3.4]; ref=18-25 kg/m2) were associated with higher mortality over the first 24 months. Similar associations were found for LTFU.
Conclusion
Mortality and LTFU are high among ART-receiving HIV-2-infected individuals and higher in men than in women. There is a critical need to further determine the causes of poor retention and implement sex specific solutions that improve outcomes in HIV-2 ART programs.
Keywords: HIV-2, sex, antiretroviral therapy, mortality, loss to follow-up, West Africa
Introduction
HIV-2 infection is mainly localized in West Africa, with a limited spread to other parts of the world (1). People infected with HIV-2 experience a longer asymptomatic phase, a slower immunologic progression and a lower virologic replication, compared with those infected with HIV-1 (2–4). Because of the intrinsic resistance of HIV-2 to non-nucleoside reverse transcriptase inhibitors (NNRTIs), treatment options in resource-limited settings for HIV-2 infected individuals are limited and this may affect treatment sequencing in case of failure and long term outcomes of these patients (5–7).
Similar to HIV-1 infection, more women than men are infected with HIV-2 (8–10), with a significantly advanced age at diagnosis and ART initiation among HIV-2 infected individuals compared with HIV-1 (11). In resource-limited settings, higher mortality (12–14) and loss to follow-up (LTFU) rates have been reported in men than in women among HIV-1 infected individuals (9,15–17), as well as an association between LTFU and mortality (18). Many possible explanations such as baseline characteristics (19,20), late presentation (79% of HIV-1 infected individuals initiating ART with CD4 count <200/mm3), high viral load (> 5 log) and suboptimal immunologic response (14,21) have been suggested, but the underlying causes of these findings remain poorly understood.
The relationship between antiretroviral therapy (ART) outcomes and sex, as well as age have been well described among HIV-1 infected individuals in resource-limited settings, but very poorly studied in HIV-2 infected individuals because of lack of power related to a limited sample size of HIV-2 cohorts in each country. Considering the different disease pattern of HIV-2 infection compared with HIV-1, we evaluated mortality, LTFU and associated determinants among ART receiving HIV-2 infected individuals.
Methods
Study design, site and population
This analysis was conducted among patients in the IeDEA-WA HIV-2 cohort that has been previously described (22). This dynamic cohort is currently made up of 5,193 HIV-2 and HIV-1/HIV-2 dually reactive individuals followed up in 15 West African clinics, in seven countries (Benin, Burkina Faso, Cote d’Ivoire, Guinea-Bissau, Mali, Senegal, and Togo). Patients were included in this cohort based on the results of the HIV testing performed according to the national algorithms of each participating country. The HIV diagnosis and the initial classification of patients of the IeDEA-WA HIV-2 cohort have been described elsewhere (24). The study population considered for this analysis was HIV-2 mono-infected patients only, aged 16 years old and above, initiating ART between February 1997 and September 2014, and followed up till May 15, 2015. The routine care of patients (ART and biological tests) follows the national HIV treatment guidelines of each country, most of them recommending ART initiation below 350 CD4/mm3 (22).
Variables and definition
Baseline characteristics used in this analysis refer to measurements recorded immediately before ART initiation for specific variables such as demographics (age, gender), disease progression (CD4 cell count, WHO clinical stage) as well as clinical and biological parameters of patients (weight, haemoglobin). The following variables were transformed into categorical ones: age (16-39; 40-49; ≥50 years), initial CD4 count (0-99; 100-199; 200-349; ≥350 cells/mm3), haemoglobin at enrolment (<7.5; 7.5-9.9; 10-12; >12 g/dL), body mass index (BMI) at enrolment (<18; 18-25; >25 kg/m2) and WHO clinical stage (I-II and III-IV).
Outcomes and definition
The primary outcome was all cause mortality based on the living status reported at the closing date (May 15, 2015) of the cohort’s database for this analysis, after an active search by home visit, phone tracking of patients or calling a close relative of the participant when the contact details were available.
Secondary outcome was LTFU, defined as a patient who was neither reported dead nor transferred-out, and whose date of last visit was >6 months prior to the closing date of the cohort’s database for this analysis (May 15, 2015). Patients returning into care before the closing date of the cohort were not LTFU even when they had missed more than three consecutive visits (HIV care visits are usually programmed every three months).
Data collection and management
A dedicated case report file (CRF) was used to collect enrolment and follow-up data for all HIV-2 infected individuals included in the cohort. Follow-up characteristics (death or LTFU) were checked by social workers and notified to doctors who reported in the CRF. Data of each CRF were recorded in a local database built with Microsoft Access software (Microsoft Access 2007).
Statistical analysis
Baseline characteristics and summary outcomes were compared between men and women and between age groups. Differences between proportions and medians were tested with Pearson’s chi-squared test or Mann-Whitney test. Probability and median survival time from the date of ART initiation to death or LTFU was estimated by Kaplan-Meier methods. Cox proportional hazards regression models were used to assess crude and adjusted hazard ratios between baseline characteristics and outcomes (death or LTFU), over the first 24 months after ART initiation. For patients neither dead nor LTFU, the closing date of the follow up was 15th May 2015. For patients who experienced death or LTFU during the observation period, the censor date was the date of the first event, meaning date of death for dead patients and date of the last visit for patients LTFU). Interactions between adjustment factors and sex or age were tested and possible confounders were checked. Univariable Cox models were performed to assess covariate effect, manual backwards selection method was performed to select significant covariates in a multivariable Cox model. The level of significance for all analyses was a two sided P-value of less than 0.05. All statistical analyses were performed with the statistical software package, Stata® 11.0 software (Stata Corp, College Station, Texas USA).
