Abstract
Background
Management of neonatal parenteral protein intake for preterm infants is challenging and requires daily modifications of the dose to account for the infant’s postnatal age, birth weight, current weight, and the volume and protein concentration of concurrent enteral nutrition. The objective of this study was to create and evaluate the Parenteral Protein Calculator (PPC), a clinical decision support system to improve the accuracy of protein intake in preterm infants who require parenteral nutrition (PN).
Materials and Methods
We integrated the PPC into the computerized provider order entry system, and tested it in a randomized controlled trial (routine or PPC). Infants were eligible if they were 3 days old or less, had birth weight equal to or less than 1,500 grams, and had no inborn error of metabolism. The primary outcome was the appropriate total protein intake (TPI) defined as target protein dose ± 0.5 gram/Kg.
Results
We randomly allocated 42 infants; for 221 PN days in the control group and 211 in the PPC group. TPI in the PPC group was more accurate compared to the control group, appropriate protein dosing odds ratio = 5.8 [2.7 – 12.4]. Absolute deviation from protein target was 0.41 gram/Kg [0.24–0.58], lower in the PPC group.
Conclusion
The PPC improved appropriate protein dosing in premature infants receiving parenteral nutrition. Further studies are needed to test whether clinical decision support systems will reduce uremia and improve growth; and to replicate similar findings in the cases of other PN nutrients.
Keywords: Parenteral nutrition, protein intake, clinical decision support system, medication dosing, uremia
Introduction
The goal of postnatal nutrition in premature infants is to approximate intrauterine growth and nutrient accretion.1 This goal remains elusive particularly for very low birth weight (VLBW) infants (birth weight below 1,500 grams) due to their high rate of protein turnover and catabolism during the first weeks of life.2 Consequently, most extremely low birth weight infants (birth weight below 1,000 grams) suffer significant growth restriction compared to intrauterine growth over the course of their Neonatal Intensive Care Unit (NICU) stay.3 Protein deficits contribute substantially to poor growth, but can be minimized when adequate daily amounts of enteral and parenteral protein intakes are provided.4 However, greater than recommended protein intake can produce abnormal concentrations of amino acids and blood urea nitrogen (BUN), and may lead to cholestasis.5 Excessive protein may considerably stress the capacity of the immature kidney to maintain plasma osmolality within normal limits,6 and it may lead to uremia,7 and eventually poor neurodevelopmental outcome.8 Thus, achieving the appropriate balance of protein intake is critically important.
Management of parenteral protein intake is complex.9 It requires clinicians with expertise in nutritional support, and prescriptions modified daily accounting for the infant’s postnatal age, birth weight, current weight, as well as the volume and protein concentration of concurrent enteral nutrition.10 Compared to undirected prescriptions by individual physicians, the use of standardized computerized PN protocols in preterm neonates may result in a higher provision of protein and energy, improved weight gain, and a better biochemical profile.11 The Parenteral Nutrition (PN) Consensus Safety Recommendations published by the American Society of Parenteral and Enteral Nutrition recommended healthcare organizations to develop clinical decision support systems (CDSSs) for the use within Computerized Provider Order Entry (CPOE) when prescribing PN.12 CDSS have been successfully used to standardize treatments13 and improve outcomes in many conditions,14 including tools created to validate the prescription of PN nutrients.15,16 However, to our knowledge, no CDSS has been developed to accurately recommend the age specific protein that integrates enteral and parenteral amounts for premature infants.
Our objective was to create and implement the Parenteral Protein Calculator (PPC), a CPOE integrated CDSS with the aim to improve protein intake in preterm infants, who require PN. We hypothesized that implementation of the PPC would result in a more accurate delivery of recommended daily total protein intake (TPI) in VLBW infants compared to conventional CPOE ordering.
Methods
Intervention
Before the beginning of the study, ordering providers calculated the parenteral protein dose at the time of ordering. Resources available to support prescription process included NICU protocols made available online, as well as quick access notes placed in providers’ work area. Providers were also able to consult with NICU nutritionists and NICU pharmacists.
