Synopsis
LCIS is both a risk factor and a non-obligate precursor of breast carcinoma. The relative risk of invasive carcinoma after a diagnosis of classic LCIS is approximately 9-10 times that of the general population. LCIS and ILC share common copy number alterations and somatic mutations. A subset of LCIS is clonally related to synchronous or subsequent invasive breast carcinoma. Classic LCIS diagnosed on core biopsy with concordant imaging and pathologic findings does not mandate surgical excision. The margin status of classic LCIS is not reported. Active surveillance and chemoprevention are management options for classic LCIS. The identification of variant LCIS, in a needle core biopsy specimen mandates surgical excision, regardless of radiologic-pathologic concordance. The presence of variant LCIS close to the surgical margin of a resection specimen is reported, and re-excision should be considered.
Keywords: pleomorphic lobular carcinoma in situ, variant lobular carcinoma in situ, E-cadherin, p120, CDH1, core biopsy
Foote and Stewart first described LCIS in 1941 as a rare form of mammary cancer originating in lobules and terminal ducts 1. They reported all the key morphologic features of LCIS that still hold true and accurate today. 1) LCIS is an incidental microscopic finding: “There is no way in which a clinical diagnosis of lobular carcinoma in situ can be made”… “There is no way by which it can be recognized grossly”. 2) LCIS has characteristic morphologic features: “The cells lose polarity, varying in shape while maintaining surprisingly uniform size”. 3) LCIS is multifocal: “it is always a disease of multiple foci”. The aforementioned features characterize the so called “classic” form of LCIS. Even though classic LCIS constitutes both a risk factor and a non-obligate precursor of invasive breast cancer, it is currently managed as a benign lesion, and does not require complete removal and/or evaluation of margin status. Hormonal chemoprevention is recommended for patients with classic LCIS. In the 8th edition of the TNM staging by the American Joint Committee on Cancer (AJCC)2, LCIS is no longer staged as Tis3.
Epidemiology
Classic LCIS (LCIS) usually is an incidental finding in a breast needle core biopsy or surgical excision specimen targeting another lesion. It is therefore difficult to estimate the actual incidence of LCIS. LCIS is identified in 0.5-1.5% of benign breast biopsies 4-6 and in 1.8-2.5% of all breast biopsies4, 7. In a population-based study using data from the Surveillance, Epidemiology, and End Results (SEER) program 8 the incidence of LCIS in women without prior history of in situ or invasive breast carcinoma increased from 0.90/100,000 person-year in 1978-1980 to 3.19/100,000 person-year in 1996-1998 8, 9. The increased incidence of LCIS is likely due to the increased use of mammographic screening and biopsy of mammographically indeterminate or suspicious lesions. Age-specific incidence analysis revealed that the magnitude of the increase was highest among women ages ≥50 years, the age group most likely to participate in routine mammographic screening9.
Clinical features
LCIS occurs predominantly in premenopausal women, with mean and median age at diagnosis of 49 and 50 years, respectively (range 20s-80s)10-13. LCIS is multicentric in 60-80% of patients 14 and bilateral in 20-60% 11, 15, 16. Classic LCIS is clinically and mammographically occult, although recent studies report an association with grouped amorphous or granular mammographic calcifications 13, 17, or heterogeneous non-mass-like enhancement with persistent enhancement kinetics on MRI17. LCIS variants, such as pleomorphic LCIS and LCIS with central necrosis, are usually detected mammographically due to associated pleomorphic calcifications, or can present as a mass lesion with or without associated calcifications 13, 18-25.
Histopathology
Classic LCIS
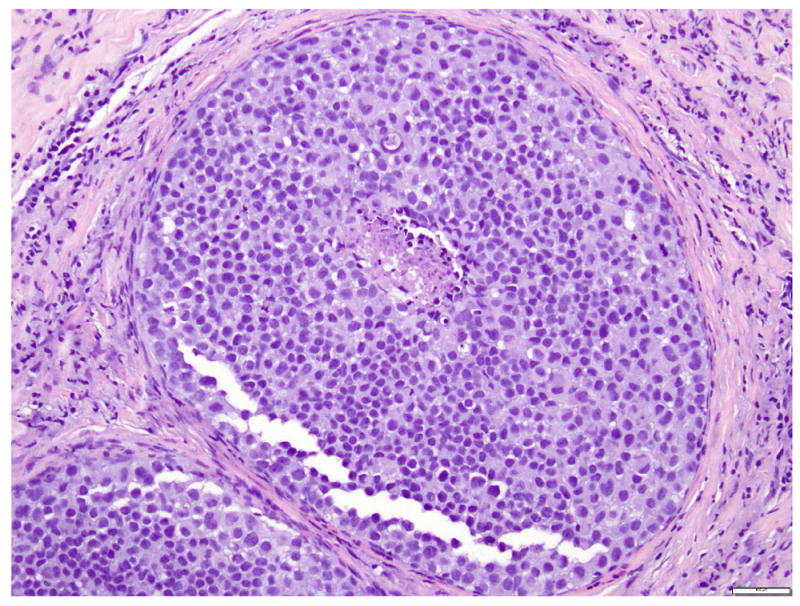
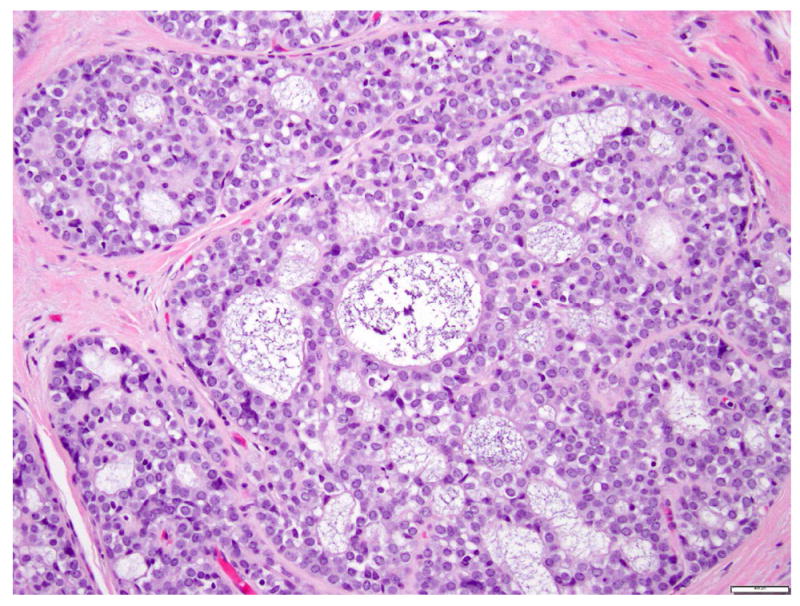
LCIS is a proliferation centered in the terminal ductal lobular units (TDLUs), and consists of neoplastic cells that fill and expand most (>50%) of the acini (Fig. 1). Pagetoid extension into the terminal ducts with growth of LCIS cells underneath the ductal epithelium is also common. The cross-section of a duct with pagetoid involvement by LCIS has a characteristic “cloverleaf” pattern (Fig. 2). Classic LCIS is a monomorphic, dyshesive proliferation of non-polarized cells with round to oval shape, inconspicuous cytoplasm. The nuclei are located in the center of the cells, and are small, round to oval, with smooth nuclear membrane and inconspicuous nucleoli (Fig. 3). Cell borders are indistinct. Intracytoplasmic vacuoles are common, and signet-ring cell formation can occur (Fig. 4). Mitotic activity is absent to exceedingly rare.
Fig 1. Lobular carcinoma in situ, classic type.
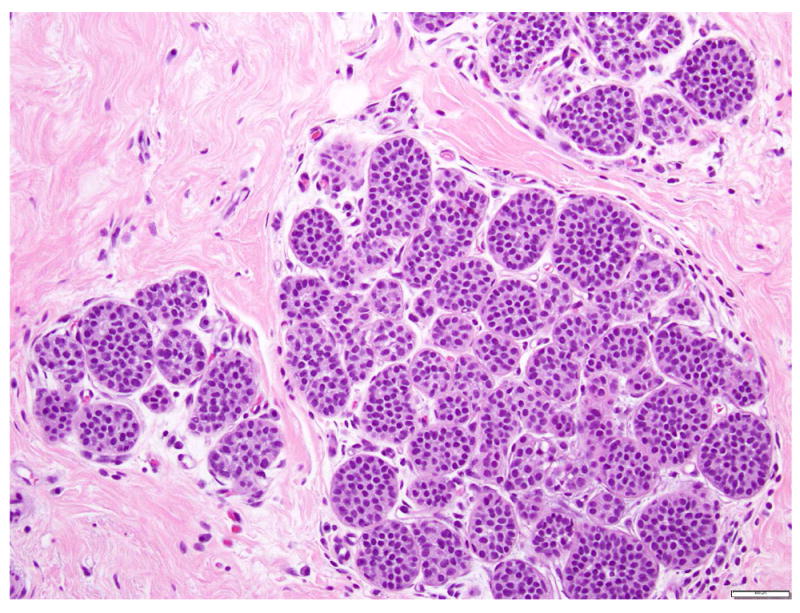
The acini are expanded by monomorphic, evenly spaced dyshesive cells with low grade nuclear atypia. Magnification 200x.
Fig 2. Lobular carcinoma in situ, classic type, with Pagetoid growth in a duct.
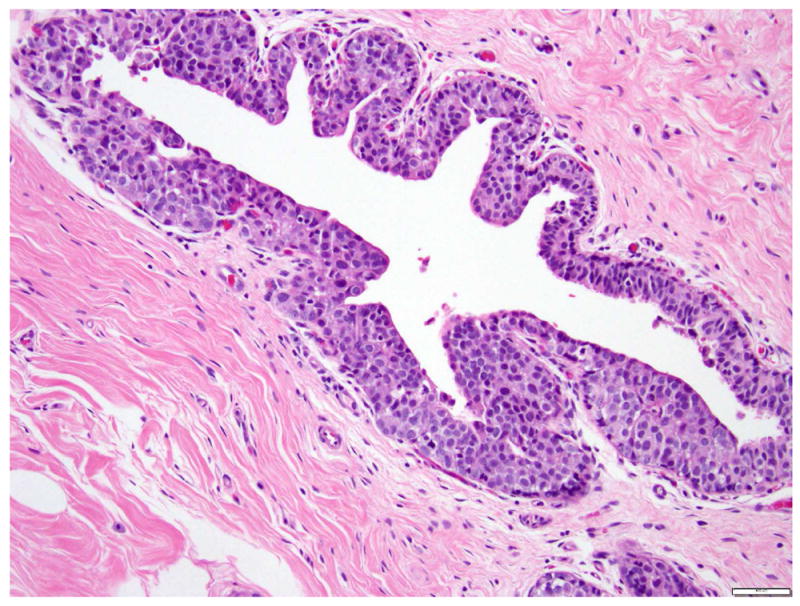
Magnification 200x.
Fig 3. Lobular carcinoma in situ, classic type with small cells (type A cells).
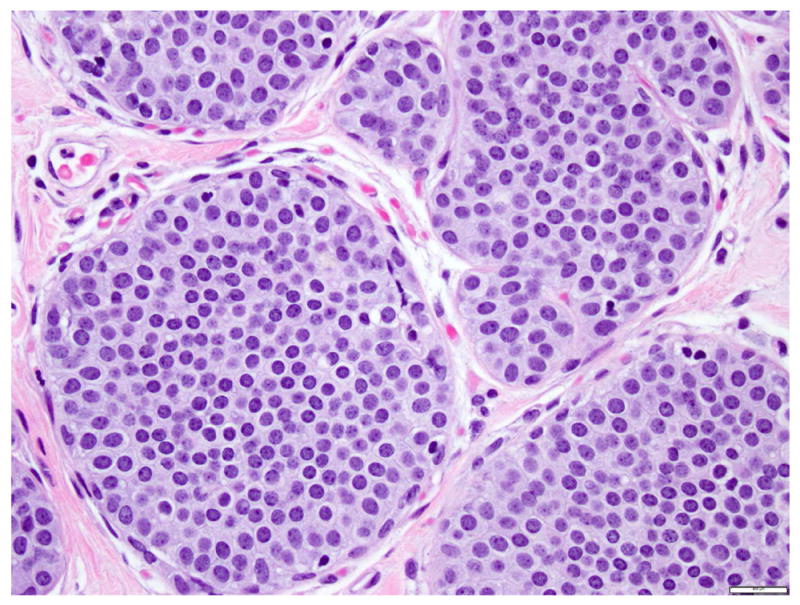
Dyshesive and nonpolarized cells, with scant cytoplasm, monotonous, round to oval nuclei, with regular nuclear membrane, uniform chromatin, and inconspicuous nucleoli. Magnification 400x.