Ethics
The IeDEA-WA HIV-2 cohort’s protocol has been approved by the national ethic committee of each participating country. All the patients gave their written consent or fingerprint if illiterate before being included in the cohort.
Results
Study population
As of May 15, 2015, the IeDEA-WA HIV-2 cohort comprised 5,193 individuals, including 2,878 (55.4%) HIV-2 infected patients and 2,315 (44.6%) HIV-1/HIV-2 dually reactive patients. Among them 1,825 HIV-2 singly infected patients met the eligibility criteria for this analysis, including 1,102 (60.4%) women. Median age at ART initiation was 45.4 years (interquartile range IQR [38.0 - 52.0]). Most of the patients came from Côte d’Ivoire (42.3%), Guinea-Bissau (23.2%) and Burkina Faso (18.5%) with the remaining 16.0% coming from Benin, Mali, Senegal and Togo.
Baseline characteristics
Table 1 summarizes the baseline characteristics of the study population. At ART initiation, men were older in median than women (49 vs. 43 years p= 0.000). The baseline clinical stage was reported for 877 (48%) patients and 663 of them (75.5%) were classified WHO stage I or II. The median CD4 cell count at baseline was 185 cells/mm3 (IQR [95-297]), lower in men than women (160 IQR [79-262] vs. 204 cells/mm3 IQR [105-337]; p= 0.000). At ART initiation, more men than women had a BMI<18kg/m2, (12.2% vs. 9.3%, p =0.015). The most commonly prescribed ART regimens were protease inhibitor (PI)-based regimens (66.6%), followed by triple nucleoside reverse transcriptase inhibitors (NRTI)-based regimens (9.3%) and 436 (23.9%) patients initiated a suboptimal ART regimen (mainly NNRTI-based regimen) due to HIV-type misclassification at enrolment.
Table 1.
Baseline characteristics of patients in the IeDEA HIV-2 Cohort according to sex from 2002 to 2014
| Baseline characteristics | HIV-2 infected patients initiating ART | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Men | Women | Total | P* | ||||
| 723 | (39.6%) | 1 102 | (60.4%) | 1 825 | (100%) | ||
| Age years, median (IQR) | 48.8 [42.9 – 54.6] | 42.6 [35.9 – 49.8] | 45.4 [38.0 – 52.0] | 0.000 | |||
| 16–39 | 131 | 18.1 | 437 | 39.7 | 568 | 31.1 | |
| 40–49 | 288 | 39.8 | 396 | 35.9 | 684 | 37.5 | |
| ≥50 | 304 | 42.1 | 269 | 24.4 | 573 | 31.4 | |
| WHO Clinical stage n (%) | 0.180 | ||||||
| I and II | 261 | 36.1 | 402 | 36.5 | 663 | 36.3 | |
| III and IV | 97 | 13.4 | 117 | 10.6 | 214 | 11.7 | |
| Missing | 365 | 50.5 | 583 | 52.9 | 948 | 52.0 | |
| Initial CD4 cell count, median (IQR) cells/mm3 | 160 [79 – 262] | 204 [105 – 337] | 185 [95 – 297] | 0.000 | |||
| 0 – 49 | 73 | 10.1 | 86 | 7.8 | 159 | 8.7 | |
| 50 – 99 | 88 | 12.2 | 99 | 9.0 | 187 | 10.2 | |
| 100 – 199 | 148 | 20.5 | 201 | 18.2 | 349 | 19.1 | |
| 200 – 349 | 136 | 18.8 | 227 | 20.6 | 363 | 19.9 | |
| ≥350 | 60 | 8.3 | 179 | 16.2 | 239 | 13.1 | |
| Missing | 218 | 30.2 | 310 | 28.1 | 528 | 28.9 | |
| Initial ART regimen | 0.018 | ||||||
| PI-based regimen | 507 | 70.1 | 709 | 64.3 | 1216 | 66.6 | |
| Triple NRTI-based regimen | 68 | 9.4 | 105 | 9.5 | 173 | 9.5 | |
| Sub optimal ART regimen** | 148 | 20.5 | 288 | 26.2 | 436 | 23.9 | |
| BMI, median (IQR) Kg/m2 | 0.015 | ||||||
| <18 | 88 | 12.2 | 103 | 9.3 | 191 | 10.4 | |
| 18 – 25 | 199 | 27.5 | 273 | 24.8 | 472 | 25.9 | |
| >25 | 52 | 7.2 | 117 | 10.6 | 169 | 9.3 | |
| Missing | 384 | 53.1 | 609 | 55.3 | 993 | 54.4 | |
| Haemoglobin level n (%) g/dL | 0.000 | ||||||
| <7.5 | 51 | 7.1 | 81 | 7.4 | 132 | 7.2 | |
| 7.5 – 9.9 | 98 | 13.5 | 216 | 19.6 | 314 | 17.21 | |
| 10 – 12 | 134 | 18.5 | 266 | 24.1 | 400 | 21.9 | |
| >12 | 168 | 23.2 | 128 | 11.6 | 296 | 16.2 | |
| Missing | 272 | 37.6 | 411 | 37.3 | 683 | 37.4 | |
| Year of ART initiation | 0.000 | ||||||
| <2007 | 249 | 34.4 | 277 | 25.1 | 526 | 28.8 | |
| 2007–2010 | 201 | 27.8 | 345 | 31.3 | 546 | 29.9 | |
| 2011–2014 | 273 | 37.8 | 480 | 43.6 | 753 | 41.3 | |
IQR: Interquartile Range; ART: Antiretroviral therapy; BMI: Body mass index; Hb: Haemoglobin; p*: p-value indicating a significant difference between men and women for the considered variable;
Sub optimal regimen indicative of patients initially miss-diagnosed and treated as HIV-1.
