Abstract
Background: Up to 10% of women are exposed to selective serotonin reuptake inhibitors (SSRIs) during pregnancy. Information on their effect on birthweight and gestational age remains conflicting. The aim of this sibling-controlled prospective cohort study is to address shared geneticand family-level confounding to investigate the effects of prenatal SSRI exposure and maternal depression on birthweight and gestational age.
Methods: We used the Norwegian Mother and Child Cohort Study (MoBa) and the Medical Birth Registry of Norway (MBRN). Our study population consisted of 27 756 siblings; 194 were prenatally exposed to SSRIs and 27 500 were unexposed to any antidepressant medication. Random and fixed effects analysis with propensity score adjustment was used to evaluate the effectson birthweight and gestational age.
Results: SSRI exposure during two or more trimesters was associated with a decrease in birthweight of 205 g [95% confidence interval (CI) −372 to − 38] and a decrease in gestational length of 4.9 days (95% CI − 9.1 to − 1.4). Neither maternal SSRI use in one trimester, lifetime history of major depression nor depressive symptoms during pregnancy were associated with these pregnancy outcomes (for non-pharmacologically treated depression in two periods in pregnancy, +5 g (95% CI − 56 to + 67) and +4.9 days (95% CI − 4.7 to + 14.7), respectively).
Conclusions: Prenatal exposure to SSRIs during two or more trimesters may decrease birthweight and gestational length. Our results indicate that neither maternal depression nor shared genetics and family environment fully explain this association.
Keywords: SSRIs, depression, birthweight, gestational age, sibling design
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are the first-line drug treatment option of moderate to severe depression.1,2 Approximately 20% of women of childbearing age are diagnosed with depression and other mood-affective disorders, and up to 5% of pregnant women are diagnosed with major depressive disorder.3–8 It is therefore not surprising that between one and 10% of pregnant women living in high-income countries use or have SSRIs prescribed during pregnancy,9–13 and the safety of SSRIs during this period continues to be extensively studied.14,15
Information on the effect of SSRIs on birthweight and gestational age at birth remains conflicting.14,15 In three meta-analyses comprising in total over 30 000 pregnancies exposed to antidepressants and spanning study sizes from less than 100 to over 1 million, an increased risk of low gestational age (< 37 weeks) at birth after second and third trimester SSRI exposure has been reported. The pooled effect estimates, odds ratios and relative risks ranged between 1.53 and 1.69 with 95% confidence intervals between 1.38 and 1.88.16–18 A pooled mean of − 0.5 weeks of gestational age at birth (95% CI −0.64 to − 0.25) was also found.18 An association with low birthweight, with a pooled risk ratio (RR) of 1.44 (95% CI 1.21 to 1.70), was reported by only one of the meta-analyses.16 The pooled mean of − 74 g of birthweight (95% CI − 117 to − 31) found by Ross et al. ceased to have statistical significance after comparison with non-medicated depression,18 and the authors suggest that maternal depression mediated this association. A recent population-based cohort study with a within-family design reported an association between dispensed SSRI medication during pregnancy and reduction in gestational length of 2.3 days (95% CI −3.8 to − 0.8). No effect on birthweight when was reported.19
Trying to summarize the effect of non-medicated depression on pregnancy outcome, two recent meta-analyses reported conflicting results.20,21 The first one reported a pooled RR for low gestational age at birth and low birthweight of 1.18 (95% CI 1.08 to 1.28) and 1.17 (95% CI 1.06 to 1.30), respectively.20 The other meta-analysis could only confirm a statistically significant association with low gestational age at birth with a pooled odds ratio (OR) of 1.37 (95% CI 1.04 to 1.81), but not low birthweight.21 The high degree of heterogeneity (I2> 60%) between the studies included in the meta-analyses leads to a challenging clinical decision making scenario.
As thousands of women worldwide are being prescribed antidepressants during pregnancy every year, even a small increased risk for low birthweight or premature delivery could have a significant impact from a public health perspective. There is therefore a great need for studies on the fetal safety of antidepressants with the ability to address both confounding by the underlying maternal depression as well as genetic and family-environmental confounding factors. Using a cohort design, we previously found that exposure to antidepressants was not associated with increased risk of low birthweight or preterm birth in the Norwegian Mother and Child Cohort.6 However, we did find an association between maternal depression and preterm birth (OR 1.13, 95% CI 1.03 to 1.25).
The aim of the present study was to investigate the effect of prenatal SSRI exposure and maternal depression on birthweight and gestational age at birth de novo.In an attempt to adjust for shared genetics and family environmental factors, we employed a sibling design.As siblings share the same mother and on average 50% of their genes, putative confounding factors related to maternal factors are minimized in a discordant sibling design. This counterfactual condition can be considered analogous to a natural experiment.22 Moreover, by applying a fixed effects model, we had the advantage of reducing residual confounding by addressing the unmeasurable and unknown family-level differences that may be a source of bias.23
Methods
Study design
This sibling-controlled observational prospective cohort study was based on the Norwegian Mother and Child Cohort Study (MoBa) and the Medical Birth Registry of Norway (MBRN). MoBa and MBRN were linked via the 11-digit maternal identification number assigned to every resident of Norway.
MoBa is an ongoing observational prospective cohort study conducted by the Norwegian Institute of Public Health (NIPH).24 The principal objective of MoBa is to evaluate the effect of a vast array of prenatal exposures on the health of the child.
All pregnant women living in Norway who gave birth between 1999 and 2008 were invited to participate in MoBa. There were no exclusion criteria, and the participation rate was 40.6%.25 Information on maternal medical, socio-demographic and lifestyle characteristics before and during pregnancy was obtained from three self-administered questionnaires. The first questionnaire was completed at gestational week 17, Qw17, covering the period between 6 months preceding pregnancy and gestational week 18. The second and third questionnaires were completed at gestational week 30, Qw30, and at 6 months postpartum, Q6mPP, and covered the second and third trimester of pregnancy until delivery. The response rates were 94.9% (Qw17), 91.0% (Qw17) and 84.8%, respectively (Q6mPP).26 The current study is based on version six of the quality-assured data files released for research in 2012.