Fig 4. Lobular carcinoma in situ with signet ring cells.
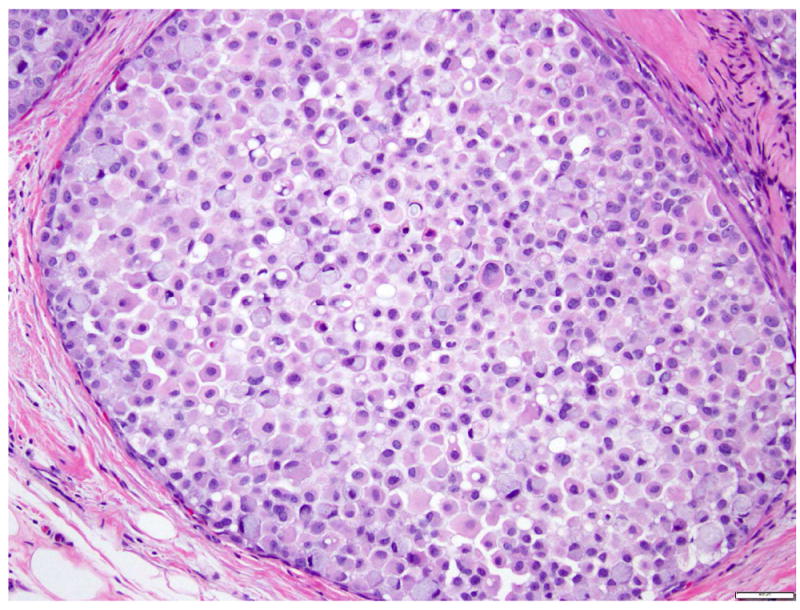
Magnification 400x.
The cells of classic LCIS can have scant cytoplasm (“small cells” or “type A” cells) 10 or be a bit larger (“large cell” type or “type B”) 10, with slightly more abundant cytoplasm, slightly bigger nuclei and more prominent nucleoli (Fig. 5). The two cell types can coexist in the same patient, in the same breast, and even in the same lobule. In the absence of necrosis or marked nuclear pleomorphism, LCIS composed predominantly of large cells is best classified as classic LCIS.
Fig 5. Lobular carcinoma in situ, classic type with large cells (type B cells).
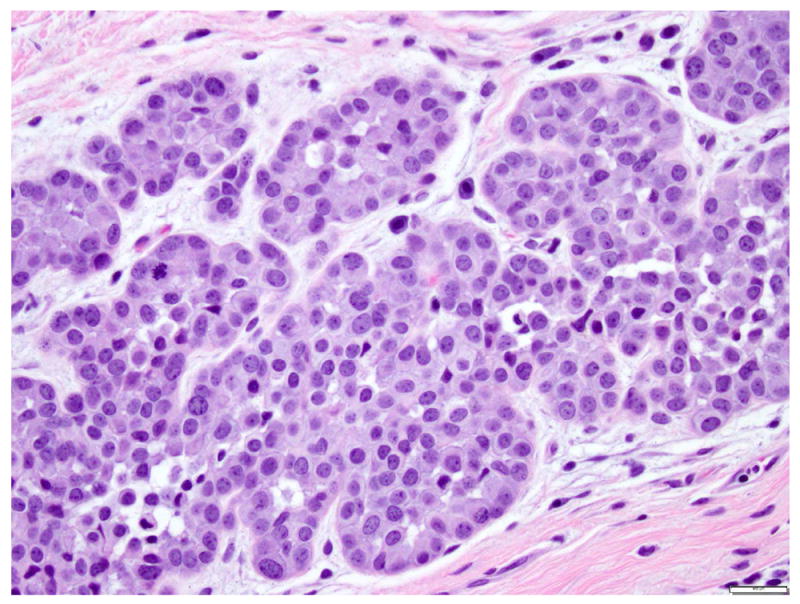
The cells are slightly larger than Type A cells (compare with Figure 3), have more cytoplasm, slightly larger but uniform nuclei, with scattered nucleoli. Magnification 400x.
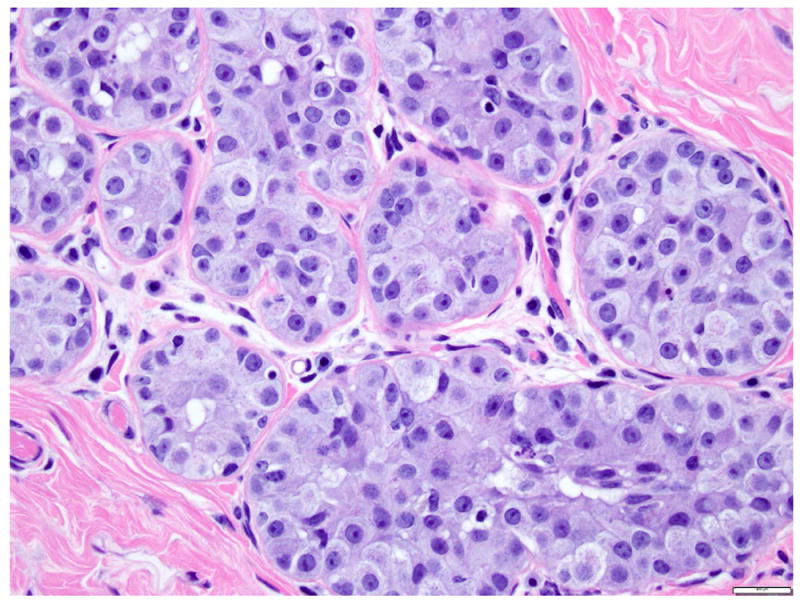
The term lobular neoplasia refers to a morphologic spectrum of lesions including atypical lobular hyperplasia (ALH) and classic LCIS. The cells composing ALH are morphologically indistinguishable from those of classic LCIS, but the proliferation is limited to less than 50% of the acini of the terminal duct lobular units and none of the acini is markedly expanded (Fig. 6) 6, 26. ALH has similar genetic alterations and immunohistochemical profile as classic LCIS, and the two lesions often coexist. ALH is associated with a 4-to-5 fold increase in the risk of subsequent breast carcinoma.
Fig 6. Atypical lobular hyperplasia.
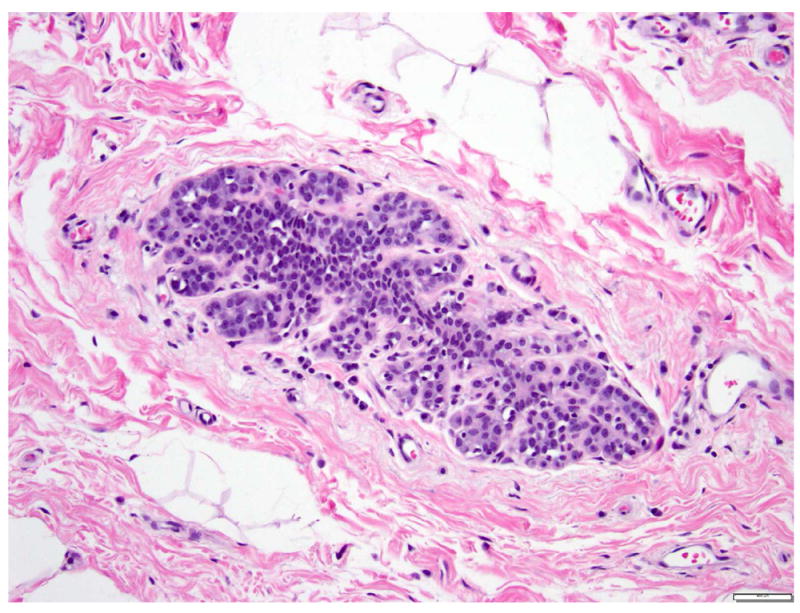
Small round dyshesive cells, cytologically similar to the cells of classic LCIS, involve less than 50% of the acinar spaces. Magnification 200x.
LCIS Variants
Pleomorphic LCIS (P-LCIS)
The term “pleomorphic” was first used to qualify a variant of invasive lobular carcinoma (ILC) showing the infiltrative pattern characteristic of classic ILC (single cell files or targetoid arrangement of dyshesive cells), but having marked nuclear pleomorphism 27-30. Compared to classic ILC, pleomorphic ILC (P-ILC) consists of larger cells with round to ovoid shape, abundant cytoplasm, and large hyperchromatic nuclei that tend to be located slightly off-center, and have prominent nucleoli (Fig. 7). Binucleation is common. The cytoplasm is often eosinophilic, and slightly granular or foamy. Intracytoplasmic vacuoles similar to those seen in classic ILC are common. Mitotic activity is evident. Some P-ILC show apocrine differentiation.
Fig 7. Pleomorphic invasive lobular carcinoma.
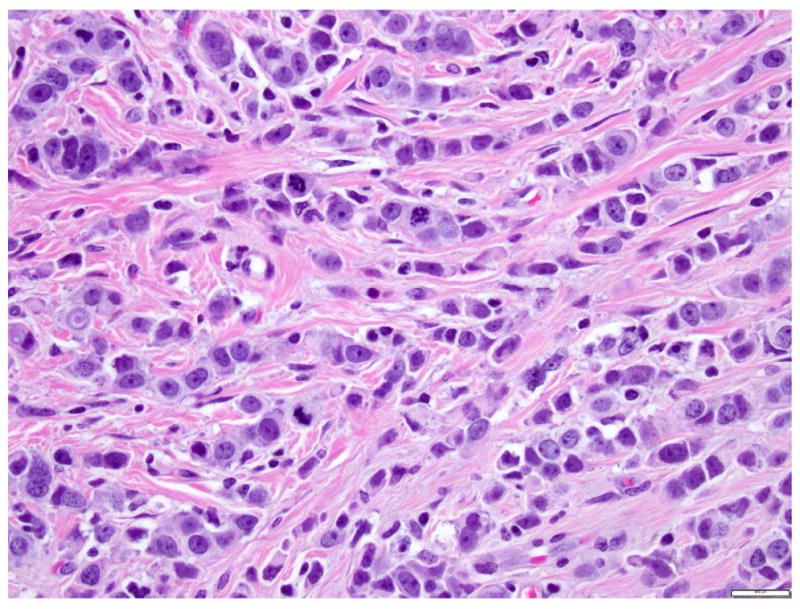
Invasive lobular carcinoma with single file growth pattern, dyshesive cells, and marked nuclear pleomorphism. Magnification 400x.
Pleomorphic LCIS (P-LCIS) was first described by Frost et al in 1996 31. The authors reported a case of PILC with extensive in situ component that was morphologically similar to the P-ILC, centered in the lobules or with pagetoid extension into ducts. Middleton et al identified P-LCIS in 17 of 38 cases (45%) of P-ILC 32. Sneige et al 22 studied 10 cases of P-LCIS without associated invasive carcinoma and 14 cases of P-LCIS with associated P-ILC, and found the histologic features of P-LCIS to be similar in cases with and without invasive carcinoma. Classic LCIS coexisted with P-LCIS in over 40% of the cases 22. Central necrosis and calcifications are common in P-LCIS (Fig. 8), and mitoses are evident. In contrast to classic LCIS, P-LCIS is often detected mammographically as an area of calcifications, architectural distortion, and less frequently, as a mass lesion with or without associated calcifications 20, 22, 23, 25, 33, 34. Patients with PLCIS tend to be significantly older than patients with classic LCIS 20, 23, 34, and most are postmenopausal 20, 23, 25. Some authors further categorize P-LCIS into apocrine and non-apocrine types 23. The cells of apocrine P-LCIS have abundant eosinophilic cytoplasm, and are frequently binucleated. Apocrine P-LCIS seems to be more common in postmenopausal women 23.
Fig 8. Lobular carcinoma in situ, pleomorphic type.
Dyshesive proliferation of round to oval cells with abundant cytoplasm, large eccentric nuclei with irregular nuclear membrane, coarse chromatin, and prominent nucleoli. Foci of necrosis with calcifications are common. Magnification 200x.
Due to its solid growth pattern, marked nuclear pleomorphism, and presence of necrosis and calcifications, P-LCIS closely mimics ductal carcinoma in situ (DCIS) (Fig. 9). However, the cells of P-LCIS are dyshesive, lack true cell polarity, and do not form secondary lumina. Immunohistochemical stains for E-cadherin and p120 can help resolve the differential diagnosis of P-LCIS vs DCIS in ambiguous cases (see Immunohistochemistry).