Follow up characteristics and immunological response
The median follow-up duration was 28.8 months (IQR [9.8-58.9]) with no difference between sex and age groups, and the at-risk period was estimated to 5 915 person-years of observation (pyo). The median CD4 count was 185 cells /mm3 at baseline, and increased to 263 cells /mm3, 283 cells /mm3 and 290 cells /mm3, at six, 12 and 24 months respectively. The median CD4 count was higher in woman than in men at six months (289 [172 - 472] vs. 229 [150 - 344]; p=0.0003), 12 months (292 [196 - 475] vs. 273 [180 - 396]; p=0.033) and 24 months (337 [195 - 530] vs. 259 [170 - 379]; p=0.0001).
Mortality
There were 221 (12.1%) deaths during the observation period and the overall mortality rate was 3.7 per 100 pyo (95% CI [3.2-4.2]). The cumulative estimated risk of death at six, 12 and 24 months after ART initiation was 4.1 (95% CI [3.2-5.1]), 5.5% (95% CI [4.4-6.5]) and 7.7% (95% CI [6.4-8.9]), respectively. Over the first 24 months after ART initiation (2687 pyo), 140 (7.7%) patients dead and the mortality rate was 5.2 per 100 pyo (95% CI [4.4-6.1], higher in men than in women (7.4/100 pyo; 95% CI [5.9-9.3], vs. 3.9/100 pyo; 95% CI [3.0-4.9], Log rank test p=0.001) (Fig. 1). Mortality was also associated with age at ART initiation (log-rang test p-value=0.026). Figure 1b shows a higher probability of death among people initiating ART at 50 years or older compared with those initiating ART at a younger age.
Figure 1.
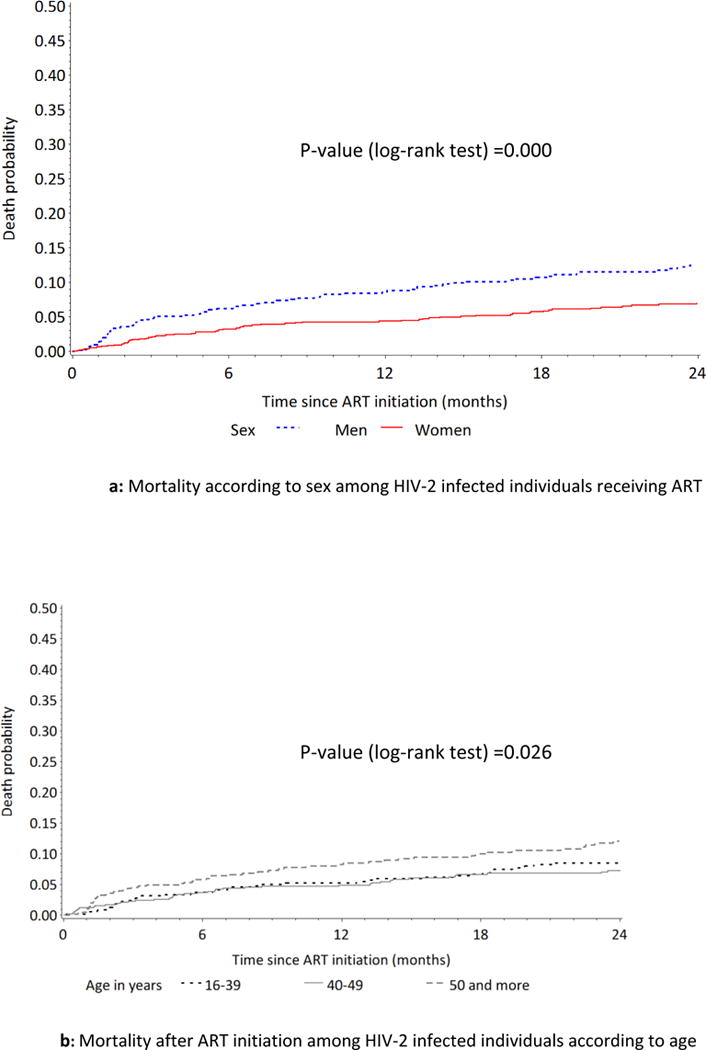
Effect of sex and age on mortality among ART-receiving HIV-2 infected individuals in West Africa.