MBRN encompasses pregnancy outcomes in Norway after the 12th gestational week. Information on maternal health both before and during pregnancy, the course of pregnancy and pregnancy complications, delivery and postpartum complications and interventions, and the health of the neonate is available from standardized mandatory forms completed by midwives and obstetricians and/or gynaecologists at each delivery and from antepartum obstetric records completed by general physicians, gynaecologists or midwives throughout pregnancy.
Study population
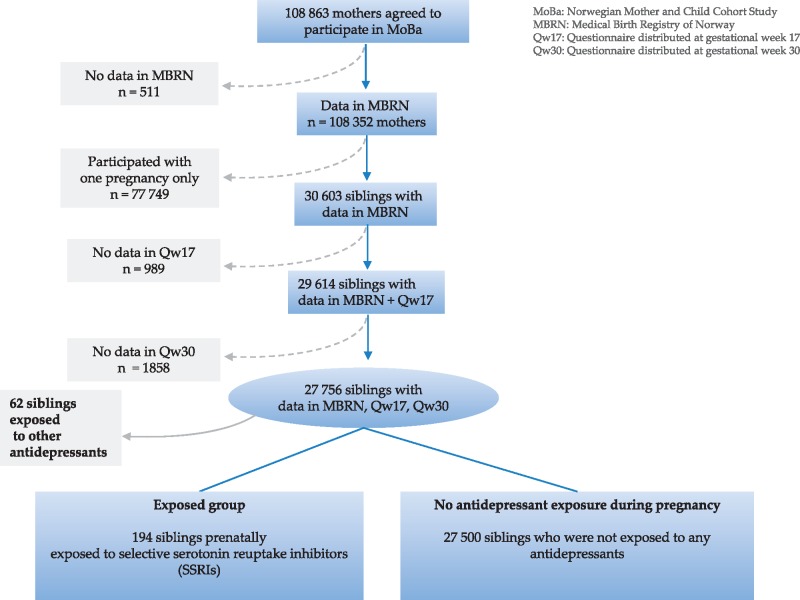
A total of 27 756 siblings with data in MBRN and both MoBa questionnaires were included in this study. Multiple pregnancies were excluded. The study population was subdivided into the exposed group (women using SSRIs during pregnancy) and the unexposed group (women who did not use any antidepressant medication during pregnancy and who either did not have symptoms of depression or did). Women using non-SSRI antidepressants (n = 62) were excluded from the study. A flow chart of the study population is shown in Figure 1.
Figure 1.
The study population.
SSRI exposure during pregnancy
Information on the type and timing of SSRI use was available from the two MoBa questionnaires answered during pregnancy (Qw17 and Qw30) and one MoBa questionnaire answered 6 months postpartum (Q6mPP). The indications specifically named to increase the reporting of SSRIs included not only depression and anxiety but also eating and sleepingdisorders, other psychiatric illnesses, unusual tiredness and other illnesses. For each indication, the following exposure windows could be specified: gestational weeks 0 to 4, 5 to 8, 9 to 12, and 13 + (Qw17), weeks 13 to 16, 17 to 20, 21 to 24, 25 to 28, and 29 + (Qw30) and week 30 to delivery (Q6mPP). Drug exposure was classified and grouped according to the Anatomical Therapeutic Chemical (ATC) Classification System developed by the World Health Organization.27 SSRI exposure was defined as exposure to a drug belonging to ATC class N06AB. The following explanatory exposure variables were then created: ‘SSRI exposure anytime during pregnancy’, ‘SSRI exposure during one trimester’ and ‘SSRI exposure during two or more trimesters’.
Maternal depressive symptoms
The Hopkins Symptom Checklist SCL-25 is a widely used self-rating scale designed to measure symptoms of depression and anxiety and is acceptable as a screening instrument for depression as defined by the ICD-10.28,29 We used the validated short version SCL-5 answered in Qw17 and Qw30.30,31 SCL-5 consists of the following questions: ‘Have you been bothered by any of the following during the last two weeks: (1) feeling fearful, (2) nervousness or shakiness inside, (3) feeling hopeless about the future, (4) feeling blue, and (5) worrying too much about things’.30,32 Each item could be scored as ‘not at all’ (score 1) to ‘extremely’ (score 4). The total sum score was divided by the number of items. Women who scored a raw sum score of 2.0 or above in SCL-5 in either Qw17 or Qw30 were classified as women with depressive symptoms in one pregnancy period. Women who scored a raw sum score of 2.0 or above in SCL-5 in both Qw17 and Qw30 were classified as women with depressive symptoms in two pregnancy periods. SCL-5 had adequate internal reliability of > 0.83.
Women were also classified as having a lifetime history of major depression (major depression that occurred at any point in time in their life and irrespective of whether or not they had depressive symptoms during the current pregnancy) using answers to the lifetime occurrence of five key depressive symptoms chosen from the nine symptomatic criteria for major depression in the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) (sad mood, change in appetite, loss of energy, feelings of guilt or worthlessness, and problems with concentration).The key depressive symptoms were (1) sad mood, (2) change in appetite, (3) loss of energy, (4) feelings of guilt or worthlessness and (5) problems in concentration. They were then asked whether any three of these symptoms co-occurred in their life for at least 2 weeks.33
Birthweight and gestational length
The outcome variables in our study, birthweight in grams and gestational length in weeks were derived from MBRN. Birthweight outside 3.5 standard deviations (SD) from the gender-specific mean at each pregnancy week (0.5%) and gestational length exceeding 44 weeks (0.9%) were recoded as missing.
Potential confounding variables
The following measured confounding variables were included in the propensity score variable that was used as adjusting variable for SSRI exposure probability: symptoms of depression at gestational week 17, lifetime history of major depression, maternal age, parity, birth order, marital status, level of education, smoking daily during the past 3 months of pregnancy, alcohol intake at least once a week during pregnancy, acute musculoskeletal pain during pregnancy, rheumatoid disorders during pregnancy, migraine and headache during pregnancy and use of hypnotics, opioids and anxiolytics during pregnancy. All variables were categorized as presented in Tables 1 and 2, except for symptoms of depression at gestational week 17, maternal age and birth order which were used as continuous variables in the analyses. The same propensity score variable was used as adjusting variable for depression probability but did not include depressive symptoms at gestational week 17 or lifetime history of major depression and did include SSRI exposure during pregnancy.