We created the PPC, a software tool, whose algorithm is described in Figure 1, and integrated it into the CPOE PN page in the Vanderbilt University Medical Center’s (VUMC) CPOE. To ensure safety of the intervention, the team, who built the PPC, was comprised of experts in pharmaceutical and/or clinical informatics including pharmacists, pediatricians, a neonatologist, and a neonatology fellow. The development process lasted approximately 4 months and consisted of scope definition, requirements analysis, resources identification, management approval, software design, implementation, quality assurance, and integrated testing.
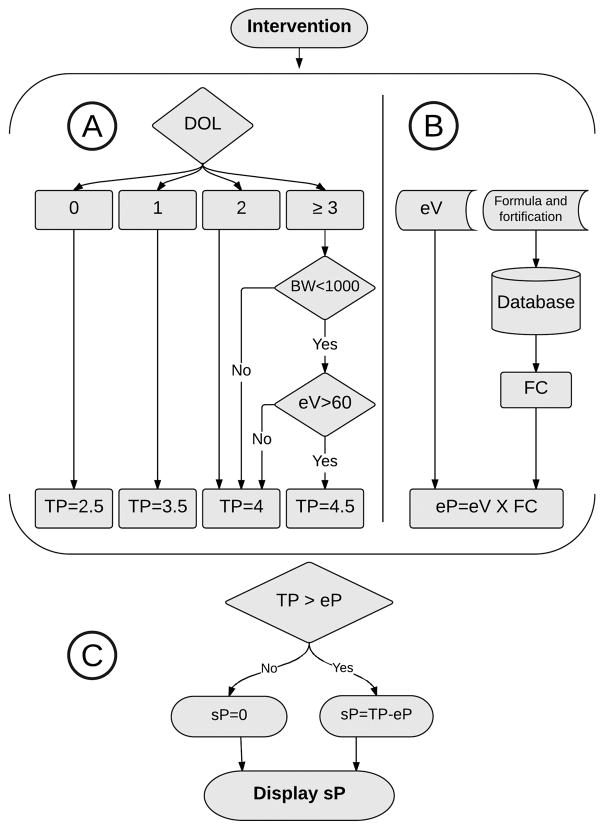
Figure 1.
Parenteral Protein Calculator procedure algorithm. PPC initially determines the total protein target (A) and concurrent enteral protein (B). Parenteral protein is then calculated and displayed (C). DOL= Day of life, eV= Enteral volume, BW= Birth weight, FC= Concentration of protein in formula (gram/mL), TP= Total protein intake (gram/Kg/day), eP= Protein intake from enteral route (gram/Kg/day), sP= Suggested parenteral protein (gram/Kg/day).
The nutrition protocols for VLBW infants at VUMC utilize guidelines from the American Academy of Pediatrics Nutrition Handbook,17 a recent Cochrane Library review,4 and randomized clinical trials.18 Those protocols recommend initiating protein at 2.5 gram/Kg/day on the first day of life (DOL) and increasing it over two days to a maximum of 4 gram/Kg/day for most infants, and 4.5 gram/Kg/day for extremely low birth weight infants, who are mainly nourished by enteral route. The PPC uses infant’s specific data stored in the CPOE in a structured format including DOL, birth weight, and volume of enteral nutrition to identify the required daily total protein. It then produces a suggested parenteral protein by calculating the total daily protein and subtracting the enteral protein using the feeding volume, feeding frequency, and type of formula and fortification. The suggested parenteral protein value is displayed in the protein field for the provider at the time of PN ordering if the infant is in the intervention group. The provider also had the ability to display calculation steps used by the PPC on the ordering screen. No other changes were made to the PN ordering system.