Fig 9.
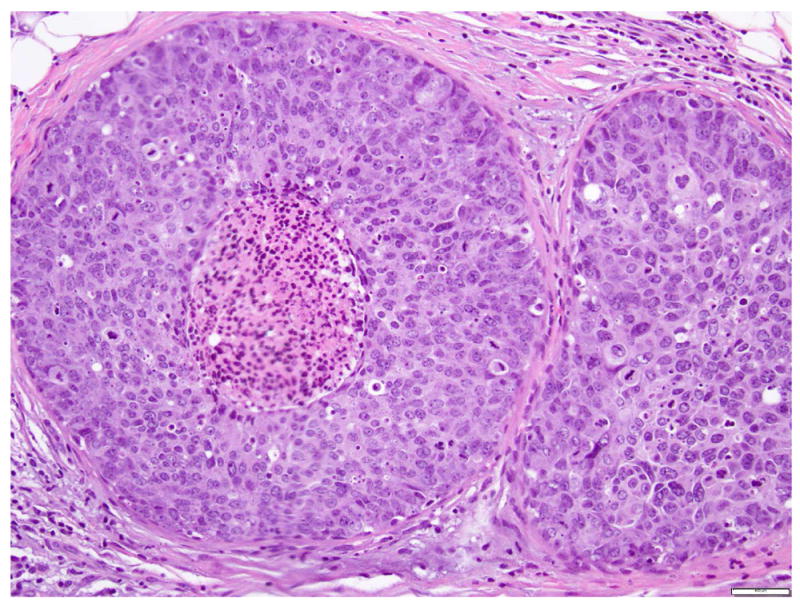
Ductal carcinoma in situ, with solid growth pattern, high nuclear grade and necrosis. Magnification 200x.
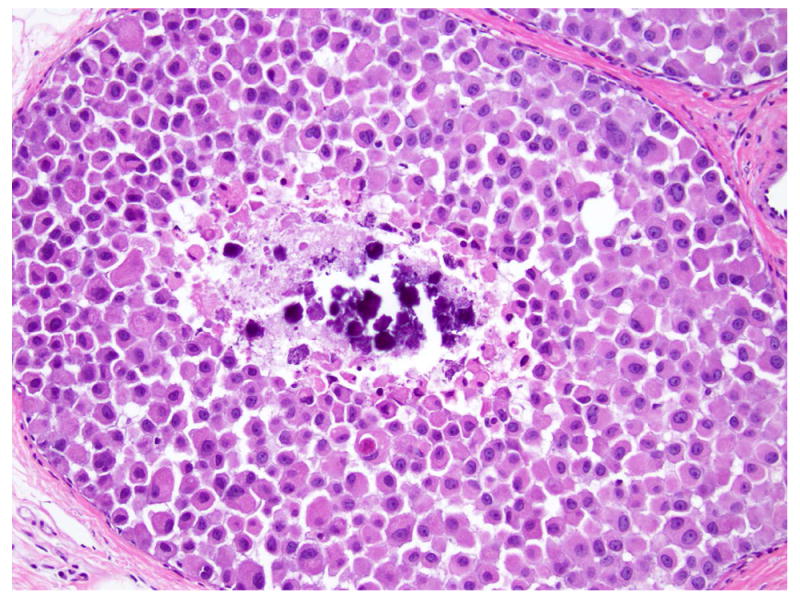
LCIS with necrosis (also known as “florid” LCIS)
LCIS with necrosis refers to a proliferative lesion that exhibits the cytologic features of classic LCIS, including cell dyshesion and type A or type B morphology, but is characterized by massive expansion of the acini (at least 50 cells across the diameter of an acinus) and central necrosis (Fig. 10), which are incompatible with the diagnosis of classic LCIS. The necrotic foci often harbor calcifications. This lesion is also referred as “florid” LCIS 24, 35. LCIS with necrosis also tends to occur at an older age than classic LCIS, and is commonly associated with invasive carcinoma 19. Fadare et al reported 18 cases of LCIS with comedo type necrosis, 12 (67%) of which had associated invasive carcinoma, most commonly ILC with classic morphology 19. Like P-LCIS, LCIS with necrosis closely mimics solid DCIS. Among the 18 cases of LCIS with comedo type necrosis reported by Fadare et al, two had initial diagnosis of DCIS on core biopsy 19. Immunohistochemical stains for E-cadherin and p120 can be useful to confirm the diagnosis in cases with ambiguous morphology (see Immunohistochemistry).
Fig 10. Lobular carcinoma in situ with central necrosis.
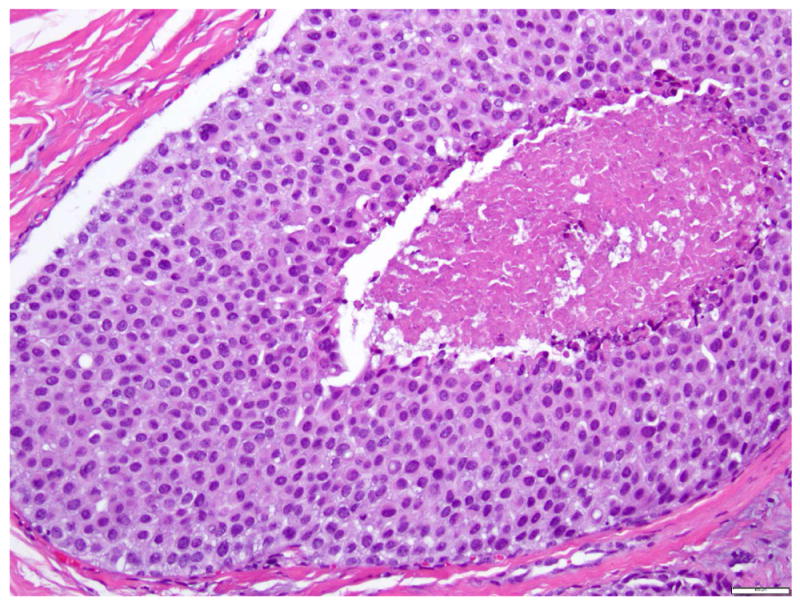
This proliferation of cells morphologically indistinguishable from those of classic LCIS, is associated with massive acinar expansion (50 or more cells across the diameter of an expanded acinus) and central necrosis. Magnification 200x.
Immunohistochemistry
E-cadherin
Rasbridge et al first reported the loss of E-cadherin expression in ILC in 1993 36. In the same year, several other investigators reported similar findings, as well as complete or partial loss of E-cadherin in LCIS and ALH 37, 38. E-cadherin, a member of the cadherin family, is a calcium dependent cell-cell adhesion glycoprotein in epithelial cells 39, 40. E-cadherin is encoded by the CDH1 gene, located on chromosome 16q22.1 41. Using polymerase chain reaction (PCR)/single strand conformation polymorphism (SSCP) assay, Berx et al detected truncation mutations in the extracellular domain of Ecadherin, in combination with loss of heterozygosity (LOH) of chromosome 16q22.1 containing the CDH1 gene in over 50% of the ILC examined 41, 42. The same truncating mutations and LOH of the wild-type allele was also identified in LCIS 43.
By immunohistochemistry, normal mammary glands, DCIS, and most invasive ductal carcinomas (IDCs) show strong and continuous membranous staining for E-cadherin (Fig. 11a-b). In contrast, ILC and LCIS, including all LCIS variants, are characterized by loss or aberrant expression of the E-cadherin protein (Fig. 12a-b). Immunohistochemical stain for E-cadherin is routinely used to distinguish lobular from ductal lesions, and is especially useful in separating LCIS variants from DCIS in cases with ambiguous morphology (Fig. 13a-b, Fig. 14a-b). Most cases of LCIS demonstrate complete loss of E-cadherin staining (Fig. 14b), but in some cases of LCIS, attenuated E-cadherin expression is observed, with scattered cells showing dot-like discontinuous and weak membranous staining or patchy cytoplasmic staining (Fig. 13b).
Fig 11. Ductal carcinoma in situ, extending to lobules.

a. H&E stain. b. Immunohistochemical stain for Ecadherin. The cells show strong membranous staining for E-cadherin, consistent with ductal phenotype. Magnification 200x.
Fig 12. Lobular carcinoma in situ, pleomorphic type, with central necrosis.
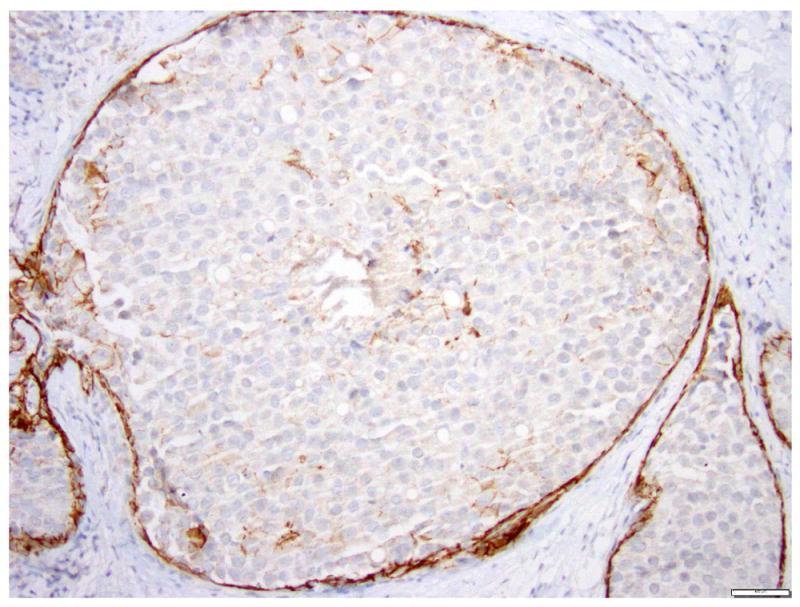
a. H&E stain. b. Immunohistochemical stain for E-cadherin. The LCIS cells are negative for E-cadherin. Magnification 200x.
Fig 13. Pleomorphic lobular carcinoma in situ, apocrine type.
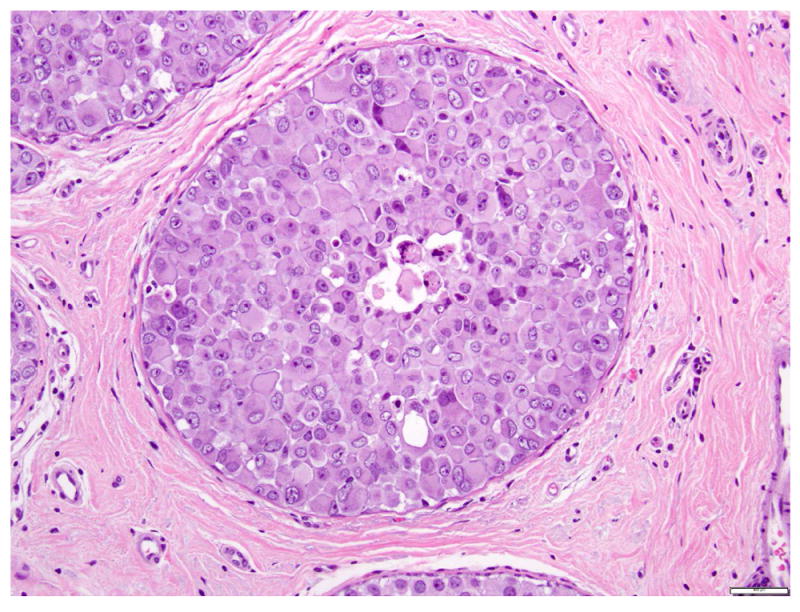
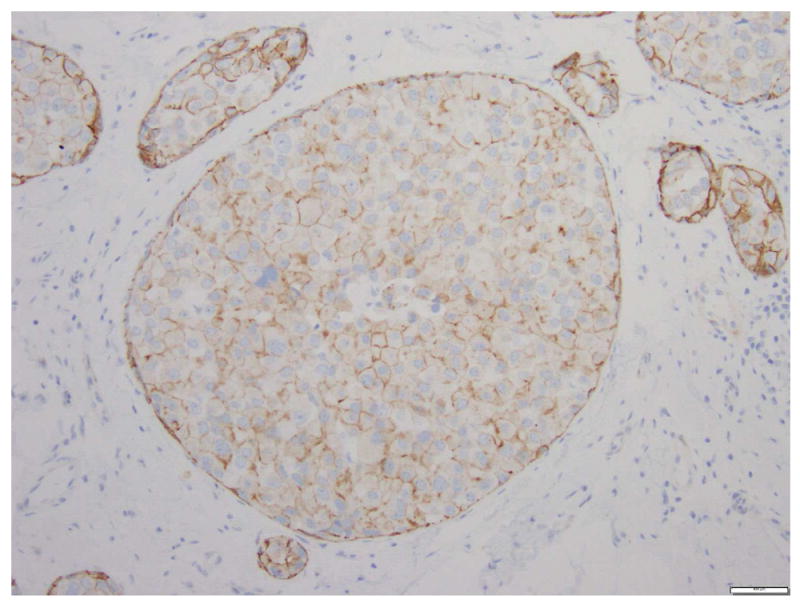
a. H&E stain. b. Immunohistochemical stain for E-cadherin. The cells are dyshesive, with abundant eosinophilic granular cytoplasm, large nuclei, and prominent nucleoli. Instead of complete loss of E-cadherin expression, the cells composing PLCIS in this case show focal incomplete, attenuated, and granular membranous staining for E-cadherin. Magnification 200x.