Table 1.
Socio-demographic characteristics of the exposed and the unexposed women
| SSRI exposure during pregnancy |
SSRI exposure during one trimester |
SSRI exposure during two or more trimesters |
No antidepressant exposure during pregnancy |
|||||
|---|---|---|---|---|---|---|---|---|
| N = 194 | N = 94 | N = 84 | N = 27500 | |||||
| Maternal age, mean (SD) (years) | 29.3 (4.5) | 29.0 (4.5) | 30 (4.4) | 30.1 (4.1) | ||||
| n | % of N | n | % of N | n | % of N | n | % of N | |
| Parity | ||||||||
| 0 | 87 | 44.8 | 46 | 48.9 | 29 | 34.5 | 10599 | 38.5 |
| 1 | 72 | 37.1 | 32 | 34.0 | 39 | 46.4 | 12124 | 44.1 |
| >1 | 35 | 18.0 | 16 | 17.0 | 16 | 19.0 | 4777 | 17.4 |
| Marital status with father of child | ||||||||
| Married/ cohabiting | 179 | 92.3 | 85 | 90.4 | 78 | 92.9 | 27188 | 98.9 |
| Other | 15 | 7.7* | 9 | 9.6* | 6 | 7.1* | 312 | 1.1 |
| Education | ||||||||
| Primary | 3 | 1.5 | 1 | 1.1 | 2 | 2.4 | 286 | 1.0 |
| Secondary | 72 | 37.1 | 36 | 38.3 | 28 | 33.3 | 6348 | 23.1 |
| Tertiary | 115 | 59.3* | 55 | 58.5** | 52 | 61.9*** | 20407 | 74.2 |
| Folic acid intake before and during pregnancy | 66 | 34.0 | 32 | 34.0 | 27 | 32.1 | 10885 | 39.6 |
| Smoking daily at the end of pregnancy | 10 | 5.1** | 5 | 5.3*** | 4 | 4.8 | 673 | 2.4 |
| Alcohol intake of ≥ 1 unit per week during pregnancy | 10 | 5.1 | 3 | 3.2 | 6 | 7.1*** | 957 | 3.5 |
There were 16 women who reported using SSRIs during pregnancy without specifying during which trimester they used them.
Pearson’s χ2 test P < 0.001 when compared with no antidepressants.
Pearson’s χ2 test P < 0.01 when compared with no antidepressants.
Pearson’s χ2 test P < 0.05 when compared with no antidepressants.
Table 2.
Maternal medical characteristics of the exposed and the unexposed women
| SSRI exposure during pregnancy |
SSRI exposure during one trimester |
SSRI exposure during two or more trimesters |
No antidepressant exposure during pregnancy |
|||||
|---|---|---|---|---|---|---|---|---|
|
N = 194 |
N = 94 |
N = 84 |
N = 27500 |
|||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Symptoms of depression in pregnancye | ||||||||
| Gestational week 17 | 1.8 (0.7) | 1.8 (0.7) | 1.9 (0.8) | 1.2 (0.3) | ||||
| Gestational week 30 | 1.8 (0.7) | 1.8 (0.7) | 1.7 (0.8) | 1.2 (0.3) | ||||
| n | % of N | n | % of N | n | % of N | n | % of N | |
| Lifetime history of major depressionf | 170 | 87.6* | 82 | 87.2* | 74 | 88.1* | 7869 | 28.6 |
| Musculoskeletal pain | 184 | 94.8*** | 90 | 95.7 | 80 | 95.2 | 24968 | 90.8 |
| Co-medication | ||||||||
| Paracetamol | 104 | 53.6 | 53 | 56.4 | 40 | 47.6 | 13029 | 47.4 |
| Nonsteroidal anti-inflammatory drugsd | 19 | 9.8*** | 10 | 10.6 | 8 | 9.5 | 1706 | 6.2 |
| Anxiolyticsa | 16 | 8.2* | 6 | 6.4* | 6 | 7.1* | 114 | 0.4 |
| Opioid analgesicsc | 9 | 4.6** | 3 | 3.2 | 5 | 6.0** | 562 | 2.0 |
| Hypnoticsb | 9 | 4.6* | 4 | 4.3* | 4 | 4.8* | 76 | 0.3 |
| Sick leave > 14 days | 78 | 40.2** | 37 | 39.4 | 35 | 41.7 | 9014 | 32.8 |
| Migraine and/or headache | 74 | 38.1 | 42 | 44.7*** | 27 | 32.1 | 9428 | 34.3 |
| Antepartum bleeding | ||||||||
| First trimester | 28 | 14.4 | 10 | 10.6 | 16 | 19.0 | 4262 | 15.5 |
| Second and/ or third trimester | 14 | 7.2 | 4 | 4.3 | 8 | 9.5 | 1880 | 6.8 |
| Rheumatoid disorders | 14 | 7.2* | 10 | 10.6* | 3 | 3.6 | 844 | 3.1 |
| Urinary tract infection and/ or pyelonephritis | 12 | 6.2 | 6 | 6.4 | 3 | 3.6 | 1511 | 5.5 |
| Asthma | 11 | 5.7 | 5 | 5.3 | 5 | 6.0 | 1879 | 6.8 |
| Thyroid disorders | 10 | 5.1** | 4 | 4.3 | 3 | 3.6 | 654 | 2.4 |
| High blood pressure | ||||||||
| Pre-eclampsia and/or eclampsia | 9 | 4.6 | 3 | 3.2 | 3 | 3.6 | 913 | 3.3 |
| Systolic blood pressure > 140 mmHg in the first trimester | 6 | 3.1 | 5 | 5.3 | 2 | 2.4 | 942 | 3.4 |
Anxiolytics: diazepam, oxazepam, alprazolam, hydroxyzine, buspirone.
Hypnotics: nitrazepam, flunitrazepam, zopiclone, zolpidem.