Study Design, screening, and randomization
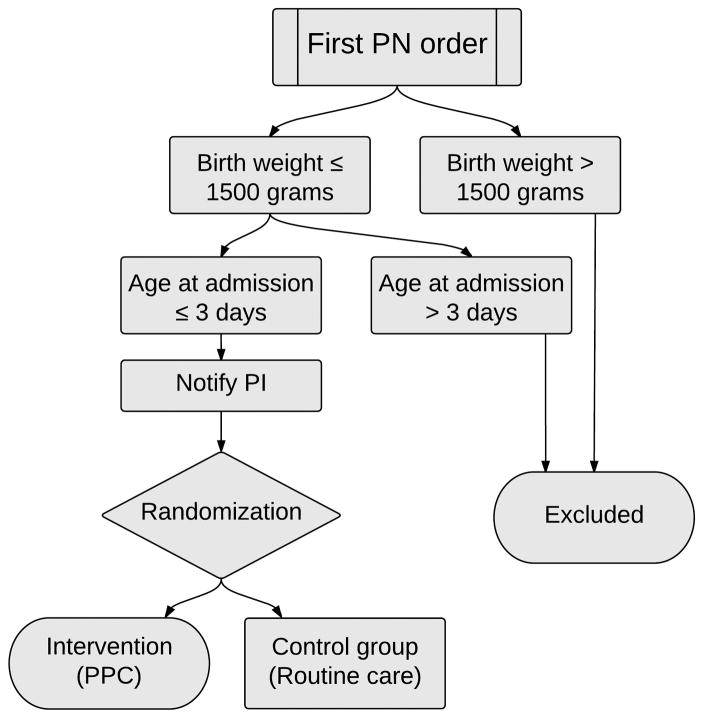
We conducted an open-label randomized controlled trial (Figure 2). Infants were eligible if they had a birth weight of less than 1,500 grams, were admitted to any VUMC NICU in the first three days of life, and were on PN before achieving full enteral feeding. To screen for eligibility and randomize eligible infants, randomization software was integrated into the PPC and ran automatically at the time of initiating a PN order. For infants randomized to the intervention group the protein field in the PN ordering page was pre-populated with the recommended parenteral protein value. The provider could elect to keep the value and submit it along with the full PN order, or could modify it as desired. Infants randomized to the control group received conventional care, with no recommended value in the protein field of the PN page.
Figure 2.
The algorithm for eligibility checking and randomization. PN=Parenteral nutrition, PI=Primary investigator, PPC=Parenteral Protein Calculator.
Data Collection, and outcomes
The values of administered enteral and parenteral protein were collected daily from each infant’s flow sheet and added resulting in the value of TPI. To conduct intent-to-treat analysis, we collected this data for all infants regardless of how the provider handled the value suggested on the PN page. The total protein goal was subtracted from TPI and the result recorded daily as protein deviation. Since recent literature 4 in neonatal protein intake compared protein doses at increments of 0.5–1 gram/Kg/day, we considered a range of 1 gram/Kg/day around protein target clinically acceptable. We defined underdosing as a protein deviation value below −0.5 gram/Kg, appropriate dosing as a deviation between −0.5 and +0.5 gram/Kg inclusive, and overdosing as a deviation value above +0.5 gram/Kg. We defined uremia as BUN greater than 60 mg/dL. Vanderbilt University Medical Center’s Institutional Review Board approved this study with a waiver of informed consent for lack of feasibility to consent parents due to the limited time available between birth and initiation of parenteral nutrition.
We collected data on PN solution osmolarity, total volume, administration route (peripheral versus central), and minimum volume (The lowest PN solution volume required to compound all ordered PN ingredients). The PN ordering system calculates osmolarity and displays its value at the time of ordering. Because NICU protocols allow for a maximum PN osmolarity of 900 mOsm/Liter when administered peripherally (and no maximum limit when administered centrally), we considered PN osmolarity of 850 mOsm/Liter high justifying limiting protein dose to maintain appropriate osmolarity. Similarly, we considered PN solution volume at the minimum volume + 5 mL low justifying limiting protein dose to maintain adequate solution volume. Therefore, we considered underdosing appropriate if the PN solution (1) was administered peripherally and had an osmolarity higher than 850 mOsm/Liter, or (2) had a volume less than the minimum volume + 5 mL. Underdosing without either of these two conditions met was deemed inappropriate.