Factors associated with mortality
Table 2 shows the results of the multivariable model assessing factors associated with mortality. Male sex was associated with higher mortality than female sex (adjusted hazard ratio (aHR) = 1.98; 95%CI [1.40-2.82], p<0.001). A borderline significant association appeared between mortality and age >=50 years (aHR=1.44; 95%CI [0.96-2.16]) compared with age 40-49 years. Regarding clinical and biological baseline characteristics, initiating ART between 2007 and 2010 (aHR =1.72; 95%CI [1.11-2.66]) or between 2011 and 2014 (aHR = 2.16; 95%CI [1.33-3.51]) was associated with higher mortality compared with initiating treatment before 2007). CD4 count at ART initiation between 100 and 199 cells/mm3 (aHR = 3.25; 95% CI [1.34-7.85]), compared with CD4 count ≥350 cells/mm3 was significantly associated with higher mortality. Similar associations with mortality were found for initial haemoglobin level between 9.9 and 7.5 g/dl (aHR 2.38; 95%CI [1.30-4.35]) compared with initial haemoglobin level ≥12 g/dl and with initial BMI <18 kg/m2 (aHR= 2.06; 95%CI [1.24-3.40]), compared with BMI between 18 and 25 kg/m2. Mortality was neither associated with the country of origin of each specific cohort nor with the initial ART regimen, after adjusting on other baseline characteristics.
Table 2.
Effect of sex, age and other factors on mortality and loss to follow up during the first 24 months after ART initiation (multivariable final Cox models)
| Variables | Death | Loss to follow up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| (N) | Rate/100 Pyo | aHR (CI 95%) | p-value | n (%) | aHR (CI 95%) | p-value |
| Sex | 0.000 | 0.058 | ||||
| Women (1102) | 3.8 (3.0–4.9) | 1 | 279 (25.3) | 1 | ||
| Men (723) | 7.4 (5.9–9.3) | 1.98 (1.39;2.82) | 190 (26.3) | 1.20 (0.99;1.47) | ||
| Age at ART initiation (years) | 0.076 | 0.028 | ||||
| 40–49 (684) | 4.1 (3.0–5.5) | 1 | 152 (22.2) | 1 | ||
| 16–39 (568) | 4.8 (3.6–6.6) | 1.36 (0.88;2.10) | 155 (27.3) | 1.28 (1.02;1.61) | ||
| ≥50 (573) | 7.1 (5.4–9.2) | 1.44 (0.96;2.16) | 162 (28.3) | 1.28 (1.02;1.60) | ||
| Year of ART initiation | 0.002 | 0.000 | ||||
| <2007 (526) | 3.6 (2.5–5.0) | 1 | 98 (18.6) | 1 | ||
| 2007–10 (546) | 5.4 (4.2–7.0) | 1.72 (1.11;2.66) | 193 (25.6) | 1.47 (1.15;1.88) | ||
| 2011–14 (753) | 7.0 (5.2–9.4) | 2.16 (1.33;3.51) | 178 (32.6) | 2.33 (1.79;3.02) | ||
| Initial WHO clinical stage | – | – | ||||
| I,II (663) | 4.7 (3.6–6.2) | – | 154 (21.9) | – | ||
| III,IV (214) | 9.1 (6.2–13.2) | – | 56 (26.2) | – | ||
| Missing (948) | 4.7 (3.7–6.1) | – | 268 (28.3) | – | ||
| Initial CD4 cell count (cells/μL) | 0.001 | – | ||||
| ≥350 (239) | 1.7 (0.8–3.7) | 1 | 71 (29.7) | – | ||
| 200–349 (363) | 3.6 (2.3–5.6) | 1.79 (0.72;4.49) | 99 (27.3) | – | ||
| 100–199 (349) | 6.6 (4.7–9.3) | 3.25 (1.34;7.85) | 92 (26.4) | – | ||
| 50–99 (197) | 9.4 (6.4–13.9) | 4.55 (1.88;11.34) | 46 (24.6) | – | ||
| 0–49 (159) | 8.1 (5.1–12.9) | 3.59 (1.39;9.27) | 42 (26.4) | – | ||
| Missing (528) | 4.8 (3.5–6.6) | 3.10 (1.24;7.74) | 119 (22.5) | – | ||
| Initial haemoglobin (g/dL) | 0.005 | 0.034 | ||||
| >12 (296) | 3.5 (2.1–5.6) | 1 | 67 (22.6) | 1 | ||
| 10–12 (400) | 5.6 (4.0–7.9) | 1.66 (0.90;3.69) | 105 (26.3) | 1.22 (0.89;1.67) | ||
| 7.5–9.9 (314) | 9.1 (6.7–12.5) | 2.38 (1.30;4.35) | 82 (26.1) | 1.15 (0.83;1.61 | ||
| <7.5 (132) | 4.3 (2.0–9.0) | 1.10 (0.44;2.74) | 57 (43.2) | 1.48 (1.03;2.15) | ||
| Missing (683) | 4.3 (3.2–5.7) | 1.29 (0.68;2.47) | 158 (23.1) | 0.97 (0.75;1.30) | ||
| Initial BMI (kg/m2) | 0.005 | 0.003 | ||||
| 18–25 (472) | 4.7 (3.3–6.5) | 1 | 84 (17.8) | 1 | ||
| <18 (191) | 11.6 (8.0–16.7) | 2.06 (1.24;3.40) | 49 (25.7) | 1.69 (1.19;2.41) | ||
| >25 (169) | 3.3(1.7–6.3) | 1.00 (0.47;2.12) | 21 (12.4) | 0.70 (0.43;1.14) | ||
| Missing (993) | 4.7 (3.7–6.0) | 1.02 (0.67;1.55) | 215 (31.7) | 2.03 (1.59;2.60) | ||
ART: Antiretroviral therapy; BMI: Body mass index; aHR: adjusted hazard ratio; pyo: person years of observation.