Opioid analgesics: codeine alone and in combination with paracetamol, morphine, tramadol, oxycodone, buprenorphine, dextropropoxyphene.
NSAIDs: ibuprofen, diclofenac, naproxen, meloxicam, piroxicam, indomethacin.
Measured by the Mean Hopkins symptom checklist SCL-5 value.
Measured using answers to the lifetime occurrence of five key depressive symptoms chosen from the nine symptomatic criteria for major depression in the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R).
Pearson’s χ2 test P < 0.001 when compared with no antidepressants.
Pearson’s χ2 test P < 0.01 when compared with no antidepressants.
Pearson’s χ2 test P < 0.05 when compared withno antidepressants.
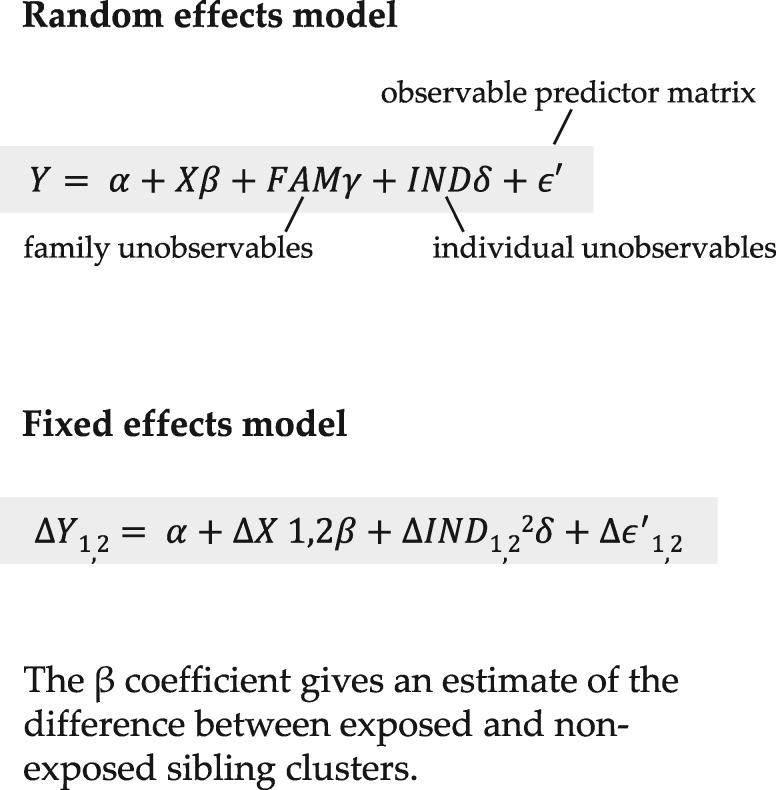
The shared familial characteristics were addressed by the fixed-effects design of the analysis (see below).
Statistical analysis
Statistical analyses were performed with Stata statistical software, version 13.
The analyses on the effect of SSRI exposure on birthweight and gestational length were carried out in three steps. First, a crude random effects analysis was performed to provide an estimate of the association between SSRI exposure during pregnancy and pregnancy outcome for the whole cohort of 27 756 siblings. This estimate is comparable to that obtained from a non-sibling design. Second, the propensity score variable was used as the adjusting variable for SSRI exposure probability in the adjusted random effects model. To create the propensity score, we used logistic regression in which SSRI exposure was the outcome variable and the variables considered material or theoretical confounders were predictors. The propensity score variable thus created was included as a co-variable in the final analysis. Third, fixed effects analysis was used to adjust for unmeasurable family-level effects alone in addition to the propensity score adjustment. The reference category consisted of women who were not exposed to antidepressants during pregnancy. Sub-analyses were performed to investigate the association between SSRI exposure in only one trimester and SSRI exposure in two or more trimesters and the pregnancy outcomes. In both adjusted models, a stratified analysis on term infants only (infants born in gestational week 37 or thereafter, n = 26 577) was performed to account for the possible effect of gestational length on birthweight.
The analyses on the effect of SSRI exposure on gestational length were carried out following the same procedure. Separate analyses on the effect of (i) lifetime history of major depression, (ii) depressive symptoms in one pregnancy period and (iii) two pregnancy periods on birthweight and gestational length were also carried out following the same steps.
Three separate analyses were performed using three different depressive symptoms exposure variables respectively. The reference category for each analysis included the women who were not members of the specific exposure group analysed to maintain as much similarity between the groups as possible.
Results
Of the 27 756 siblings included in this study, 194 were prenatally exposed to SSRIs—94 during one trimester and 84 during two or more trimesters.Of the remaining 27 562 siblings, 62 were exposed to other antidepressants during pregnancy and 27 500 were not exposed to any antidepressant during pregnancy. There were25 506 mothers who had no depressive symptoms during pregnancy, 2110 mothers who had depressive symptoms recorded in one pregnancy period (gestational week 17 or 30) and 508 mothers who had depressive symptoms recorded in two pregnancy periods (gestational weeks 17 and 30). Irrespective of presence or absence of depressive symptoms, 7450 mothers had a lifetime history of major depression.
The characteristics of the mothers who used SSRIs, and mothers who did not use any antidepressants during pregnancy are presented in Tables 1 and 2. A significantly higher proportion of those using SSRIs during pregnancy lacked a life partner, attained lower levels of education, smoked and consumed alcohol during pregnancy, compared with the mothers who did not use any antidepressants (Table 1). Likewise, a significantly higher proportion of those using SSRIs during pregnancy suffered from a number of medical conditions and used psychoactive and analgesic medications (Table 2).
The mean birthweight of infants exposed to SSRIs any time during pregnancy, during one trimester and during two or more trimesters was 3550 g (SD 606), 3660 g (SD 695) and 3459 g (SD 477), respectively. The mean birthweight of infants born to women with a lifetime history of major depression, with depressive symptoms during one pregnancy period or with depressive symptoms during two pregnancy periods was 3620 g (SD 538), 3600 g (SD 575) and 3573 g (SD 548), respectively. The mean birthweight of infants born to women who did not use antidepressants during pregnancy was 3636 g (SD 527).