Our primary outcome was appropriate protein dosing which we analyzed as a binary variable (whether or not the protein dosing fell within target range), and on a continuous scale as absolute deviation from target. Secondary outcomes included underdosing and overdosing, absolute deviation, uremia, and the number of days to regain birth weight.
Sample size and statistical analysis
For baseline dosing information, we collected data retrospectively from the Vanderbilt NICU. Appropriate dosing occurred in 37% of prescriptions, with a standard deviation of 14%, and a mean of 14 PN days per infant. Based on a power analysis, to detect two additional days in appropriate dosing per infant (14% increase) with 90% power and an alpha of 0.05, the study needed 22 subjects in each group.
Descriptive statistics were presented as median with interquartile range or frequency (percentage) where appropriate. We compared patients’ demographic and prescription characteristics between the control and PPC groups using Wilcoxon rank sum test for continuous variables and Pearson’s chi-squared test for one categorical variable (sex).
We fit multiple logistic regression model to assess difference in primary and secondary outcomes between the two groups with adjustment of post menstrual age and access. Since each subject was measured multiple times, we used cluster sandwich covariance estimator with the patient ID as a cluster in order to adjust the variance in our model to account for these repeated measurements. We plotted all prescriptions’ deviation referenced to the target protein for each prescription. Analyses were performed using statistical software R version 3.3.0. p value of < 0.05 was considered statistically significant.
Results
Between November 2015 and January 2016, 42 VLBW infants were admitted in the first three days of life to one of VUMC NICUs. None of the infants had an inborn error of metabolism and all infants in the cohort were started on PN before achieving full enteral feeds. We randomized 42 Infants (23 control, and 19 PPC) generating 432 prescriptions (221 control, and 211 PPC). Infants’ baseline data showed no significant difference in gestational age, birth weight, sex, number of PN days, and percentage of days with central access (Table 1).
Table 1.
Patients’ characteristics
| Control | PPC | p value | |
|---|---|---|---|
| Patients characteristics: | |||
| Number of patients | 23 | 19 | |
| Gestational age (weeks.days) | 28.4 (26.4 – 30.2) | 28.3 (27.5 – 29.2) | 0.87 |
| Birth weight (grams) | 1110 (790 – 1190) | 1070 (720 −1220) | 0.65 |
| Male sex (%) | 10 (43) | 6 (32) | 0.43 |
| Number of PN days | 8 (4.5 – 12.5) | 8 (7.0 – 14.0) | 0.42 |
| Percentage of days with central access (%) | 100 (73 – 100) | 88 (70 – 100) | 0.61 |
Values are expressed as median (interquartile), or n (%)
PN, Parenteral Nutrition
The median percentage of appropriate dosing was greater in the PPC group than in the control group, 88% vs. 56% respectively, (Table 2). (Odds ratio (OR) [95% confidence interval (CI)] = 5.8 [2.7 – 12.4], Table 3). Overdosing was almost eliminated in the PPC group with only one overdose PN day in the PPC compared to a total of 27 overdose PN days in control, OR [95% CI] = 0.035 [0.005 – 0.267].
Table 2.
Dosing classes
| Control | PPC | |
|---|---|---|
| Number of patients | 23 | 19 |
| Appropriate dosing (%) | 56 (23 – 77) | 88 (75 – 100) |
| Overdosing (%) | 8 (0 – 20) | 0 (0 – 0) |
| Underdosing (%) | 29 (0 – 53) | 8 (0 – 25) |
| Inappropriate underdosing (%) | 73 (0 – 100) | 0 (0 – 25) |
| Appropriate underdosing (%) | 27 (0 – 50) | 100 (75 – 100) |
| Absolute deviation (gram/Kg) | 0.6 (0.43 – 0.86) | 0.16 (–0.09 – 0.42) |
Values are expressed as median [interquartile]
Table 3.