Fig 14. Lobular carcinoma in situ with massive acinar expansion and central necrosis.

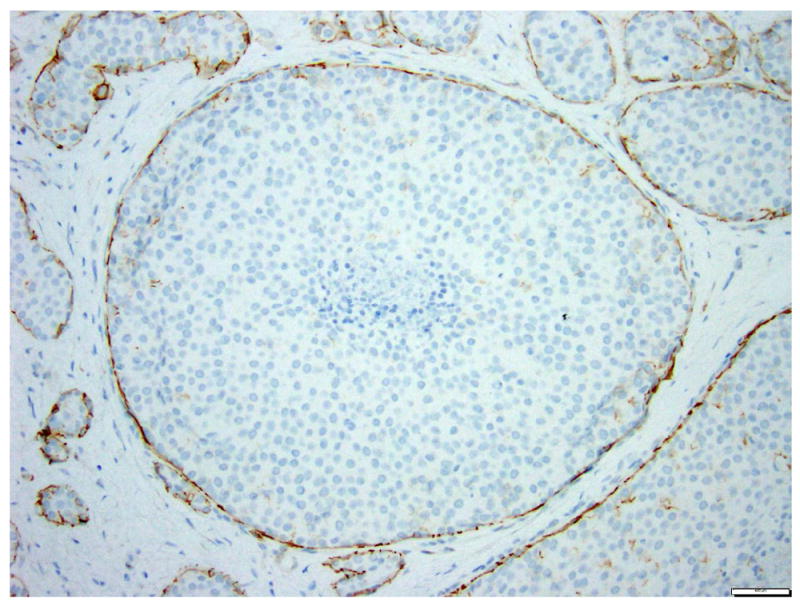
a. H&E stain. b. Immunohistochemical stain for E-cadherin. This variant form of lobular carcinoma in situ closely mimics solid DCIS. The LCIS cells are completely negative for E-cadherin. Magnification 200x.
The results of immunohistochemical staining should always be interpreted in the context of morphologic findings. Aberrant E-cadherin staining patterns have been documented in invasive mammary carcinoma and LCIS 44-48. In particular, the finding of membranous E-cadherin with attenuated intensity does not preclude the diagnosis of LCIS in cases with conventional lobular morphology, and is attributed to presence of dysfunctional E-cadherin protein 45, 47.
p120-catenin
p120, a tyrosine kinase substrate, interacts with E-cadherin and catenins and is required for cadherinmediated cell-cell adhesion 49-52. Cadherins are both necessary and sufficient to recruit p120 to the cell membrane 52. In cells lacking functional cadherins, p120 is located in the cytoplasm (Fig. 15a-b) 52. Sarrio et al reported cytoplasmic localization of p120 in all stages of lobular neoplasia, including ALH, LCIS, ILC, and metastatic lobular carcinoma 53. The authors analyzed 326 breast biopsies using tissue microarrays, including 219 IDC, 69 ILC, 29 ALH and 9 LCIS 53. Immunohistochemical stain for p120 showed diffuse cytoplasmic staining in 90% (26/29) of ALH and 100% (9/9) of LCIS 53. In contrast, ductal carcinoma showed reduced membranous staining for p120 but no cytoplasmic distribution 53. Cytoplasmic location of p120 was significantly associated with the absence of E-cadherin expression. Using an MDA-231 breast cancer cell line which is negative for E-cadherin due to promoter hypermethylation, the authors demonstrated that restored expression of E-cadherin induced a shift of p120 protein from the cytoplasm to the membrane, where p120 colocalized with re-expressed E-cadherin 53. Dabbs et al evaluated the diagnostic utility of p120 54 in 64 ILC (49 classic and 15 pleomorphic) and 62 IDC. They documented loss of E-cadherin and intense cytoplasmic staining of p120 in all ILC cases. The immunostaining pattern for E-cadherin and p120 correlated with histology in 100% of the cases 54. Based on these findings, the authors concluded cytoplasmic staining of p120 is a useful, positive marker for lobular neoplasia 54. Diffuse cytoplasmic staining pattern for p120 is also observed in P-LCIS 55.
Fig 15. A case with both lobular carcinoma in situ (left) and ductal carcinoma in situ (right).
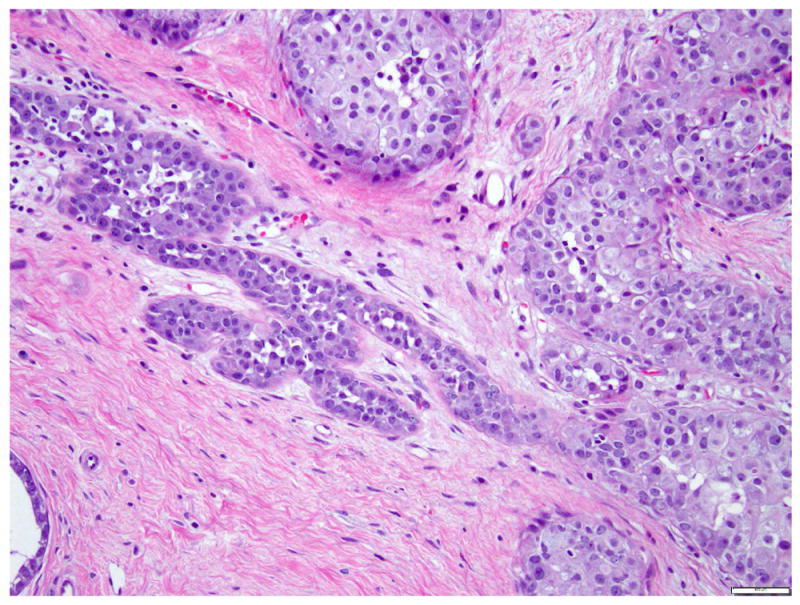
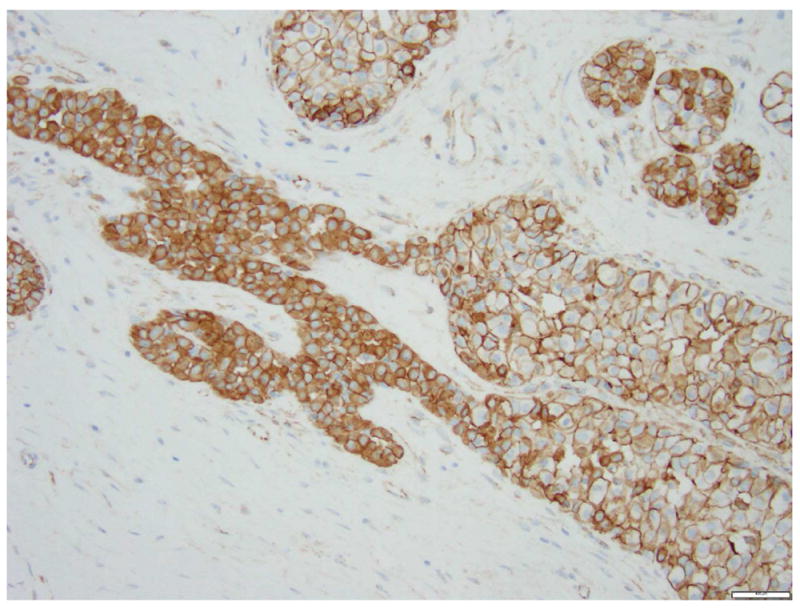
a. H&E stain. b. Immunohistochemical stain for p120. It demonstrates cytoplasmic expression of p120 in the lobular carcinoma in situ and membranous staining in ductal carcinoma in situ. Magnification 200x.
Estrogen receptor (ER), progesterone receptor (PR) and HER2
Classic LCIS is usually positive for ER and/or PR and negative for HER2 56. Immunohistochemical stains were performed in a subset of LCIS samples from patients enrolled in National Surgical Adjuvant Breast Project (NSABP) Protocol B-17 56, and all cases tested were positive for ER and PR, and negative for HER2 56. In P-LCIS, ER is positive in 72-100% of the cases, PR in 50-100% of cases, and HER2 is overexpressed in 1-41% of the cases 20, 22, 23, 32, 33. In a study of 31 cases of P-LCIS, including 13 P-LCIS with apocrine morphology 23, ER and PR expression was detected in all non-apocrine P-LCIS, but only in 20% of apocrine P-LCIS 23. HER2 overexpression was identified in 13% of all P-LCIS, and was restricted to the apocrine P-LCIS 23. At present, there is no recommendation to test and report ER, PR and HER2 status in LCIS.
Differential Diagnosis and Diagnostic Pitfalls
LCIS variants mimic solid DCIS
P-LCIS and LCIS with necrosis exhibit histologic features usually seen in DCIS, which may lead to possible misdiagnosis as DCIS with solid morphology. Sullivan et al retrospectively performed E-cadherin staining in a series of core biopsies with original diagnoses of solid DCIS 57. Ten of 75 (13.3%) cases were reclassified as LCIS, including 9 LCIS variant (5 P-LCIS and 4 LCIS with necrosis) and 1 classic LCIS 57. LCIS variants display some morphologic features characteristic of LCIS, such as dyshesive growth, lack of microacinar formation, intracytoplasmic lumina and signet ring cell morphology. The identification of any of these morphologic features in an intraductal solid proliferation should raise the differential diagnosis of LCIS variant. The latter diagnosis can be confirmed using immunohistochemical stains for Ecadherin and p120. LCIS shows loss of membranous staining for E-cadherin and diffuse cytoplasmic accumulation of p120. The management of patients with variant LCIS and no invasive carcinoma remains highly controversial, especially with respect to radiation therapy. Information regarding the clinical follow-up of patients with diagnosis of P-LCIS remains extremely limited, but a few small series suggest that P-LCIS tends to recur locally with or without associated invasion. Some series found lower recurrence rates in patients treated with radiation therapy 20, 25, 34.
Classic LCIS involving collagenous spherulosis mimics low grade cribriform DCIS
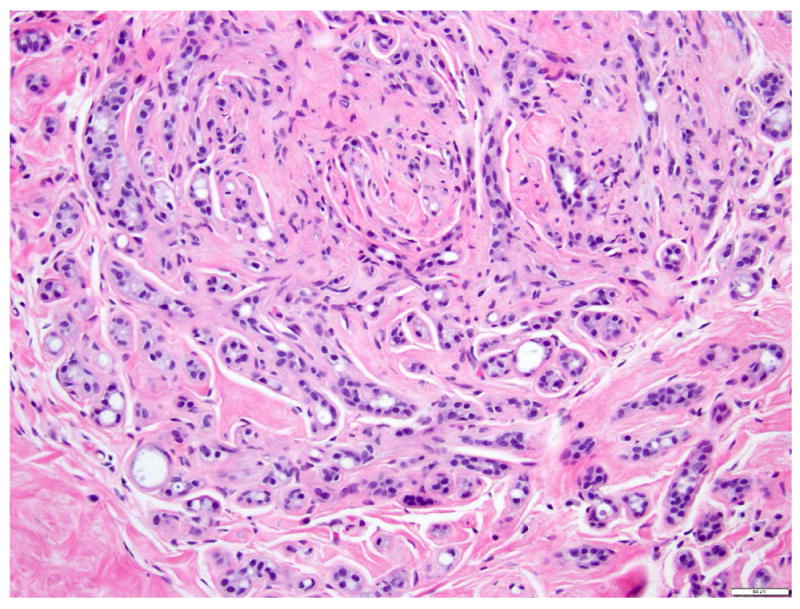
Collagenous spherulosis, first described by Clement et al in 1987 58, is a benign lesion consisting of globoid deposits of variably collagenized matrix surrounded by basement membrane and myoepithelium. This alteration is often associated with myoepithelial hyperplasia. Collagenous spherulosis is usually an incidental microscopic finding, but in some cases can be detected mammographically due to associated calcifications59. LCIS often involves foci of collagenous spherulosis, in a pattern that can mimic low grade cribriform DCIS (Fig. 16a-b) 59-62. The key morphologic features distinguishing LCIS in collagenous spherulosis from low grade cribriform DCIS, first reported by Sgroi and Koerner 60, include the myoepithelial nature of the cells surrounding the spaces, the presence of basement membrane-like material within the pseudo-glandular spaces, and the dyshesive growth of the neoplastic cells which show cytomorphology of classic LCIS. Resetkova et al reviewed 59 cases of collagenous spherulosis, and found LCIS involving collagenous spherulosis in 15 of 59 cases 59, including four cases initially misinterpreted as DCIS by the submitting pathologist 59.