The mean gestational age at birth for the SSRI-exposed any time during pregnancy, during one trimester and during two or more trimesters was 39.1 weeks (SD 2.0), 39.4 weeks (SD 2.2) and 38.9 weeks (SD 1.8), respectively. The mean gestational age at birth of infants born to women with a lifetime history of major depression, with depressive symptoms during one pregnancy period and with depressive symptoms during two pregnancy periods was 39.5 weeks (SD 1.7), 39.4 weeks (SD 2.0) and 39.3 weeks (SD 1.8), respectively. The mean gestational age of infants born to women who did not use antidepressants during pregnancy was 39.7 weeks (SD 1.7).The distribution of birthweights and gestational ages at birth of infants exposed to SSRIs, born to mothers with a lifetime history of major depression, with depressive symptoms during pregnancy and with no antidepressant exposureduring pregnancy are shown in e-Figures 2 and 3 (available as Supplementary data at IJE online). The intra-class correlation for gestational length and for birthweight was 0.39 and 0.21, respectively, indicating a moderate effect of familial factors. Tables 3 and 4 show the effect of SSRIs on birthweight and gestational age at birth.
Table 3a.
Associations of SSRI exposure with birthweight
| Exposure during pregnancy | Random effects | Random effects | Fixed effects |
|---|---|---|---|
| Crude model | Adjusted modela | Adjusted modela | |
| Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | |
| No antidepressant exposure during pregnancy | Ref | Ref | Ref |
| Any time | −91 (−163 to −19) | −73 (−146 to 1) | −93 (−193 to 7) |
| One trimester | 11 (−86 to 109) | 28 (−70 to 126) | 4 (−116 to 124) |
| Two or more trimesters | −183 (−295 to −70) | −161 (−275 to −47) | −205 (−372 to −38) |
Table 3b.
Associations of depressive symptoms with birthweight
| Maternal depression | Random effects | Random effects | Fixed effects |
|---|---|---|---|
| Crude model | Adjusted modela | Adjusted modela | |
| Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | Mean difference (β) (95%CI) | |
| Lifetime history of major depression | −21 (−35 to −7) | −17 (−31 to −3) | −19 (−41 to 3) |
| Depressive symptoms in one pregnancy period | −34 (−56 to −11) | −29 (−52 to −6) | −16 (−47 to 15) |
| Depressive symptoms in two pregnancy periods | −31 (−78 to 15) | −29 (−75 to 17) | −3 (−64 to 59) |
All values are given in grams. Significant findings shown in bold.
The β obtained in the random effects linear regression is representative of the β that would be obtained in a linear regression performed on the whole cohort. The fixed effects linear regression model addresses unmeasured and residual family-level confounding.
Three separate analyses were performed using three different depressive symptoms exposure variables, respectively. The reference category for each analysis included the women who were not members of the specific exposure group analysed.
Propensity score pain during pregnancy, rheumatoid disorders during pregnancy, migraine and headache during pregnancy, use of hypnotics, opioids and anxiolytics during pregnancy).
Table 4a.
Associations of SSRI exposure with gestational age
| Exposure during pregnancy | Random effects | Random effects | Fixed effects |
|---|---|---|---|
| Crude model | Adjusted modela | Adjusted modela | |
| Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | |
| No antidepressant exposure during pregnancy | Ref | Ref | Ref |
| Any time | −2.9 (−4.6 to −1.3) | −2.7 (−4.3 to −1.0) | −3.1 (−5.5 to −0.6) |
| One trimester | −1.4 (−3.5 to 0.7) | −0.7 (−3.5 to 1.4) | −1.4 (−4.2 to 1.4) |
| Two or more trimesters | −4.9 (−7.0 to −2.1) | −4.2 (−7.0 to −1.4) | −4.9 (−9.1 to −1.4) |
Table 4b.
Associations of depressive symptoms with gestational age
| Maternal depression | Random effects | Random effects | Fixed effects |
|---|---|---|---|
| Crude model | Adjusted modela | Adjusted modela | |
| Mean difference (β) (95%CI) | Mean difference (β) (95% CI) | Mean difference (β) (95% CI) | |
| Lifetime history of major depression | 0.0 (−0.7 to 0.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.7) |
| Depressive symptoms in one pregnancy period | −1.0 (−1.5 to −0.7) | −0.7 (−1.4 to 0.0) | 0.0 (−0.7 to 0.0) |
| Depressive symptoms in two pregnancy periods | −1.0 (−2.1 to 0.0) | −0.7(−1.4 to 0.0) | 0.7 (−0.7 to 2.1) |
All values are given in days. Significant findings shown in bold.
The β obtained in the random effects linear regression is representative of the β that would be obtained in a linear regression performed on the whole cohort. The fixed effects linear regression model addresses unmeasured and residual family-level confounding.
Three separate analyses were performed using three different depressive symptoms exposure variables, respectively..The reference category for each analysis included the women who were not membersof the specific exposure group analysed.
Propensity score variable for SSRI exposure/ depression occurrence during pregnancy included in all models (symptoms of depression at gestational week 17 and lifetime history of major depression (SSRI exposure), SSRI exposure (depressive symptoms), maternal age, parity, birth order, marital status, education, smoking, alcohol intake, acute musculoskeletal pain during pregnancy, rheumatoid disorders during pregnancy, migraine and headache during pregnancy, use of hypnotics, opioids and anxiolytics during pregnancy).
In the propensity score-adjusted random effects model, infants exposed to SSRIs during two or more trimesters weighed 161 g less than infants whose mothers were not exposed to any antidepressants (β: −161 g, 95% CI − 275 g to − 47 g).
In the fixed effects model, allowing for adjustment for familial factors, infants exposed to SSRIs during two or more trimesters weighed 205 g less than infants whose mothers were not exposed to any antidepressants during pregnancy (β: −205 g, 95% CI − 372 to − 38). We did not find an effect of SSRI exposure in one trimester on birthweight (Table 3a). The stratified analysis on term infants did notattenuate the above association (e-Table 3a, available as Supplementary data at IJE online).