Odds ratio for primary and secondary outcomes
| OR [95th Confidence Interval] | p value | ||
|---|---|---|---|
| Appropriate dosing, intervention:control | 5.8 | [2.7 – 12.4] | <0.001 |
| Overdosing, intervention:control | 0.035 | [–0.005 – 0.267] | 0.001 |
| Underdosing, intervention:control | 0.28 | [–0.12 – 0.66] | 0.004 |
| Uremia | 0.27 | [0.07 – 1.06] | 0.061 |
| Days to regain birth weight | 0.38 | [0.10 – 1.41] | 0.147 |
Values are expressed as odds ratio [95% confidence interval]. Underdosing includes appropriate and inappropriate underdosing
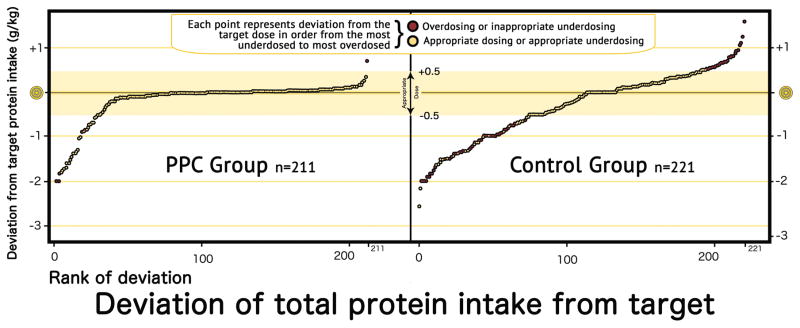
Both overall underdosing and its inappropriate underdosing portion were lower in the PPC group compared to the control group (8% vs. 29%, and 0% vs. 73%, respectively). (Overall underdosing OR [95% CI] = 0.28 [0.12 – 0.66], Figure 3). There was no significant difference in the frequency of appropriate underdosing due to high osmolarity or to low PN solution volume. Among underdosed PN, the mean percentage of inappropriate underdosing was higher in the control group compared to the PPC group.
Figure 3.
Protein deviation, ranked from lowest below target to highest above target for both groups. PPC=Parenteral Protein Calculator.
The PPC reduced the mean absolute deviation from the target dose of 0.6 gram/Kg in the control group to 0.16 gram/Kg in the PPC group. The multiple linear regression model demonstrated 0.41 gram/Kg [0.24 – 0.58] lower deviation in the PPC group compared to the control group.
The reductions in overdosing was associated with a trend for a decrease in the observations of uremia OR [95% CI] = 0.27 [0.07 – 1.06]; the improved underdosing was also associated with a trend for faster regain of birth weight from 9.6 [7.2 – 12.8] (median [interquartile]) in PPC group to 8.0 [7.2 – 9.0] in control group, OR [95% CI] = 0.38 [0.10 – 1.41] however, these trends were not statistically significant.
Discussion
The attempts to create CDSSs to prevent improper PN prescriptions corresponded to the earliest use of PN in neonatal care,19 both of which began in the 1970s. Since then, numerous reports have been published to describe calculation assistance and validation for ordered dosages.15,16 Our study is the first randomized, prospective clinical trial to evaluate an advanced integrated CDSS that utilizes structured data within the EHR to forecast the optimal parenteral protein dose based on the clinical variants routinely used by ordering providers and taking into account enteral feedings. The PPC resulted in a significant increase in appropriate dosing, as well as a remarkable decrease in inappropriate underdosing and overdosing. Because providers may elect to limit the total or parenteral protein due to concern for intolerance, the PPC was more effective in avoiding overdosing than underdosing. Additionally, we observed a trend to improve uremia and weight gain during the phase of parenteral nutrition, however, these findings although approaching statistical significance did not achieve our p value cut off of 0.05 possibly because our study was not powered to detect such differences.