Loss to follow-up
After 24 months, 469 (25.7%) patients were LTFU and the estimated risk of being LTFU at six, 12 and 24 months after ART initiation was 10.8% (95% CI [9.4-12.3]), 16.7% (95% CI [14.9-18.4]) and 25.7% (95% CI [23.7-18.7]) respectively. The probability of being LTFU after 24 months on ART did not significantly vary according to sex (log-rank test p-value=0.310), but varied according to age, with people aged 16-39 and those aged >=50 having higher probability (log-rank test p-value= 0.007) of being LTFU than those aged 40-49 (Fig. 2).
Figure 2.
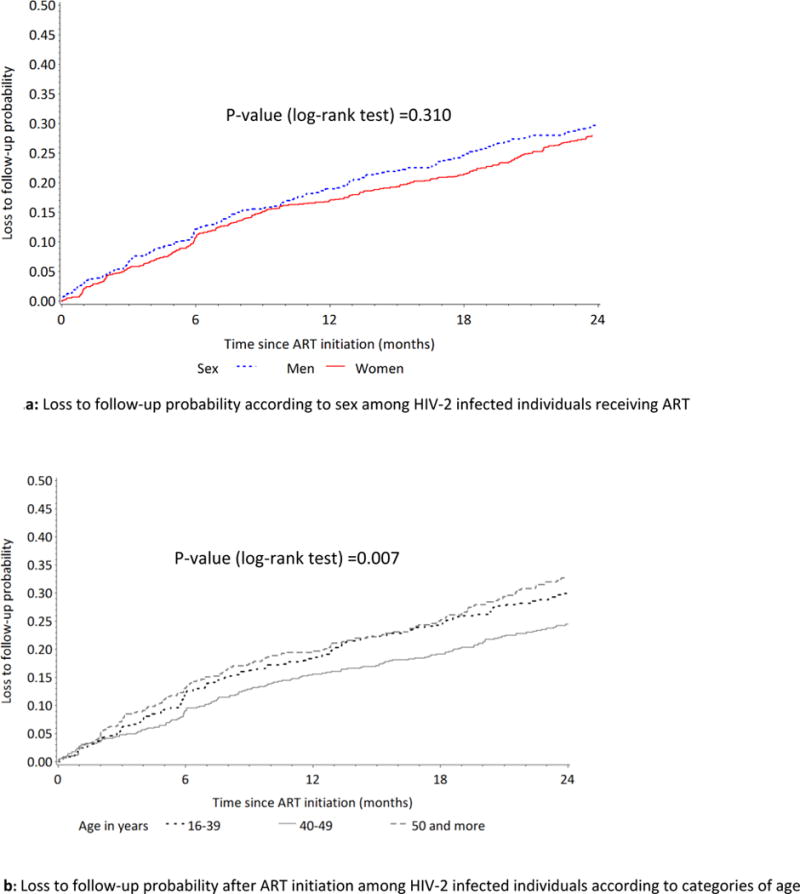
Effect of sex and age on loss to follow-up probability among ART-receiving HIV-2 infected individuals in West Africa.
Factors associated with loss to follow-up
In a multivariable model, after adjusting for demographic, clinical and laboratory baseline characteristics, male sex (aHR = 1.20; 95% CI [0.99 - 1.47], p-value = 0.058) and being aged 16-39 or >=50, compared with 40-49 years old (aHR = 1.28; 95% CI [1.02 - 1.61]) was associated with LTFU (Table 2). In addition, ART initiation in 2007-2010 (aHR = 1.47; 95% CI [1.15 - 1.88]) or in 2011-2014 (aHR = 2.33; 95% CI [1.79 – 3.02]) compared with before 2007, was associated with LTFU. Initial haemoglobin level <7.5 g/dL (aHR = 1.48; 95% CI [1.03– 2.15]) compared with >12 g/dL and initial BMI <18 Kg/m2 (aHR = 1.69; 95% CI [1.19 – 2.41]) compared with 18–25 Kg/m2, were also associated with LTFU, while initial WHO stage and CD4 cell count were not associated with LTFU.
Death and LTFU
During the first 24 months of follow-up, 609 (33.4%) individuals died (n=140) or became LTFU (n=469). The retention probability was 85.0% (95% CI [83.4-86.6] at six months, 77.9% (95% CI [75.9-79.8%]) at 12 months and 66.6% (95%CI [64.5-68.8]) at 24 months. The retention probability at 24 months was higher among women compared with men (68.9% (95% CI [66.0-71.6%]) vs. 63.2% (95% CI [59.6-66.7%]) respectively, p-value =0.007). Retention was also higher among patients aged 16 to 39 (65.5%; 95% CI [61.4-69.4]) and those aged 40 to 49 (71.4%; 95% CI [67.9-74.8]) compared with those aged >=50 (61.9%; 95% CI [57.8-65.9]) (Fig. 3).
Figure 3.