In the adjusted random effects model, infants exposed to SSRIs during two or more trimesters were born 4.2 days earlier than infants whose mothers were not exposed to any antidepressants (β: −4.2 days; 95% CI − 7.0 to − 1.4). In the fixed effects model, allowing for adjustment for familial factors, infants exposed to SSRIs during two or more trimesters were born 4.9 days earlier than infants whose mothers were not exposed to any antidepressantsduring pregnancy (β: −4.9 days, 95% CI –9.1 to − 1.4) (Table 4a).
We did find associations between prenatal exposure to depressive symptoms on the above pregnancy outcomes in the random effects models. However, these effects were attenuated when we accounted for shared genetic and familial confounding in the fixed effects models (Tables 3b, 4b;e-Table 3b, available as Supplementary data at IJE online).
Discussion
The main finding of this study was that prenatal SSRI exposure was associated with a decrease in birthweight of 205 g and a decrease of gestational length of 4.9 days following prenatal exposure to SSRIs in two or more trimesters. No association between maternal SSRI use in one trimester, a lifetime history of major depression or depressive symptoms and these pregnancy outcomes was found.
Several previous studies have reported associations between antidepressant exposure and lower birthweight or being small for gestational age;34–40 however, an equal number of studies failed to confirm these findings.19,38,41–46 Only one of these studies took shared familial confounding into account.19 Viktorin et al. found a smaller decrease in gestational age than we did (2.2 days vs 4.9 days, although confidence intervals overlapped) when compared with non-exposed, non-depressed controls. The discrepancy between our results could be due to differences in exposure ascertainment: our study used maternal self-report, which is unlikely to produce false negatives (i.e. women who report SSRI use but who were truly unexposed), whereas their study relied on prescription dispensing, a method that is likely to over-estimate women exposed to SSRIs. It is therefore possible that non-differential misclassification, and its expected bias towards the null, can explain the differences in effect estimate. Moreover, it should also be taken into account that their results are standardized values of birthweight adjusted for gestational length. We did not include gestational length in our model, but it is worth noting that the association we found for birthweight remained present in term infants. With respect to the lack of association between depression and birthweight, our results can be corroborated by two recent meta-analyses.20,21
The biological mechanisms by which long-term SSRI exposure may affect birthweight remain unknown. A possible explanation could be the same factors as are causing weight loss during the first 6–10 months of treatment initiation in adults.47 Our findings on gestational length point to a decrease at birth of 5 days following prenatal exposure to SSRIs in two or more trimesters.
No association between antidepressant exposure and preterm birth (gestational age at birth of < 37 weeks) was found in the former cohort study from our group.6 This may be explained by the fact that we previously assessed prematurity and were unable to detect small effects on gestational length that are visible when this outcome is analysed in its continuous form. Interestingly, our finding corresponds rather accurately to that of Lund et al. where a mean decrease in 5 days of gestational length was reported after SSRI exposure during pregnancy.40 Also several other studies have reported an association between use of antidepressants, mainly SSRIs, and lower gestational age at birth 19,34,37,38,41,48–51 or preterm birth (gestational age at birth of < 37 weeks).35,51–53 Of these, only five accounted for any underlying psychiatric illness,19,39,43,51,54 and one of the studies, by Vitkorin et al., applied the within-family design.19 The exact biological mechanism by which SSRIs may affect gestational length also remains unknown. One possible explanation could be the elevated estriol levels seen in pregnant women treated with antidepressants,55 as this effect has been shown to be associated with an increased risk of preterm birth.56
To our best knowledge, this is the first sibling study on the effect of self-reported prenatal SSRI exposure on birthweight and gestational length.57,58 By applying this design we could take advantage of controlling for factors such as shared genetics and the same family environmental factors across pregnancies.22 Not only were we able to rule out such otherwise potentially confounding factors but, by applying a fixed effects model, we had the advantage of reducing residual confounding by addressing unmeasurable and unknown family-level differences.23 Moreover, the vast amount of detailed information available in both the MoBa and the MBRN enabled us to adjust for non-familial confounding factors including, most importantly, symptoms of depression as a continuous variable as well as smoking, co-medication during pregnancy and maternal health during pregnancy, which were all implemented in a propensity score variable for SSRI exposure. Application of propensity scores is akin to the rationale of the randomized experiment; exposed and unexposed cases are balanced on the factors related to exposure (i.e. maternal use of SSRI or maternal depression). Maternal reporting of SSRI intake was found to be highly consistent with the dispensing of these drugs in a previous study where 93% of women who reported use during pregnancy had a prescription in the Norwegian Prescription Database during pregnancy or the preceding 90 days.59 Exposure misclassification may nevertheless have occurred because non-adherent pregnant women may have over-estimated their SSRI use. However, it is worth remembering that self-reported drug use lies generally closer to actual drug use than what would have been obtained had the respondents been interviewed.
Analysing the possible effect of depression, both a lifetime history of major depression and symptoms of depression during pregnancy, enabled us to examine the effect of the underlying maternal illness. Even though confounding by indication is more or less unavoidable in studies of drug exposure on pregnancy outcomes, we have as much as possible tried to control for this factor. Moreover, we were able to take into account the symptom severity of the underlying maternal illness. The low participation rate of 40% in MoBa is often a source of concern with respect to selection bias, especially with regard to prevalence estimates (women younger than 25 years, those without a life-partner, those with a parity > 0 and smokers are all under-represented, whereas mothers who take folic acid are over-represented). On the other hand, the fact that only minor differences (below 2% in absolute differences in socio-demographic variables) have been reported between MoBa participants and the general Norwegian population of pregnant women strengthens the representativeness of our material.24,25 Finally, the prospective nature of data collection in MoBa reduced the risk of recall bias to a minimum.