Fig 16. Lobular carcinoma in situ involving collagenous spherulosis mimicking cribriform ductal carcinoma in situ.

a. H&E stain. b. Immunohistochemical stain for E-cadherin. Note the basement membrane-like material in the lumen and the dyshesive growth pattern of the neoplastic cells. The absent of immunoreactivity for E-cadherin in the neoplastic cells confirms the lobular phenotype. Magnification 200x.
LCIS involving sclerosing adenosis mimics invasive lobular carcinoma
Sclerosing adenosis is a benign sclerosing alteration of the TDLU, characterized by distortion of acini and glands due to abundant deposition of periglandular basement membrane and stromal sclerosis (Fig. 17). Myoepithelial hyperplasia, and epithelial calcifications are common. Sclerosing adenosis can be a focal finding, or it may diffusely involve the breast. Sclerosing adenosis is often detected mammographically due to associated calcifications or a mass lesion. Classic LCIS often involves foci of sclerosing adenosis, in an arrangement that can closely simulate invasive carcinoma (Fig. 18a-b). The absence of stromal desmoplasia, the presence of a thick basement membrane, and retained myoepithelial cells around the sclerosed glands and tubules are useful diagnostic clues. Immunohistochemical stains for myoepithelial markers can be used in difficult cases (Fig. 18b).
Fig 17. Sclerosing adenosis.
Magnification 200x.
Fig 18. LCIS involving sclerosing adenosis.
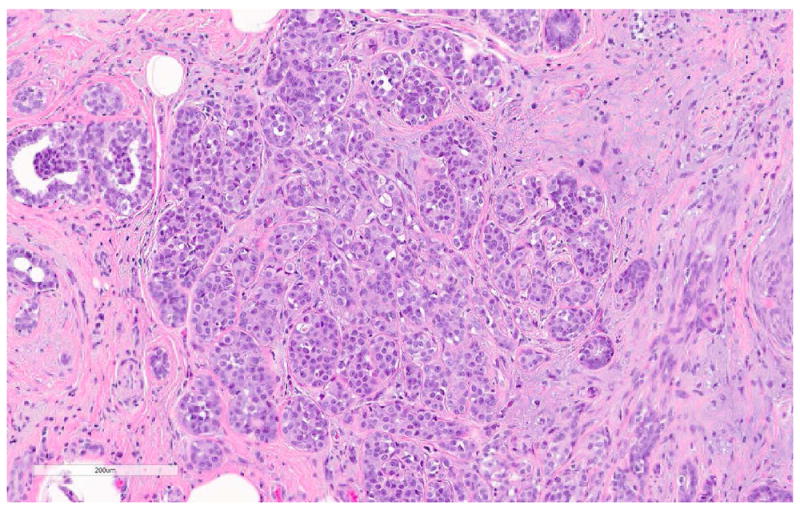
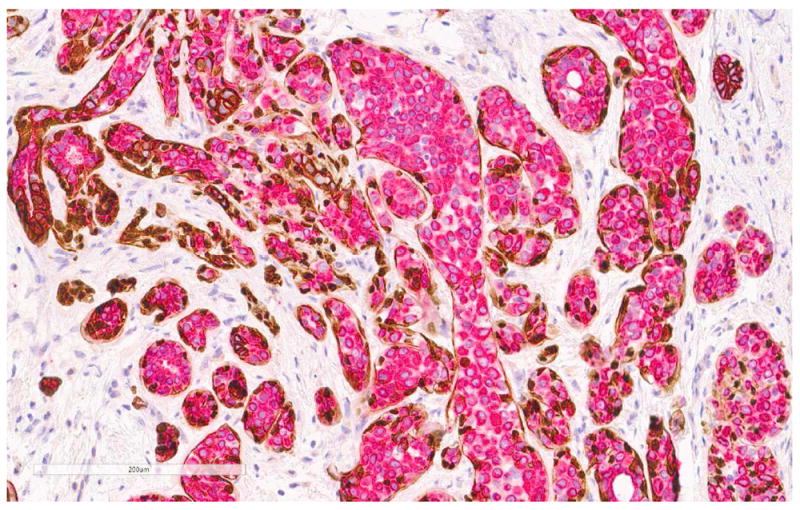
a. H&E stain. b. Immunohistochemical stain for ADH5. ADH5 stain demonstrates the presence of myoepithelial cells surrounding adenosis and LCIS
Genetics
Lakhani et al first reported loss of heterozygosity (LOH) on chromosome 16q, 17q, 17p, and 13q in LCIS, using microdissection, polymorphic DNA markers and PCR assay 63. Comparative genomic hybridization (CGH) analysis of LCIS revealed a low average rate of copy number changes and no evidence of amplifications. The most frequent chromosome alteration in LCIS is the loss of 16q, followed by gain of 1q 64-68. Other recurrent genetic alterations include loss of 16p, 17p, 22q 64. LCIS and ALH have similar patterns of genomic alterations64. LCIS/ALH and ILC share some common chromosome alterations, however, the average number of copy number changes in LCIS/ALH is significantly lower than in ILC and invasive carcinoma in general 64. Loss of 16q is also the most common genetic alteration in low grade DCIS 69-71, tubular carcinoma, well-differentiated invasive carcinoma 72, and premalignant lesion such as atypical ductal hyperplasia (ADH) 73, 74 and columnar cell changes with atypia 75. In contrast, high grade DCIS and poorly differentiated invasive carcinoma, show high frequency of amplifications (17q12, 11q13) and a higher average rate of genetic imbalances, suggesting distinct evolutional pathways between low grade and high grade tumors 70. Comparing copy number alterations of LCIS, DCIS and associated invasive carcinoma, Buerger et al proposed that LCIS and low grade DCIS are closely related neoplastic lesions evolving from a single cell clone 65. Columnar cell changes, LCIS/ALH, low grade DCIS, tubular carcinoma, ILC and well-differentiated IDC not only frequently coexist 76, 77, but also exhibit similar immunophenotypes 78 and clonal relationship 79, 80, supporting the hypothesis that these lesions are members of a family of low grade lesions of the breast, including precursor lesions, in situ and invasive carcinoma with low grade morphology (see article by Collins in this issue of The Clinics) 76, 78 (REF COLLINS).
P-LCIS shares similar genomic alterations as classic LCIS, including 16q loss and 1q gain 23, 81, but shows higher numbers of genetic alterations. In particular, apocrine P-LCIS displays additional recurrent changes, such as amplification of the HER2 gene at 17q11.2-17q12, gain of 16p, loss of 8p and amplification of cyclin D1 gene at 11q13.323.
Genomic analyses reveal a clonal relationship and similar mutation profile between LCIS and synchronous or metachronous invasive carcinoma, supporting the role of LCIS as a precursor to invasive carcinoma 67, 82-86. Hwang et al analyzed 24 samples containing synchronous ipsilateral LCIS and ILC by microdissection and array CGH 67, and found 16q loss and 1q gain to be the most common alterations in LCIS and ILC, occurring in 100% and 90% of paired samples respectively 67. In 14 of 24 cases, synchronous LCIS and ILC were found to be more similar to each other than to any of the other lesion pairings, calculated by weighted similarity scores 67. Aulmann et al analyzed 9 paired cases of LCIS and subsequent ipsilateral invasive breast carcinoma (5 ILCs and 4 IDC) by microdissection and mitochondrial D-loop sequencing 82. Clonal relationships were observed in 3 of the 5 LCIS/ILC pairs, whereas all 4 IDC appear to be clonally unrelated to the LCIS 82. Andrade et al assessed clonal relationships by SNP array in 17 cases of LCIS and synchronous carcinoma, including 9 ILC, 4 DCIS, and 4 IDC. Seven (41%) pairs across all matched lesion types were found to be clonal 83. These studies demonstrated that at least a subset of LCIS represents a precursor of invasive carcinoma.
Targeted capture massively parallel sequencing and whole exome sequencing analyses identified a similar repertoire of somatic mutations in synchronous LCIS and ILC 84-86. The most frequent somatic mutations in LCIS and ILC are CDH1 (56% and 66%, respectively), PIK3CA (41% and 52%, respectively), and CBFB (12% and 19% respectively) 84. The mutation of CDH1 gene is in keeping with the loss of Ecadherin expression, a hallmark of lobular carcinoma. In addition to allel loss at CDH1 locus (16q22.1) and somatic mutations of CDH1 gene, epigenetic alterations such as promoter hypermethylation can also lead to downregulation or silencing of E-cadherin 87-90.
Germline mutation of CDH1 is associated with familial early-onset, poorly differentiated, diffuse gastric cancer 91 and increased risk of lobular breast cancer 92-95. In women with CDH1 germline mutation, the estimated cumulative risk by age 80 years is over 80% for gastric cancer, and 39-60% for breast cancer 94-97. Annual breast MRI starting at age of 30 years is recommended for breast cancer surveillance in this group of women 98.
Natural history and prognosis
LCIS is a risk factor and a non-obligate morphologic precursor of invasive breast carcinoma. McDivitt et al reported a follow-up study of 50 patients with LCIS treated with local excision 99. The cumulative risk of subsequent invasive breast carcinoma was 8% after 5 years, 15% after 10 years, 27% after 15 years, 35% after 20 years, and over 50% after 23 years 99. The cumulative risk of contralateral breast cancer was 10% after 10 years, 15% after 15 years, and 25% after 20 years 99. Several other long term follow-up studies also indicated an increased incidence of breast cancer in both the ipsilateral and contralateral breasts in patients with LCIS 4-6, 10, 100, 101. In a recent long term follow-up study of 1060 women with LCIS and median follow-up of 81 months (range, 6 to 368 months), 150 patients developed 168 breast cancers (63% ipsilateral, 25% contralateral, 12% bilateral). The annual incidence of breast carcinoma in women with LCIS was 2% 12. The subsequent breast carcinoma included DCIS (35%), IDC (29%), ILC (27%), and other types of invasive carcinoma (9%). The relative risk of subsequent breast carcinoma in patients with LCIS is 9-10 times greater than that in the general population 5, 6, 101. The relative risk of invasive breast carcinoma after diagnosis ALH is 3-5 times that of general population 6, 26, 102, 103, approximately one-half that of LCIS 6. Analysis of Surveillance, Epidemiology, and End Results (SEER) data revealed the minimum cumulative risk of developing invasive breast carcinoma after LCIS was 7.1% at 10 years, with equal predisposition in both breasts104. In a long term follow-up study of 161 women with index diagnosis of ALH, 25 (16%) developed invasive breast cancer, which arose in the ipsilateral breast in 17 cases (68%) and in the contralateral breast in 5 cases (20%) with known laterality 103. The relative risk of breast cancer in women with ALH and no other atypical lesion was 2.6 (95% CI 1.7-3.9, p<0.0001)103. Most invasive carcinomas that developed in women with ALH had low Nottingham grade and excellent survival105. The distinction between ALH and LCIS remains valuable for the purpose of patient counseling.
Management
Historically, mastectomy was recommended for women with LCIS, based on the observation that there is an increased risk of subsequent invasive breast cancer 1, 7, 16, 100. Haagensen et al pioneered the concept that “when it (LCIS) occurs alone without accompanying infiltrating carcinoma, it is a distinctive benign disease which predisposes to subsequent carcinoma” and advocated a more conservative approach of close follow-up as an alternative to mastectomy 10. Currently, there is a general agreement that LCIS represents both a risk factor and a non-obligate precursor of breast cancer. Observation alone is the preferred management option. Counseling for chemoprevention with tamoxifen or aromatase inhibitors is recommended.