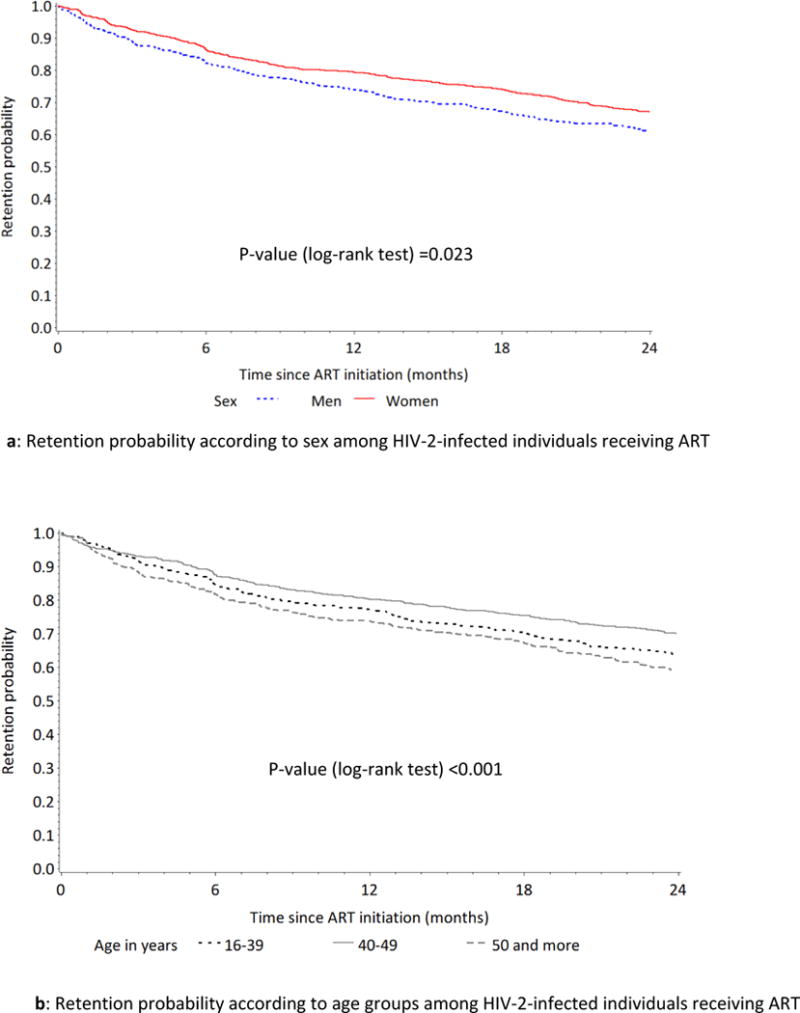
Effect of sex and age on retention among ART-receiving HIV-2 infected individuals in West Africa.
Over the first 24 months on ART, factors associated with death and LTFU in multivariable analysis after adjusting for other baseline characteristics were male sex (aHR = 1.39; 95%CI [1.17-1.65], p-value =0.000), age ≥50 years (aHR = 1.31; 95%CI [1.08-1.60], p-value = 0.006), ART initiation after 2007 (aHR = 2.21; 95%CI [1.76-2.78]), initial haemoglobin level < 7.5 g/dL (aHR = 1.49; 95%CI [1.06-2.09], p-value = 0.01), and initial BMI<18 (aHR=1.87; 95%CI [1.40-2.49]).
Discussion
This study reports on HIV-2 ART-program outcomes according to sex and age in a large West African multi-country cohort. The overall number of deaths was 221 (12.1%), for a global mortality rate of 3.7 per 100 pyo (95% CI [3.2-4.2]). Over the first 24 months after ART initiation, the mortality rate was estimated to be 5.2 per 100 pyo with a death probability of 7.7%. Men had higher mortality rate than women especially those initiating ART at an advanced age. Approximately, one quarter of the patients were LTFU after 24 months on ART, and the retention rate was only 66%. Male sex, advanced age at ART initiation, ART initiation after 2007, low initial haemoglobin level and low BMI were associated with mortality and with LTFU at 24 months, while low initial CD4 count was only associated with mortality.
In our study, among ART-receiving HIV-2-infected individuals, the crude mortality rate was high, around 12% after a median follow up duration of 28.8 months and 1/3 of deaths occurred during the first 6 months after starting ART. Thus, despite the differences in disease progression between HIV-1 and HIV-2, the estimates of mortality in ART-receiving HIV-2 infected individuals appear to be comparable with what is seen in HIV-1 infection in sub-Saharan Africa (SSA)(25–28). Like in HIV-1 infection, late presentation (20% of patients with CD4 count <100 cells/mm3) could explain this high mortality rate in HIV-2 infected individuals receiving ART. The suboptimal immune recovery (only +100 CD4 /mm3 after the first 24 months of ART), which is ~ two times lower in our cohort than that observed in HIV-1 infected individuals over the same period (27), may also play a role in this high mortality rate. In addition, men were more likely to die than women in our cohort, as has been reported in ART-receiving HIV-1 infected individuals (10,13,14). Baseline clinical and laboratory characteristics have been identified as possible factors explaining this sex-difference in mortality in HIV-1 infected individuals (12,18–20,29). Similar associated factors were found in our study, especially the CD4 counts that were lower in men than in women at ART initiation, indicating higher rates of late presentation among men. Age is another cofactor frequently cited to explain the difference in mortality among ART-receiving men and women. Many surveys identified the older age of men compared with women at ART initiation, as a reason for sex-difference in mortality (9,13,14,30). In our cohort, more men than women initiated ART after the age of 50 and this age difference could also partly explain the difference in mortality between men and women. Individuals initiating ART after 2007 in our cohort had higher mortality than those initiating ART earlier. This result contrast with findings from other HIV-1 studies, reporting a tremendous decline in mortality and LTFU with the scaling up of ART programs (12,29,30). One possible explanation for our finding could be the potential for the lower likelihood of being captured in programmatic databases for patients who started ART prior to 2007, and died or who were lost to follow up soon after entering care, compare to patients who survived.