Since sibling studies represent a fraction of the original study population, study power is inevitably reduced. However, even though results that are not significant cannot be relied upon in such cases, our 95% confidence intervals were relatively narrow and imply acceptable power. Also, changes in household composition, differences in environmental exposures, financial stressors and prescribing and diagnosis patterns that may occur between pregnancies in the same family need to be taken account as these could, both directly and indirectly, influence the intrauterine environment. Also, the sibling births taking part in the study may not be entirely representative of the entire sibling population of Norway. The MBRN data used in our study (birthweight, gestational length, maternal age, parity) had only < 1% missing values. On the other hand, approximately 9% of the siblings were excluded from the study because their mothers lacked information in Qw17 and Qw30. Although this may have affected the prevalence of medication use, the possible associations between SSRI exposure and pregnancy outcome would not be expected to be changed.60 Due to space limitations in the questionnaire, only the shorter version of the Hopkins Symptoms Checklist, SCL-5, was used to measure symptoms of depression during pregnancy. The Cronbach alpha for SCL-5 for depression was estimated to lie between 0.74 and 0.83; however, an increase in the skewness of score distribution due to a reduction in the number of items in the checklist should not be dismissed.61 Last, we did not have data on dosage of SSRI use, which would have enabled us to study dose–response effects, although the numbers of trimesters during which the fetus was exposed could be viewed as a proxy of cumulative dose.
Conclusion
Our results suggest that prenatal exposure to SSRIs during two or more trimesters may decrease birthweight and gestational length. Our results indicate that shared genetics and family environment cannot explain these associations. Neither maternal SSRI use in one trimester, a lifetime history of major depression nor depressive symptoms during pregnancy was associated with these pregnancy outcomes.
Key Messages
Prenatal exposure to SSRIs during two or more trimesters may decrease birthweight and gestational length.
Neither maternal depression nor shared genetics and family environment fully explain this association.
Figure 2.
Equations representing the random and fixed effects models.
Funding
This project has been financially supported by the Norwegian Women's Public Health Association on behalf of theNorwegian EXTRA Foundation for Health and Rehabilitation through EXTRA funds. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, National Institutes of Health/National Institute of Environmental Health Sciences (grant no. NO-ES-75558), National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant no. 1 UO1 NS 047537–01) and the Norwegian Research Council/Functional Genomics (grant no. 151918/S10).
Supplementary Material
Acknowledgements
We would like to extend our thanks to Dr Mollie Wood, Pharmaco-Epidemiology and Drug Safety Research Group, School of Pharmacy, University of Oslo, Norway, for her valuable input. We are grateful to all of the participants and their families for taking part in this study.
Conflict of interest
There are no conflicts of interest to declare.
References
- 1. (CANMAT) CNfMaAT. Treating Depressive Disorder 2014 http://www.canmat.org/cme-depression-pharma-cotherapy.php (February 2015, date last accessed).
- 2. (APA) APA. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd edn. 2010. http://www.guideline.gov/content.aspx?id = 24158 (February 2015, date last accessed).
- 3. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T.. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005;106(5 Pt 1):1071–83. [DOI] [PubMed] [Google Scholar]
- 4. Duncan AE, Munn-Chernoff MA, Hudson DL. et al. Genetic and environmental risk for major depression in African-American and European-American women. Twin Res Hum Genet 2014;1:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE.. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M.. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol 2012;32:186–94. [DOI] [PubMed] [Google Scholar]
- 7. Sandanger I, Nygard JF, Ingebrigtsen G, Sorensen T, Dalgard OS.. Prevalence, incidence and age at onset of psychiatric disorders in Norway. Soc Psychiatry Psychiatr Epidemiol 1999;34:570–79. [DOI] [PubMed] [Google Scholar]
- 8. Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K.. Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004-2006. Br J Clin Pharmacol 2008;65:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlton R, Jordan S, Pierini A. et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG 2015;122: 1010–20. [DOI] [PubMed] [Google Scholar]
- 10. Meunier MR, Bennett IM, Coco AS.. Use of antidepressant medication in the United States during pregnancy, 2002-2010. Psychiatr Serv 2013;64:1157–60. [DOI] [PubMed] [Google Scholar]
- 11. Hanley GE, Mintzes B.. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth 2014;14:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geier ML, Hills N, Gonzales M, Tum K, Finley PR.. Detection and treatment rates for perinatal depression in a state Medicaid population. CNS Spectr 2015; 20:11–19. [DOI] [PubMed] [Google Scholar]
- 13. Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT.. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 2008;65:600–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weisskopf E, Fischer CJ, Bickle Graz M. et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf 2015;14:413–27. [DOI] [PubMed] [Google Scholar]
- 15. McDonagh MS, Matthews A, Phillipi C. et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol 2014;124: 526–34. [DOI] [PubMed] [Google Scholar]
- 16. Huang H, Coleman S, Bridge JA, Yonkers K, Katon W.. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birthweight. Gen Hosp Psychiatry 2014;36:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huybrechts KF, Sanghani RS, Avorn J, Urato AC.. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLoS One 2014;9: e92778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross LE, Grigoriadis S, Mamisashvili L. et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry 2013;70:436–43. [DOI] [PubMed] [Google Scholar]
- 19. Viktorin A, Lichtenstein P, Lundholm C. et al. Selective serotonin re-uptake inhibitor use during pregnancy: association with offspring birth size and gestational age. Int J Epidemiol 2016;45:170–77. [DOI] [PubMed] [Google Scholar]
- 20. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ.. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birthweight, and intrauterine growthrestriction. Arch Gen Psychiatry 2010;67:1012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grigoriadis S, VonderPorten EH, Mamisashvili L. et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 2013;74:e321–41. [DOI] [PubMed] [Google Scholar]
- 22. D'Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P.. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health 2013;103(Suppl 1):S46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawlor A, Mishra G.. Family Matters. Oxford, UK: Oxford University Press, 2009. [Google Scholar]
- 24. Magnus P, Irgens L, Haug K. et al. Cohort profile: The Norwegian mother and child cohort study (MoBa). Int J Epidemiol 2006;35:1146–50. [DOI] [PubMed] [Google Scholar]
- 25. Nilsen RM, Vollset SE, Gjessing HK. et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 2009;23:597–608. [DOI] [PubMed] [Google Scholar]
- 26. Norwegian Institute of Public Health. Protocol II. Oslo: Norwegian Institute of Public Health, 2013. [Google Scholar]
- 27. World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2013 http://www.whocc.no/atc/structure_and_principles/.