The National Comprehensive Cancer Network (NCCN) guidelines recommend follow-up of patients with physical examinations every 6 to 12 months and annual diagnostic mammograms 106. In the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, LCIS has been removed from the staging classification system and is no longer included in the pathologic tumor in situ (pTis) category 3. Results of randomized controlled clinical trials support the use of tamoxifen or aromatase inhibitors for risk reduction among women at increased risk of breast cancer. The National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (P-1) demonstrated that subsequent risk of invasive breast cancer can be significantly reduced by tamoxifen 107. In the NSABP P-1 trial, women (N=13388) at increased risk for breast cancer were randomly assigned to receive placebo (n=6707) or 20 mg/day tamoxifen (n=6681) for 5 years. Patients with increased breast cancer risk were defined as 60 years of age or older, or 35-59 years of age with a 5-year predicted risk for breast cancer of at least 1.66%, or those with a history of LCIS 107. Tamoxifen reduced the risk of invasive breast cancer by 49% 107. The NCIC Clinical Trials Group Mammary Prevention.3 Trial (NCIC CTG MAP.3) is a randomized, placebo-controlled, double-blind trial of exemestane for breast cancer prevention in postmenopausal women 108. Postmenopausal women were eligible if they had at least one of the following risk factors: age 60 years or older, Gail risk score greater than 1.66%, prior atypical ductal or lobular hyperplasia or lobular carcinoma in situ on breast biopsy or prior DCIS treated with mastectomy. A total of 4560 women were randomized to either exemestane (2285 patients) or placebo (2275 patients) 108. At a median follow-up of 35 months, exemestane reduced the relative incidence of invasive breast cancers by 65%, from 0.55% to 0.19% 108. King et al reported a 29-year longitudinal single institution experience with LCIS 12. Among 1060 patients with LCIS without concurrent breast cancer diagnosed between 1980 and 2009, 1004 chose surveillance with (n=173) or without (n=831) chemoprevention 12. The overall cumulative cancer incidence at 15 years was 26%, with a 2% annual incidence of breast cancer. The 10-year cumulative cancer risks in women with or without chemoprevention were 7% and 21%, respectively. In multivariate analysis, chemoprevention was the only clinical factor associated with breast cancer risk reduction 12. The American Society of Clinical Oncology Clinical Practice guidelines recommend that the use of chemoprevention should be discussed as an option to reduce the risk of breast cancer in high risk patients 109.
The natural history of P-LCIS remains poorly characterized. Due to the rarity of P-LCIS without concurrent invasive carcinoma, reports of the natural history of P-LCIS are anecdotal. No randomized prospective clinical trial data are available. Currently there is no consensus regarding the treatment recommendations for P-LCIS. A survey sent to self-identified breast surgeons revealed that in cases of PLCIS present at the surgical margins, 53% of the surgeons would not re-excise, 23% sometimes re-excise, and 24% always re-excise 110. All published series on outcomes of PLCIS are retrospective and adjuvant therapy was not uniform (Table 1) 20, 25, 34, 111. According to the 2012 WHO consensus classification, “in the absence of better information on the natural history of pleomorphic LCIS, caution should be exercised in recommending more aggressive management strategies, such as excision to negative margins or mastectomy as a routine practice after a diagnostic surgical biopsy reveals pleomorphic LCIS” 111. According to the NCCN guidelines 106, “Some variants of LCIS (pleomorphic LCIS) may have a similar biological behavior to that of DCIS. Clinicians may consider complete excision with negative margins for pleomorphic LCIS, but this may lead to high mastectomy rate without proven clinical benefit. There are no data to support using radiotherapy in this setting” 106.
Table 1.
Characteristics and outcomes of patients with pure PLCIS in the published series.
| Authors (year) | No. of patients | Surgery | Margin clearance | Additional therapy | Median followup (month) | Recurrence rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| At margin | <1 mm | 1.1-2 mm | >2 mm | CP | XRT | CP + XRT | |||||
| Downs-Kelly E et al (2011) | 26 | BCS | 6 (23%) | 7 (27%) | 4 (15%) | 9 (35%) | 6 (23%) | 4 (15%) | 6 (23%) | 46 | 1 (3.8%) (had positive margin) |
| Khoury T et al (2014) | 31 | BCS 29 (94%) TM 2 (6%) | 9 (29%) | No data | No data | No data | 11 (35%) | 3 (10%) | No data | 55.6 | 6 (19.4%) (4 had positive margin) |
| Flanagan MR et al (2015) | 12 | BCS | No data | 4 (30%) | No data | No data | 3 (25%) | 1 (8%) | No data | 49 | None |
| De Brot M et al (2017) | 7 | BCS | 2 (29%) | 3 (43%) | No data | No data | 1 (14%) | 0 | 0 | 67 | 4 (57%) |
Abbreviations: BCS, breast conserving surgery; CP, chemoprevention; TM, total mastectomy; XRT, radiation therapy;
The management of patients with classic LCIS or ALH diagnosed at needle core biopsy with radiologicpathologic concordant findings is also debated and is the subject of an article by Calhoun in this issue of The Clinics (REF CALHOUN). In brief, the last decade has seen the publication of studies with careful radiologic-pathologic correlation and acceptably low upgrade rates (1-4.4%) at surgical excision of classic LCIS or ALH diagnosed at needle core biopsy with radiologic-pathologic concordant findings 133-139. A recent prospective multi-institutional trial (TBCRC 020) revealed a 1% (1/74; 95% CI 0.01-7) upgrade rate for cases with central pathology review 141. These results have demonstrated that routine excision is not indicated for patients with classic LCIS or ALH on core biopsy and concordant imaging findings. As specified in the 2016 consensus guidelines by the American Society of Breast Surgeons, “we no longer advocates routine excision of ALH or LCIS when the radiological and pathological diagnoses are concordant, and no other lesions requiring excision are present” 142.
LCIS variants (pleomorphic LCIS or LCIS with necrosis) diagnosed on core biopsy requires surgical excision. The reported upgrade rates was 25%-30% 25, 33, 57, 143. The NCCN guidelines recommend surgical excision for pleomorphic LCIS and classic LCIS with discordant imaging findings 106.
Conclusions
The relative risk of invasive carcinoma after a diagnosis of classic LCIS is approximately 9-10 times that of general population. LCIS and ILC share common copy number alterations and somatic mutations. A subset of LCIS is clonally related to synchronous or subsequent invasive breast carcinoma. Classic LCIS diagnosed at needle core biopsy with concordant imaging and pathologic findings does not mandate surgical excision. Active surveillance and chemoprevention are management options for classic LCIS. The finding of variant LCIS at needle core biopsy warrants surgical excision. The management of patients with variant LCIS and no invasive carcinoma upon excision is the subject of debate.
Supplementary Material
KEY POINTS.
LCIS is a risk factor and a non-obligate precursor lesion
LCIS shows loss of E-cadherin and diffuse cytoplasmic staining for p120 catenin
The most frequent chromosome alteration in LCIS is deletion of 16q; the most common somatic mutations in LCIS affect CDH1 (gene encoding for E-cadherin)
Surgical excision can be safely spared in patients with classic LCIS diagnosed on needle core biopsy with concordant imaging and pathologic findings
Surgical excision is recommended for LCIS with variant or pleomorphic morphology, and for classic LCIS with discordant imaging and/or pathologic findings
In a resection specimen, the margin status of classic LCIS is not reported, but it should be reported for LCIS with variant and/or pleomorphic morphology
Footnotes
DISCLOSURE:
The authors declare they have no conflict interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foote FW, Stewart FW. Lobular carcinoma in situ: A rare form of mammary cancer. Am J Pathol. 1941;17:491–496 493. doi: 10.3322/canjclin.32.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Connolly JL, D’Orsi CJ, et al. Breast. The AJCC Cancer Staging Manual. 2017:589–628. [Google Scholar]
- 3.Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017 doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler JE, Enterline HT, Roseman JM, et al. Lobular carcinoma in situ of the breast. Long-term followup. Cancer. 1974;34:554–563. doi: 10.1002/1097-0142(197409)34:3<554::aid-cncr2820340313>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Andersen JA. Lobular carcinoma in situ of the breast. An approach to rational treatment. Cancer. 1977;39:2597–2602. doi: 10.1002/1097-0142(197706)39:6<2597::aid-cncr2820390644>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Page DL, Kidd TE, Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232–1239. doi: 10.1016/0046-8177(91)90105-x. [DOI] [PubMed] [Google Scholar]
- 7.Giordano JM, Klopp CT. Lobular carcinoma in situ: incidence and treatment. Cancer. 1973;31:105–109. doi: 10.1002/1097-0142(197301)31:1<105::aid-cncr2820310114>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Anderson BO, Daling JR, Moe RE. Changing incidence of lobular carcinoma in situ of the breast. Breast Cancer Res Treat. 2002;75:259–268. doi: 10.1023/a:1019950918046. [DOI] [PubMed] [Google Scholar]
- 9.Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14:1008–1011. doi: 10.1158/1055-9965.EPI-04-0849. [DOI] [PubMed] [Google Scholar]
- 10.Haagensen CD, Lane N, Lattes R, Bodian C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer. 1978;42:737–769. doi: 10.1002/1097-0142(197808)42:2<737::aid-cncr2820420247>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Beute BJ, Kalisher L, Hutter RV. Lobular carcinoma in situ of the breast: clinical, pathologic, and mammographic features. AJR Am J Roentgenol. 1991;157:257–265. doi: 10.2214/ajr.157.2.1853802. [DOI] [PubMed] [Google Scholar]
- 12.King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol. 2015;33:3945–3952. doi: 10.1200/JCO.2015.61.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell AJ, Clements K, Dodwell DJ, et al. The radiological features, diagnosis and management of screen-detected lobular neoplasia of the breast: Findings from the Sloane Project. Breast. 2016;27:109–115. doi: 10.1016/j.breast.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Powers RW, O’Brien PH, Kreutner A., Jr Lobular carcinoma in situ. J Surg Oncol. 1980;13:269–273. doi: 10.1002/jso.2930130314. [DOI] [PubMed] [Google Scholar]
- 15.Hutter RV, Snyder RE, Lucas JC, Foote FW, Jr, Farrow JH. Clinical and pathologic correlation with mammographic findings in lobular carcinoma in situ. Cancer. 1969;23:826–839. doi: 10.1002/1097-0142(196904)23:4<826::aid-cncr2820230417>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Hutter RV. The management of patients with lobular carcinoma in situ of the breast. Cancer. 1984;53:798–802. doi: 10.1002/1097-0142(19840201)53:3+<798::aid-cncr2820531331>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Scoggins M, Krishnamurthy S, Santiago L, Yang W. Lobular carcinoma in situ of the breast: clinical, radiological, and pathological correlation. Acad Radiol. 2013;20:463–470. doi: 10.1016/j.acra.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Georgian-Smith D, Lawton TJ. Calcifications of lobular carcinoma in situ of the breast: radiologicpathologic correlation. AJR Am J Roentgenol. 2001;176:1255–1259. doi: 10.2214/ajr.176.5.1761255. [DOI] [PubMed] [Google Scholar]
- 19.Fadare O, Dadmanesh F, Alvarado-Cabrero I, et al. Lobular intraepithelial neoplasia [lobular carcinoma in situ] with comedo-type necrosis: A clinicopathologic study of 18 cases. Am J Surg Pathol. 2006;30:1445–1453. doi: 10.1097/01.pas.0000213290.58283.82. [DOI] [PubMed] [Google Scholar]
- 20.Khoury T, Karabakhtsian RG, Mattson D, et al. Pleomorphic lobular carcinoma in situ of the breast: clinicopathological review of 47 cases. Histopathology. 2014;64:981–993. doi: 10.1111/his.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapino A, Frigerio A, Peterse JL, Arisio R, Coluccia C, Bussolati G. Mammographically detected in situ lobular carcinomas of the breast. Virchows Arch. 2000;436:421–430. doi: 10.1007/s004280050469. [DOI] [PubMed] [Google Scholar]
- 22.Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol. 2002;15:1044–1050. doi: 10.1097/01.MP.0000027624.08159.19. [DOI] [PubMed] [Google Scholar]
- 23.Chen YY, Hwang ES, Roy R, et al. Genetic and phenotypic characteristics of pleomorphic lobular carcinoma in situ of the breast. Am J Surg Pathol. 2009;33:1683–1694. doi: 10.1097/PAS.0b013e3181b18a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SJ, Lal A, De Vries S, et al. Florid lobular carcinoma in situ: molecular profiling and comparison to classic lobular carcinoma in situ and pleomorphic lobular carcinoma in situ. Hum Pathol. 2013;44:1998–2009. doi: 10.1016/j.humpath.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan MR, Rendi MH, Calhoun KE, Anderson BO, Javid SH. Pleomorphic Lobular Carcinoma In Situ: Radiologic-Pathologic Features and Clinical Management. Ann Surg Oncol. 2015;22:4263–4269. doi: 10.1245/s10434-015-4552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW. Infiltrating lobular carcinoma of the breast. Histopathology. 1982;6:149–161. doi: 10.1111/j.1365-2559.1982.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 28.Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Churchill Livingstone; 1987. [Google Scholar]
- 29.Eusebi V, Magalhaes F, Azzopardi JG. Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol. 1992;23:655–662. doi: 10.1016/0046-8177(92)90321-s. [DOI] [PubMed] [Google Scholar]
- 30.Weidner N, Semple JP. Pleomorphic variant of invasive lobular carcinoma of the breast. Hum Pathol. 1992;23:1167–1171. doi: 10.1016/0046-8177(92)90035-2. [DOI] [PubMed] [Google Scholar]