Our study found a tremendous increase of LTFU in HIV-2 infected adults at 6, 12 and 24 months after ART initiation. Many other surveys from SSA reported similar or higher rates of LTFU among HIV-1 infected individuals receiving ART (14,17,18,30). In these studies, LTFU was associated with age at ART initiation, male sex, low BMI, low haemoglobin level and sometimes with low CD4 count at ART initiation (14,17,18,30). All these factors are also known to be associated with mortality in HIV-1 infected individuals, and many studies report that 30% to 40% of patients LTFU are misclassified deaths (13,18,31). Our analysis retrieved the same factors associated with LTFU among HIV-2 infected individuals receiving ART, and we speculate that a considerable number of patients LTFU in our cohort may be misclassified deaths.
Retention in care is a major outcome to assess success of ART delivery programs, since it takes into account both deaths and LTFU. A 2007 meta-analysis among HIV-1 infected patients on ART in SSA reported a retention of 75.0% at 12 months and 61.6% at 24 months (32). More recently, a 2012 systematic review on retention and LTFU among HIV infected patient in SSA reported a retention rate of 65% (95% CI [58-73]) among ART receiving individuals after three years of follow-up (33). The results in HIV-2 infected patients are similar with a retention rate of 77.9% at 12 months and 66% at 24 months and lower retention among men. This low retention in care suggests high ART discontinuation and the possible development of ART resistance. ART resistance is of concern, especially for HIV-2 infected individuals in sub-Saharan Africa, who initiate ART with a PI-based regimen, but have limited or no access to effective second-line ART (34–36).
Some limitations should be considered when interpreting our study results. First, we used data from routine HIV care that usually included significant levels of missing data even after data queries. Secondly, the death assessment was limited to hospital reports or patient’s family notification, mainly due to the weakness of the national death registries from contributing IeDEA-WA countries. Furthermore, HIV-2 viral load data was not routinely available to assess whether those who died or were LTFU had virologic failure.
This study underscores the fact that like in HIV-1 cohorts, HIV-2 ART-receiving men are more likely to die and have a lower retention rate than women. There is an urgent need to improve HIV-2 programmatic outcomes, reduce mortality and LTFU, educate clinicians and programs about low retention rates and explore interventions to improve the cascade of care for both men and women infected with HIV-2.
Supplementary Material
Acknowledgments
We thank the fieldwork teams, the study sites and Eric Balestre for their effort. We are indebted to all of the HIV-positive people who agreed to participate in this present study as well as to the health workers and PAC-CI who performed the data collection.
Funding: This work was supported by the following institutes: the US National Cancer Institute (NCI); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the US National Institute of Allergy and Infectious Diseases (NIAID) as part of IeDEA (grant 5U01AI069919).
List of Abbreviation
- aHR
Adjusted hazard ratio
- ART
Antiretroviral therapy
- BMI
Body mass index
- CCR5
C-C chemokine receptor type 5
- CD4
Cluster of differentiation 4
- CI
Confidence interval
- CRF
Case report file
- HIV-1
Human immune deficiency virus type 1
- HIV-2
Human immune deficiency virus type 2
- HRs
Hazard ratios
- IeDEA
International epidemiological database to evaluate AIDS
- IeDEA-WA
International epidemiological database to evaluate AIDS in West Africa
- IQR
Interquartile range
- LTFU
Loss to follow-up
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitor
- PI
Protease inhibitor
- SSA
Sub Saharan Africa
- USA
United states of America
- WHO
World health organization
Footnotes
Disclosure: Authors declare no conflict of interest.