We believe the results of our trial are of high clinical significance, as the decrease in protein underdosing has a well-established effect on accelerating weight gain;4 and the decrease in protein overdosing renders PN prescription a safer process by avoiding known adverse renal, metabolic, and neurodevelopmental outcomes.6–8 This pilot study demonstrates the applicability of further creating and evaluating systems capable of running the calculations on an increasing number of PN nutrients before the ordering screen is displayed.
Our study has several limitations related to the intervention itself: First, the PPC was locally developed within VUMC EHR, and further efforts are required by pediatric EHR vendors and CPOE developers to create and integrate similar CDSSs to their systems. An alternative would be to utilize interoperability techniques to create an equivalent cloud-based CDSS able to respond to queries by outside EHRs in a real-time fashion.20 Due to the lack of specific recommendations on the starting dose, stepwise increase, and the maximum dose of protein for preterm infants, different units may have different approaches that may not necessarily be similar to the PPC approach, which is based on VUMC protocols. For any web service, this would necessitate the future creation of a “group control panel” to grant institutions autonomy in customizing their own approach and periodic updates.
Other limitations related to the study included: Our trial was powered to detect an improvement in the accuracy of administered TPI, but not other clinical outcomes. It is of a clinical importance to see if the decrease in both the number of underdosing cases and the value of negative deviation would lead to improvement in weight gain, head circumference growth, incidence of uremia, or length of stay. Studies powered to detect such differences should be conducted.
Conclusion
The PPC improved appropriate protein ordering in premature infants receiving parenteral nutrition. Pediatric EHR vendors and CPOE developers should focus on creating and integrating similar tools to their EHRs. Further studies are needed to evaluate CDSSs similar to PPC for other PN nutrients and test whether they improve growth.
Clinical Relevancy Statement.
Prescribing neonatal parenteral nutrition is complex and requires coordination of the patient’s clinical condition, clinical variables, and concurrent enteral nutrition. Because of the large number of ingredients and their interactions, parenteral nutrition prescribing often results in overdosing or underdosing of specific nutrients. This pilot randomized clinical trial evaluates the impact of a clinical decision support system on prescription accuracy of parenteral protein.
Acknowledgments
Sponsor Name: Vanderbilt University, Department of Pediatrics
Funder: Supported in part by the Vanderbilt CTSA Grant UL1 TR000445 from NCATS/NIH
List of Abbreviations
- BUN
Blood urea nitrogen
- CDSS
Clinical decision support system
- CI
Confidence interval
- CPOE
Computerized Provider Order Entry
- DOL
Day of life
- EHR
Electronic Health Records
- NICU
Neonatal intensive care unit
- OR
Odds ratio
- PN
Parenteral nutrition
- PPC
Parenteral protein calculator
- TPI
Total protein intake
- VLBW
Very low birth weight
- VUMC
Vanderbilt University Medical Center
Footnotes
Conflict of Interest Disclosure:
Christoph U. Lehmann, MD received royalties from Springer Verlag for the book ‘Pediatric Informatics’. He serves as the Medical Director of the Child Health Informatics Center at the American Academy of Pediatrics, editor-in-chief of the journal Applied Clinical Informatics, and as President Elect of the International Medical Informatics Association.