- 31.Frost AR, Tsangaris TN, Silverberg SG. Pleomorphic Lobular Carcinoma In Situ. AJSP: Reviews & Reports. 1996;1:27–31. [Google Scholar]
- 32.Middleton LP, Palacios DM, Bryant BR, Krebs P, Otis CN, Merino MJ. Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am J Surg Pathol. 2000;24:1650–1656. doi: 10.1097/00000478-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Chivukula M, Haynik DM, Brufsky A, Carter G, Dabbs DJ. Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: clinical significance and immunoprofile. Am J Surg Pathol. 2008;32:1721–1726. doi: 10.1097/PAS.0b013e31817dc3a6. [DOI] [PubMed] [Google Scholar]
- 34.De Brot M, Koslow Mautner S, Muhsen S, et al. Pleomorphic lobular carcinoma in situ of the breast: a single institution experience with clinical follow-up and centralized pathology review. Breast Cancer Res Treat. 2017 doi: 10.1007/s10549-017-4334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoda SA, Brogi E, Koerner FC, Rosen PP. Rosen’s Breast Pathology. 4. Wolters Kluwer; 2014. [Google Scholar]
- 36.Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993;169:245–250. doi: 10.1002/path.1711690211. [DOI] [PubMed] [Google Scholar]
- 37.Gamallo C, Palacios J, Suarez A, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 38.Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida-Noro C, Suzuki N, Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984;101:19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]
- 40.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 41.Berx G, Staes K, van Hengel J, et al. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1) Genomics. 1995;26:281–289. doi: 10.1016/0888-7543(95)80212-5. [DOI] [PubMed] [Google Scholar]
- 42.Berx G, Cleton-Jansen AM, Strumane K, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–1925. [PubMed] [Google Scholar]
- 43.Vos CB, Cleton-Jansen AM, Berx G, et al. E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer. 1997;76:1131–1133. doi: 10.1038/bjc.1997.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harigopal M, Shin SJ, Murray MP, Tickoo SK, Brogi E, Rosen PP. Aberrant E-cadherin staining patterns in invasive mammary carcinoma. World J Surg Oncol. 2005;3:73. doi: 10.1186/1477-7819-3-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da Silva L, Parry S, Reid L, et al. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am J Surg Pathol. 2008;32:773–783. doi: 10.1097/PAS.0b013e318158d6c5. [DOI] [PubMed] [Google Scholar]
- 46.Choi YJ, Pinto MM, Hao L, Riba AK. Interobserver variability and aberrant E-cadherin immunostaining of lobular neoplasia and infiltrating lobular carcinoma. Mod Pathol. 2008;21:1224–1237. doi: 10.1038/modpathol.2008.106. [DOI] [PubMed] [Google Scholar]
- 47.Dabbs DJ, Schnitt SJ, Geyer FC, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol. 2013;37:e1–11. doi: 10.1097/PAS.0b013e3182918a2b. [DOI] [PubMed] [Google Scholar]
- 48.Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016;68:57–69. doi: 10.1111/his.12869. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibamoto S, Hayakawa M, Takeuchi K, et al. Association of p120, a tyrosine kinase substrate, with Ecadherin/ catenin complexes. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995;15:4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thoreson MA, Anastasiadis PZ, Daniel JM, et al. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarrio D, Perez-Mies B, Hardisson D, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004;23:3272–3283. doi: 10.1038/sj.onc.1207439. [DOI] [PubMed] [Google Scholar]
- 54.Dabbs DJ, Bhargava R, Chivukula M. Lobular versus ductal breast neoplasms: the diagnostic utility of p120 catenin. Am J Surg Pathol. 2007;31:427–437. doi: 10.1097/01.pas.0000213386.63160.3f. [DOI] [PubMed] [Google Scholar]
- 55.Dabbs DJ, Kaplai M, Chivukula M, Kanbour A, Kanbour-Shakir A, Carter GJ. The spectrum of morphomolecular abnormalities of the E-cadherin/catenin complex in pleomorphic lobular carcinoma of the breast. Appl Immunohistochem Mol Morphol. 2007;15:260–266. doi: 10.1097/01.pai.0000213128.78665.3c. [DOI] [PubMed] [Google Scholar]
- 56.Fisher ER, Costantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Five-year observations concerning lobular carcinoma in situ. Cancer. 1996;78:1403–1416. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1403::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan ME, Khan SA, Sullu Y, Schiller C, Susnik B. Lobular carcinoma in situ variants in breast cores: potential for misdiagnosis, upgrade rates at surgical excision, and practical implications. Arch Pathol Lab Med. 2010;134:1024–1028. doi: 10.5858/2009-0300-OA.1. [DOI] [PubMed] [Google Scholar]
- 58.Clement PB, Young RH, Azzopardi JG. Collagenous spherulosis of the breast. Am J Surg Pathol. 1987;11:411–417. doi: 10.1097/00000478-198706000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Resetkova E, Albarracin C, Sneige N. Collagenous spherulosis of breast: morphologic study of 59 cases and review of the literature. Am J Surg Pathol. 2006;30:20–27. doi: 10.1097/01.pas.0000179237.91515.81. [DOI] [PubMed] [Google Scholar]
- 60.Sgroi D, Koerner FC. Involvement of collagenous spherulosis by lobular carcinoma in situ. Potential confusion with cribriform ductal carcinoma in situ. Am J Surg Pathol. 1995;19:1366–1370. doi: 10.1097/00000478-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Hill P, Cawson J. Collagenous spherulosis with lobular carcinoma in situ: a potential diagnostic pitfall. Pathology. 2007;39:361–363. doi: 10.1080/00313020701329807. [DOI] [PubMed] [Google Scholar]
- 62.Eisenberg RE, Hoda SA. Lobular carcinoma in situ with collagenous spherulosis: clinicopathologic characteristics of 38 cases. Breast J. 2014;20:440–441. doi: 10.1111/tbj.12292. [DOI] [PubMed] [Google Scholar]
- 63.Lakhani SR, Collins N, Sloane JP, Stratton MR. Loss of heterozygosity in lobular carcinoma in situ of the breast. Clin Mol Pathol. 1995;48:M74–78. doi: 10.1136/mp.48.2.m74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu YJ, Osin P, Lakhani SR, Di Palma S, Gusterson BA, Shipley JM. Comparative genomic hybridization analysis of lobular carcinoma in situ and atypical lobular hyperplasia and potential roles for gains and losses of genetic material in breast neoplasia. Cancer Res. 1998;58:4721–4727. [PubMed] [Google Scholar]
- 65.Buerger H, Simon R, Schafer KL, et al. Genetic relation of lobular carcinoma in situ, ductal carcinoma in situ, and associated invasive carcinoma of the breast. Mol Pathol. 2000;53:118–121. doi: 10.1136/mp.53.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Etzell JE, Devries S, Chew K, et al. Loss of chromosome 16q in lobular carcinoma in situ. Hum Pathol. 2001;32:292–296. doi: 10.1053/hupa.2001.22759. [DOI] [PubMed] [Google Scholar]
- 67.Hwang ES, Nyante SJ, Yi Chen Y, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004;100:2562–2572. doi: 10.1002/cncr.20273. [DOI] [PubMed] [Google Scholar]
- 68.Mastracci TL, Shadeo A, Colby SM, et al. Genomic alterations in lobular neoplasia: a microarray comparative genomic hybridization signature for early neoplastic proliferationin the breast. Genes Chromosomes Cancer. 2006;45:1007–1017. doi: 10.1002/gcc.20368. [DOI] [PubMed] [Google Scholar]
- 69.Tsuda H, Fukutomi T, Hirohashi S. Pattern of gene alterations in intraductal breast neoplasms associated with histological type and grade. Clin Cancer Res. 1995;1:261–267. [PubMed] [Google Scholar]
- 70.Buerger H, Otterbach F, Simon R, et al. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187:396–402. doi: 10.1002/(SICI)1096-9896(199903)187:4<396::AID-PATH286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 71.Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E. Genetic progression, histological grade, and allelic loss in ductal carcinoma in situ of the breast. Cancer Res. 1996;56:5260–5265. [PubMed] [Google Scholar]
- 72.Buerger H, Otterbach F, Simon R, et al. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J Pathol. 1999;189:521–526. doi: 10.1002/(SICI)1096-9896(199912)189:4<521::AID-PATH472>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 73.Lakhani SR, Collins N, Stratton MR, Sloane JP. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol. 1995;48:611–615. doi: 10.1136/jcp.48.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuda H, Takarabe T, Akashi-Tanaka S, Fukutomi T, Hirohashi S. Pattern of chromosome 16q loss differs between an atypical proliferative lesion and an intraductal or invasive ductal carcinoma occurring subsequently in the same area of the breast. Mod Pathol. 2001;14:382–388. doi: 10.1038/modpathol.3880322. [DOI] [PubMed] [Google Scholar]
- 75.Simpson PT, Gale T, Reis-Filho JS, et al. Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol. 2005;29:734–746. doi: 10.1097/01.pas.0000157295.93914.3b. [DOI] [PubMed] [Google Scholar]
- 76.Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol. 2007;31:417–426. doi: 10.1097/01.pas.0000213368.41251.b9. [DOI] [PubMed] [Google Scholar]
- 77.Brandt SM, Young GQ, Hoda SA. The “Rosen Triad”: tubular carcinoma, lobular carcinoma in situ, and columnar cell lesions. Adv Anat Pathol. 2008;15:140–146. doi: 10.1097/PAP.0b013e31816ff313. [DOI] [PubMed] [Google Scholar]
- 78.Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32:513–523. doi: 10.1097/PAS.0b013e318161d1a5. [DOI] [PubMed] [Google Scholar]
- 79.Aulmann S, Elsawaf Z, Penzel R, Schirmacher P, Sinn HP. Invasive tubular carcinoma of the breast frequently is clonally related to flat epithelial atypia and low-grade ductal carcinoma in situ. Am J Surg Pathol. 2009;33:1646–1653. doi: 10.1097/PAS.0b013e3181adfdcf. [DOI] [PubMed] [Google Scholar]
- 80.Wagner PL, Kitabayashi N, Chen YT, Shin SJ. Clonal relationship between closely approximated lowgrade ductal and lobular lesions in the breast: a molecular study of 10 cases. Am J Clin Pathol. 2009;132:871–876. doi: 10.1309/AJCP7AK1VWFNMCSW. [DOI] [PubMed] [Google Scholar]
- 81.Reis-Filho JS, Simpson PT, Jones C, et al. Pleomorphic lobular carcinoma of the breast: role of comprehensive molecular pathology in characterization of an entity. J Pathol. 2005;207:1–13. doi: 10.1002/path.1806. [DOI] [PubMed] [Google Scholar]
- 82.Aulmann S, Penzel R, Longerich T, Funke B, Schirmacher P, Sinn HP. Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Cancer Res Treat. 2008;107:331–335. doi: 10.1007/s10549-007-9557-0. [DOI] [PubMed] [Google Scholar]
- 83.Andrade VP, Ostrovnaya I, Seshan VE, et al. Clonal relatedness between lobular carcinoma in situ and synchronous malignant lesions. Breast Cancer Res. 2012;14:R103. doi: 10.1186/bcr3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakr RA, Schizas M, Carniello JV, et al. Targeted capture massively parallel sequencing analysis of LCIS and invasive lobular cancer: Repertoire of somatic genetic alterations and clonal relationships. Mol Oncol. 2016;10:360–370. doi: 10.1016/j.molonc.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Begg CB, Ostrovnaya I, Carniello JV, et al. Clonal relationships between lobular carcinoma in situ and other breast malignancies. Breast Cancer Res. 2016;18:66. doi: 10.1186/s13058-016-0727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah V, Nowinski S, Levi D, et al. PIK3CA mutations are common in lobular carcinoma in situ, but are not a biomarker of progression. Breast Cancer Res. 2017;19:7. doi: 10.1186/s13058-016-0789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995;92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Graff JR, Herman JG, Lapidus RG, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55:5195–5199. [PubMed] [Google Scholar]
- 89.Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart IR. Multiple ways of silencing Ecadherin gene expression in lobular carcinoma of the breast. Int J Cancer. 2001;92:404–408. doi: 10.1002/ijc.1208. [DOI] [PubMed] [Google Scholar]
- 90.Sarrio D, Moreno-Bueno G, Hardisson D, et al. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106:208–215. doi: 10.1002/ijc.11197. [DOI] [PubMed] [Google Scholar]
- 91.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 92.Keller G, Vogelsang H, Becker I, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol. 1999;155:337–342. doi: 10.1016/S0002-9440(10)65129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14:249–255. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 94.Pharoah PD, Guilford P, Caldas C. International Gastric Cancer Linkage C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 95.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–2372. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 96.Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 98.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–374. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McDivitt RW, Hutter RV, Foote FW, Jr, Stewart FW. In situ lobular carcinoma. A prospective followup study indicating cumulative patient risks. JAMA. 1967;201:82–86. doi: 10.1001/jama.201.2.82. [DOI] [PubMed] [Google Scholar]
- 100.Hutter RV, Foote FW., Jr Lobular carcinoma in situ. Long term follow-up. Cancer. 1969;24:1081–1085. doi: 10.1002/1097-0142(196911)24:5<1081::aid-cncr2820240534>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 101.Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW., Jr Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2:225–251. doi: 10.1097/00000478-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 103.Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD, Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361:125–129. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- 104.Chuba PJ, Hamre MR, Yap J, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23:5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 105.McLaren BK, Schuyler PA, Sanders ME, et al. Excellent survival, cancer type, and Nottingham grade after atypical lobular hyperplasia on initial breast biopsy. Cancer. 2006;107:1227–1233. doi: 10.1002/cncr.22113. [DOI] [PubMed] [Google Scholar]
- 106.National Comprehensive Cancer Network Version 2. 2017 Available from URL: https://www.nccn.org.