Author contribution
Study design and: BT, DKE, PAC, GSG, SPE and FD
Data collection: SPE, BL, CW, SJ,
Statistical analysis: BT, EB
Manuscript drafting: BT, DKE, PAC,
Critical reading: DKE, GSG, BT, BL, EB, SPE, PAC, CW, SJ, PAC, FD
References
- 1.Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011 Mar 15;52(6):780–7. doi: 10.1093/cid/ciq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle H, Morris J, Todd J, Corrah T, Sabally S, Bangali J, et al. HIV-2-infected patients survive longer than HIV-1-infected patients. AIDS Lond Engl. 1994 Nov;8(11):1617–20. doi: 10.1097/00002030-199411000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Berry N, Ariyoshi K, Jaffar S, Sabally S, Corrah T, Tedder R, et al. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J Hum Virol. 1998 Dec;1(7):457–68. [PubMed] [Google Scholar]
- 4.MacNeil A, Sarr AD, Sankalé J-L, Meloni ST, Mboup S, Kanki P. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007 May;81(10):5325–30. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren J, Bird LE, Chamberlain PP, Stewart-Jones GB, Stuart DI, Stammers DK. Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors. Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14410–5. doi: 10.1073/pnas.222366699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther. 2004 Feb;9(1):57–65. [PubMed] [Google Scholar]
- 7.Gottlieb GS, Eholié S-P, Nkengasong JN, Jallow S, Rowland-Jones S, Whittle HC, et al. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa: AIDS. 2008 Oct;22(16):2069–72. doi: 10.1097/QAD.0b013e32830edd44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen O, da Silva Z, Sandström A, Andersen PK, Andersson S, Poulsen AG, et al. Declining HIV-2 prevalence and incidence among men in a community study from Guinea-Bissau. AIDS Lond Engl. 1998 Sep 10;12(13):1707–14. doi: 10.1097/00002030-199813000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Jespersen S, Hønge BL, Esbjörnsson J, Medina C, da Silva Té D, Correira FG, et al. Differential effects of sex in a West African cohort of HIV-1, HIV-2 and HIV-1/2 dually infected patients: men are worse off. Trop Med Int Health TM IH. 2016 Feb;21(2):253–62. doi: 10.1111/tmi.12646. [DOI] [PubMed] [Google Scholar]
- 10.Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS Lond Engl. 2013 Jan 28;27(3):417–25. doi: 10.1097/QAD.0b013e328359b89b. [DOI] [PubMed] [Google Scholar]
- 11.Drylewicz J, Matheron S, Lazaro E, Damond F, Bonnet F, Simon F, et al. Comparison of viro-immunological marker changes between HIV-1 and HIV-2-infected patients in France. AIDS Lond Engl. 2008 Feb 19;22(4):457–68. doi: 10.1097/QAD.0b013e3282f4ddfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran DA, Ngo AD, Shakeshaft A, Wilson DP, Doran C, Zhang L. Trends in and determinants of loss to follow up and early mortality in a rapid expansion of the antiretroviral treatment program in Vietnam: findings from 13 outpatient clinics. PloS One. 2013;8(9):e73181. doi: 10.1371/journal.pone.0073181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. doi: 10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS Lond Engl. 2011 Jun 1;25(9):1189–97. doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 15.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010 Sep 1;88(9):681–8. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr 1999. 2011 Jan 1;56(1):e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hønge BL, Jespersen S, Nordentoft PB, Medina C, da Silva D, da Silva ZJ, et al. Loss to follow-up occurs at all stages in the diagnostic and follow-up period among HIV-infected patients in Guinea-Bissau: a 7-year retrospective cohort study. BMJ Open. 2013;3(10):e003499. doi: 10.1136/bmjopen-2013-003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001 Nov 28;286(20):2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS Lond Engl. 2008 Oct 1;22(15):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braitstein P, Boulle A, Nash D, Brinkhof MWG, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health 2002. 2008 Feb;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 22.Ekouevi DK, Balestre E, Coffie PA, Minta D, Messou E, Sawadogo A, et al. Characteristics of HIV-2 and HIV-1/HIV-2 Dually Seropositive Adults in West Africa Presenting for Care and Antiretroviral Therapy: The IeDEA-West Africa HIV-2 Cohort Study. PloS One. 2013;8(6):e66135. doi: 10.1371/journal.pone.0066135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012 Oct;41(5):1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchounga BK, Inwoley A, Coffie PA, Minta D, Messou E, Bado G, et al. Re-testing and misclassification of HIV-2 and HIV-1&2 dually reactive patients among the HIV-2 cohort of the West African Database to evaluate AIDS collaboration. J Int AIDS Soc. 2014;17:19064. doi: 10.7448/IAS.17.1.19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harries K, Zachariah R, Manzi M, Firmenich P, Mathela R, Drabo J, et al. Baseline characteristics, response to and outcome of antiretroviral therapy among patients with HIV-1, HIV-2 and dual infection in Burkina Faso. Trans R Soc Trop Med Hyg. 2010 Feb;104(2):154–61. doi: 10.1016/j.trstmh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014 Sep;11(9):e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d’Ivoire: 2-year outcomes and determinants. AIDS Lond Engl. 2008 Apr 23;22(7):873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J J Med Assoc Malawi. 2010 Jun;22(2):49–56. doi: 10.4314/mmj.v22i2.58794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tweve EN, Kayabu D, Nassari NO, Todd J. Improvement in mortality and retention among adult HIV-infected patients in the first 12 months of antiretroviral therapy in Dodoma urban district, Tanzania. Trop Med Int Health TM IH. 2015 Jun;20(6):791–6. doi: 10.1111/tmi.12488. [DOI] [PubMed] [Google Scholar]
- 30.Vinikoor MJ, Joseph J, Mwale J, Marx MA, Goma FM, Mulenga LB, et al. Age at antiretroviral therapy initiation predicts immune recovery, death, and loss to follow-up among HIV-infected adults in urban Zambia. AIDS Res Hum Retroviruses. 2014 Oct;30(10):949–55. doi: 10.1089/aid.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tweya H, Feldacker C, Estill J, Jahn A, Ng’ambi W, Ben-Smith A, et al. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PloS One. 2013;8(9):e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007 Oct 16;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charpentier C, Eholie S, Anglaret X, Bertine M, Rouzioux C, Avettand-Fenoel V, et al. Genotypic resistance profiles of HIV-2-treated patients in West Africa. AIDS Lond Engl. 2014 May 15;28(8):1161–9. doi: 10.1097/QAD.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charpentier C, Camacho R, Ruelle J, Kaiser R, Eberle J, Gürtler L, et al. HIV-2EU: supporting standardized HIV-2 drug resistance interpretation in Europe. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013 Jun;56(11):1654–8. doi: 10.1093/cid/cit104. [DOI] [PubMed] [Google Scholar]
- 36.WHO. WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [Internet] WHO; 2013. [cited 2013 Aug 10]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