- 28. Sandanger I, Moum T, Ingebrigtsen G, Dalgard OS, Sorensen T, Bruusgaard D.. Concordance between symptom screening and diagnostic procedure: the Hopkins Symptom Checklist-25 and the Composite International Diagnostic Interview I. Soc Psychiatry Psychiatr Epidemiol 1998;33:345–54 (February 2015, date last accessed). [DOI] [PubMed] [Google Scholar]
- 29. Sandanger I, Moum T, Ingebrigtsen G, Sorensen T, Dalgard OS, Bruusgaard D. The meaning and significance of caseness: the Hopkins Symptom Checklist-25 and the Composite International Diagnostic Interview. II. Soc Psychiatry Psychiatr Epidemiol 1999;34:53–59. [DOI] [PubMed] [Google Scholar]
- 30. Strand BH, Dalgard OS, Tambs K, Rognerud M.. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry 2003;57:113–18. [DOI] [PubMed] [Google Scholar]
- 31. Fink P, Orbol E, Hansen MS, Sondergaard L, De Jonge P.. Detecting mental disorders in general hospitals by the SCL-8 scale. J Psychosom Res 2004;56:371–75. [DOI] [PubMed] [Google Scholar]
- 32. Tambs K, Moum T.. How well can a few questionnaire items indicate anxiety and depression? Acta Psychiatr Scand 1993;87: 364–67. [DOI] [PubMed] [Google Scholar]
- 33. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ.. The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry 1993;50: 863–70. [DOI] [PubMed] [Google Scholar]
- 34. Lewis AJ, Galbally M, Opie G, Buist A.. Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust N Z J Psychiatry 2010;44:482–87. [DOI] [PubMed] [Google Scholar]
- 35. Maschi S, Clavenna A, Campi R, Schiavetti B, Bernat M, Bonati M.. Neonatal outcome following pregnancy exposure to antidepressants: a prospective controlled cohort study. BJOG 2008;115:283–89. [DOI] [PubMed] [Google Scholar]
- 36. Dubnov-Raz G, Juurlink DN, Fogelman R. et al. Antenatal use of selective serotonin-reuptake inhibitors and QT interval prolongation in newborns. Pediatrics 2008;122:e710–15. [DOI] [PubMed] [Google Scholar]
- 37. Malm H, Klaukka T, Neuvonen PJ.. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 2005;106:1289–96. [DOI] [PubMed] [Google Scholar]
- 38. Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J.. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164:1206–13. [DOI] [PubMed] [Google Scholar]
- 39. Lund N, Pedersen LH, Henriksen TB.. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med 2009;163:949–54. [DOI] [PubMed] [Google Scholar]
- 40. Jensen HM, Gron R, Lidegaard O, Pedersen LH, Andersen PK, Kessing LV.. The effects of maternal depression and use of antidepressants during pregnancy on risk of a child small for gestational age. Psychopharmacology 2013;228:199–205. [DOI] [PubMed] [Google Scholar]
- 41. Wisner KL, Sit DK, Hanusa BH. et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009;166:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J.. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Women Ment Health 2004;7:193–200. [DOI] [PubMed] [Google Scholar]
- 43. Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C.. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 2006;63:898–906. [DOI] [PubMed] [Google Scholar]
- 44. Wisner KL, Bogen DL, Sit D. et al. Does fetal exposure to SSRIs or maternal depression impact infant growth? Am J Psychiatry 2013;170:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sivojelezova A, Shuhaiber S, Sarkissian L, Einarson A, Koren G.. Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. Am J Obstet Gynecol 2005;193:2004–09. [DOI] [PubMed] [Google Scholar]
- 46. Malm H, Sourander A, Gissler M. et al. Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. Am J Psychiatry 2015;172:1224–32. [DOI] [PubMed] [Google Scholar]
- 47. Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. J Clin Psychiatry 2001;3:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL.. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcohol Clin Exp Res 2010;34: 681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calderon-Margalit R, Qiu C, Ornoy A, Siscovick DS, Williams MA.. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol 2009;201:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferreira E, Carceller AM, Agogue C. et al. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics 2007;119:52–59. [DOI] [PubMed] [Google Scholar]
- 51. Simon GE, Cunningham ML, Davis RL.. Outcomes of prenatal antidepressant exposure. Am J Psychiatry 2002;159: 2055–61. [DOI] [PubMed] [Google Scholar]
- 52. Reis M, Kallen B.. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 2010;40:1723–33. [DOI] [PubMed] [Google Scholar]
- 53. Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C. et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol 2006;194:961–66. [DOI] [PubMed] [Google Scholar]
- 54. Grzeskowiak LE, Morrison JL.. Long-term effects of prenatal SSRI exposure on child growth: weighing the evidence. Am J Psychiatry 2013;170:1364. [DOI] [PubMed] [Google Scholar]
- 55. Suri R, Hellemann G, Cohen L, Aquino A, Altshuler L.. Saliva estriol levels in women with and without prenatal antidepressant treatment. Biol Psychiatry 2008;64:533–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McGregor JA, Jackson GM, Lachelin GC. et al. Salivary estriol as risk assessment for preterm labor: a prospective trial. Am J Obstet Gynecol 1995;173:1337–42. [DOI] [PubMed] [Google Scholar]
- 57. Yaghootkar H, Freathy RM.. Genetic origins of low birthweight. Curr Opin Clin Nutr Metab Care 2012;15:258–64. [DOI] [PubMed] [Google Scholar]
- 58. Bhattacharya S, Raja EA, Mirazo ER. et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol 2010;115:1125–33. [DOI] [PubMed] [Google Scholar]
- 59. Skurtveit S, Selmer R, Tverdal A, Furu K, Nystad W, Handal M.. Drug exposure: inclusion of dispensed drugs before pregnancy may lead to underestimation of risk associations. J Clin Epidemiol 2013;66:964–72. [DOI] [PubMed] [Google Scholar]
- 60. Nohr EA, Frydenberg M, Henriksen TB, Olsen J.. Does low participation in cohort studies induce bias? Epidemiology 2006;17:413–18. [DOI] [PubMed] [Google Scholar]
- 61. Tambs K, Røysamb E.. Selection of questions to short-form versions of original psychometric instruments in MoBa. Norsk Epidemiol 2014;24:195–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.