The other authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1977;60:519–5. [PubMed] [Google Scholar]
- 2.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics. 2001;107(1):e1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 4.Fenton TR, Premji SS, Al-Wassia H, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database of Systematic Reviews. 2014;(4) doi: 10.1002/14651858.CD003959.pub3. Art. No.: CD003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birth weight infant. Clinical Perinatology. 2002;29:225–44. doi: 10.1016/s0095-5108(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 6.Davies DP. Plasma osmolality and protein intake in preterm infants. Arch Dis Child. 1973;48:575–579. doi: 10.1136/adc.48.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, Ziegler E. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatric Research. 2006;59:265–270. doi: 10.1203/01.pdr.0000196376.99101.34. [DOI] [PubMed] [Google Scholar]
- 8.Goldman HI, Freudenthal R, Holland B, Karelitz S. Clinical effects of two different levels of protein intake on low-birth-weight infants. J Pediatr. 1969 Jun;74(6):881–9. doi: 10.1016/s0022-3476(69)80222-2. [DOI] [PubMed] [Google Scholar]
- 9.Mirtallo J, Canada T, Johnson D, et al. A.S.P.E.N. Board of Directors and Task Force for the Revision of Safe Practices for Parenteral Nutrition. Safe practices for parenteral nutrition [published correction appears in JPEN J Parenter Enteral Nutr. 2006;30:177] JPEN J Parenter Enteral Nutr. 2004;28:S39–S70. doi: 10.1177/0148607104028006s39. [DOI] [PubMed] [Google Scholar]
- 10.Sacks GS, Rough S, Kudsk KA. Frequency and severity of harm of medication errors related to the parenteral nutrition process in a large university teaching hospital. Pharmacotherapy. 2009;29(8):966–974. doi: 10.1592/phco.29.8.966. [DOI] [PubMed] [Google Scholar]
- 11.Skouroliakou M, Koutri K, Stathopoulou M, Vourvouhaki E, Giannopoulou I, Gounaris A. Comparison of two types of TPN prescription methods in preterm neonates. Pharm World Sci. 2009;31:202–8. doi: 10.1007/s11096-009-9281-4. [DOI] [PubMed] [Google Scholar]
- 12.Ayers P, Adams S, Boullata J, Gervasio J, Holcombe B, Kraft MD, Marshall N, Neal A, Sacks G, Seres DS, Worthington P American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN J Parenter Enteral Nutr. 2014;38(3):296–333. doi: 10.1177/0148607113511992. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann CU, Miller MR. Standardization and the practice of medicine. J Perinatol. 2004;24:135–6. doi: 10.1038/sj.jp.7211060. [DOI] [PubMed] [Google Scholar]
- 14.Moja L, Kwag KH, Lytras T, et al. Effectiveness of computerized decision support systems linked to electronic health records: a systematic review and meta-analysis. Am J Public Health. 2014;104(12):e12–22. doi: 10.2105/AJPH.2014.302164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann CU, Conner KG, Cox JM. Preventing provider errors: online total parenteral nutrition calculator. Pediatrics. 2004 Apr;113(4):748–53. doi: 10.1542/peds.113.4.748. [DOI] [PubMed] [Google Scholar]
- 16.Maat B, Rademaker CM, Oostveen MI, Krediet TG, Egberts TC, Bollen CW. The effect of a computerized prescribing and calculating system on hypo- and hyperglycemias and on prescribing time efficiency in neonatal intensive care patients. JPEN J Parenter Enteral Nutr. 2013 Jan;37(1):85–91. doi: 10.1177/0148607112444608. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman RE AAP Committee on Nutrition. Pediatric Nutrition Handbook. 6. Elk Grove Village, IL: American Academy of Pediatrics; 2009. [Google Scholar]
- 18.Embleton ND, Cooke RJ. Protein requirements in preterm infants: effect of different levels of protein intake on growth and body composition. Pediatric Research. 2005;58:855–60. doi: 10.1203/01.PDR.0000182586.46532.7C. [DOI] [PubMed] [Google Scholar]
- 19.Wright PD, Shearing G, Rich AJ, Johnston ID. The role of a computer in the management of clinical parenteral nutrition. JPEN J Parenter Enteral Nutr. 1978;2:652–657. doi: 10.1177/014860717800200506. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KB, Lee CK, Spooner SA, Davison CL, Helmke JS, Weinberg ST. Automated dose-rounding recommendations for pediatric medications. Pediatrics. 2011;128:e422–e428. doi: 10.1542/peds.2011-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]