- 107.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 108.Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 109.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 110.Blair SL, Emerson DK, Kulkarni S, Hwang ES, Malcarne V, Ollila DW. Breast surgeon’s survey: no consensus for surgical treatment of pleomorphic lobular carcinoma in situ. Breast J. 2013;19:116–118. doi: 10.1111/tbj.12062. [DOI] [PubMed] [Google Scholar]
- 111.Downs-Kelly E, Bell D, Perkins GH, Sneige N, Middleton LP. Clinical implications of margin involvement by pleomorphic lobular carcinoma in situ. Arch Pathol Lab Med. 2011;135:737–743. doi: 10.5858/2010-0204-OA.1. [DOI] [PubMed] [Google Scholar]
- 112.WHO Classification of Tumours of the Breast. 4. the International Agency for Research on Cancer (IARC); 2012. [Google Scholar]
- 113.Liberman L, Sama M, Susnik B, et al. Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings. AJR Am J Roentgenol. 1999;173:291–299. doi: 10.2214/ajr.173.2.10430122. [DOI] [PubMed] [Google Scholar]
- 114.Berg WA, Mrose HE, Ioffe OB. Atypical lobular hyperplasia or lobular carcinoma in situ at coreneedle breast biopsy. Radiology. 2001;218:503–509. doi: 10.1148/radiology.218.2.r01fe32503. [DOI] [PubMed] [Google Scholar]
- 115.O’Driscoll D, Britton P, Bobrow L, Wishart GC, Sinnatamby R, Warren R. Lobular carcinoma in situ on core biopsy-what is the clinical significance? Clin Radiol. 2001;56:216–220. doi: 10.1053/crad.2000.0615. [DOI] [PubMed] [Google Scholar]
- 116.Shin SJ, Rosen PP. Excisional biopsy should be performed if lobular carcinoma in situ is seen on needle core biopsy. Arch Pathol Lab Med. 2002;126:697–701. doi: 10.5858/2002-126-0697-EBSBPI. [DOI] [PubMed] [Google Scholar]
- 117.Middleton LP, Grant S, Stephens T, Stelling CB, Sneige N, Sahin AA. Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol. 2003;16:120–129. doi: 10.1097/01.MP.0000051930.68104.92. [DOI] [PubMed] [Google Scholar]
- 118.Foster MC, Helvie MA, Gregory NE, Rebner M, Nees AV, Paramagul C. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004;231:813–819. doi: 10.1148/radiol.2313030874. [DOI] [PubMed] [Google Scholar]
- 119.Arpino G, Allred DC, Mohsin SK, Weiss HL, Conrow D, Elledge RM. Lobular neoplasia on core-needle biopsy--clinical significance. Cancer. 2004;101:242–250. doi: 10.1002/cncr.20318. [DOI] [PubMed] [Google Scholar]
- 120.Elsheikh TM, Silverman JF. Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol. 2005;29:534–543. doi: 10.1097/01.pas.0000152566.78066.d1. [DOI] [PubMed] [Google Scholar]
- 121.Mahoney MC, Robinson-Smith TM, Shaughnessy EA. Lobular neoplasia at 11-gauge vacuumassisted stereotactic biopsy: correlation with surgical excisional biopsy and mammographic follow-up. AJR Am J Roentgenol. 2006;187:949–954. doi: 10.2214/AJR.05.0710. [DOI] [PubMed] [Google Scholar]
- 122.Karabakhtsian RG, Johnson R, Sumkin J, Dabbs DJ. The clinical significance of lobular neoplasia on breast core biopsy. Am J Surg Pathol. 2007;31:717–723. doi: 10.1097/01.pas.0000213408.41182.1f. [DOI] [PubMed] [Google Scholar]
- 123.Brem RF, Lechner MC, Jackman RJ, et al. Lobular neoplasia at percutaneous breast biopsy: variables associated with carcinoma at surgical excision. AJR Am J Roentgenol. 2008;190:637–641. doi: 10.2214/AJR.07.2768. [DOI] [PubMed] [Google Scholar]
- 124.Cangiarella J, Guth A, Axelrod D, et al. Is surgical excision necessary for the management of atypical lobular hyperplasia and lobular carcinoma in situ diagnosed on core needle biopsy?: a report of 38 cases and review of the literature. Arch Pathol Lab Med. 2008;132:979–983. doi: 10.5858/2008-132-979-ISENFT. [DOI] [PubMed] [Google Scholar]
- 125.Londero V, Zuiani C, Linda A, Vianello E, Furlan A, Bazzocchi M. Lobular neoplasia: core needle breast biopsy underestimation of malignancy in relation to radiologic and pathologic features. Breast. 2008;17:623–630. doi: 10.1016/j.breast.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 126.Polom K, Murawa D, Pawelska A, Murawa P. Atypical lobular hyperplasia and lobular carcinoma in situ without other high-risk lesions diagnosed on vacuum-assisted core needle biopsy. The problem of excisional biopsy. Tumori. 2009;95:32–35. doi: 10.1177/030089160909500106. [DOI] [PubMed] [Google Scholar]
- 127.O’Neil M, Madan R, Tawfik OW, Thomas PA, Fan F. Lobular carcinoma in situ/atypical lobular hyperplasia on breast needle biopsies: does it warrant surgical excisional biopsy? A study of 27 cases. Ann Diagn Pathol. 2010;14:251–255. doi: 10.1016/j.anndiagpath.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Destounis SV, Murphy PF, Seifert PJ, et al. Management of patients diagnosed with lobular carcinoma in situ at needle core biopsy at a community-based outpatient facility. AJR Am J Roentgenol. 2012;198:281–287. doi: 10.2214/AJR.11.7043. [DOI] [PubMed] [Google Scholar]
- 129.Ibrahim N, Bessissow A, Lalonde L, et al. Surgical outcome of biopsy-proven lobular neoplasia: is there any difference between lobular carcinoma in situ and atypical lobular hyperplasia? AJR Am J Roentgenol. 2012;198:288–291. doi: 10.2214/AJR.11.7212. [DOI] [PubMed] [Google Scholar]
- 130.Zhao C, Desouki MM, Florea A, Mohammed K, Li X, Dabbs D. Pathologic findings of follow-up surgical excision for lobular neoplasia on breast core biopsy performed for calcification. Am J Clin Pathol. 2012;138:72–78. doi: 10.1309/AJCPYG48TUTFIBMR. [DOI] [PubMed] [Google Scholar]
- 131.Niell B, Specht M, Gerade B, Rafferty E. Is excisional biopsy required after a breast core biopsy yields lobular neoplasia? AJR Am J Roentgenol. 2012;199:929–935. doi: 10.2214/AJR.11.8447. [DOI] [PubMed] [Google Scholar]
- 132.Bianchi S, Bendinelli B, Castellano I, et al. Morphological parameters of lobular in situ neoplasia in stereotactic 11-gauge vacuum-assisted needle core biopsy do not predict the presence of malignancy on subsequent surgical excision. Histopathology. 2013;63:83–95. doi: 10.1111/his.12139. [DOI] [PubMed] [Google Scholar]
- 133.Nagi CS, O’Donnell JE, Tismenetsky M, Bleiweiss IJ, Jaffer SM. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112:2152–2158. doi: 10.1002/cncr.23415. [DOI] [PubMed] [Google Scholar]
- 134.Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21:1208–1216. doi: 10.1038/modpathol.2008.134. [DOI] [PubMed] [Google Scholar]
- 135.Rendi MH, Dintzis SM, Lehman CD, Calhoun KE, Allison KH. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol. 2012;19:914–921. doi: 10.1245/s10434-011-2034-3. [DOI] [PubMed] [Google Scholar]
- 136.Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M, Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119:1073–1079. doi: 10.1002/cncr.27841. [DOI] [PubMed] [Google Scholar]
- 137.Chaudhary S, Lawrence L, McGinty G, Kostroff K, Bhuiya T. Classic lobular neoplasia on core biopsy: a clinical and radio-pathologic correlation study with follow-up excision biopsy. Mod Pathol. 2013;26:762–771. doi: 10.1038/modpathol.2012.221. [DOI] [PubMed] [Google Scholar]
- 138.D’Alfonso TM, Wang K, Chiu YL, Shin SJ. Pathologic upgrade rates on subsequent excision when lobular carcinoma in situ is the primary diagnosis in the needle core biopsy with special attention to the radiographic target. Arch Pathol Lab Med. 2013;137:927–935. doi: 10.5858/arpa.2012-0297-OA. [DOI] [PubMed] [Google Scholar]
- 139.Susnik B, Day D, Abeln E, et al. Surgical Outcomes of Lobular Neoplasia Diagnosed in Core Biopsy: Prospective Study of 316 Cases. Clin Breast Cancer. 2016;16:507–513. doi: 10.1016/j.clbc.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Menon S, Porter GJ, Evans AJ, et al. The significance of lobular neoplasia on needle core biopsy of the breast. Virchows Arch. 2008;452:473–479. doi: 10.1007/s00428-008-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakhlis F, Gilmore L, Gelman R, et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020) Ann Surg Oncol. 2016;23:722–728. doi: 10.1245/s10434-015-4922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.the American Society of Breast Surgeons Consensus Statements. Available from URL: https://www.breastsurgeons.org/new_layout/about/statements/index.php#consensus_statements.
- 143.Carder PJ, Shaaban A, Alizadeh Y, Kumarasuwamy V, Liston JC, Sharma N. Screen-detected pleomorphic lobular carcinoma in situ (PLCIS): risk of concurrent invasive malignancy following a core biopsy diagnosis. Histopathology. 2010;57:472–478. doi: 10.1111/j.1365-2559.2010.03634.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


