Abstract
Background: The role of body fatness in the aetiology of breast cancer is complex. We evaluated the independent and synergistic effects of body fatness, at different stages throughout a woman's life course, on premenopausal breast cancer risk.
Methods: Premenopausal participants of the Nurses’ Health Study II (NHSII) were followed from 1991 up to 2009. Body fatness factors including birthweight, somatotype (a 9-level pictogram with level 1 being the leanest) at ages 5 and 10 years and body mass index (BMI) at age 18 were collected at baseline. Current BMI was updated biennially. Multivariate Cox regression models were used to evaluate the association between each body fatness factor as well as cross-classification of all factors and the incidence of breast cancer.
Results: Based on 1574 incident premenopausal breast cancer cases and 1 133 893 person-years of follow-up, a lower incidence was associated with lower birthweight: hazard ratio (HR) [95% confidence interval (CI)] = 0.74 (0.58–0.95) for <2.5kg vs 3.9+kg, P for trend < 0.001; higher somatotype at age 5: HR=0.57 (95% CI 0.44–0.73) for 5–9 vs 1, P fortrend < 0.0001]; and at age 10: HR=0.61 (95% CI 0.49–0.75) for 5–9 vs 1, P for trend < 0.0001]; and BMI at age 18: HR=0.67 (95% 0.47–0.95) for ≥ 27.5 kg/m2 vs < 18.5 kg/m2, P for trend = 0.009], after adjusting for age and body fatness measures earlier in life and other risk factors, respectively. No significant interaction between body fatness measures was found. Women with the lowest birthweight, the highest somatotype at ages 5 and 10 and the highest BMI at age 18 and currently had a 72% (95% CI 54%-83%) lower incidence of invasive premenopausal breast cancer than women with the opposite extreme of each body fatness indicator.
Conclusion: The lowest incidence of premenopausal breast cancer was associated with the lowest birthweight and the highest childhood, adolescent and early adult body fatness.
Keywords: Body fatness, premenopausal breast cancer, life course, birthweight, somatotype, body mass index, interaction
Key Messages
Lower birthweight, higher somatotype at ages 5 and 10 years and higher BMI at age 18 were each independently associated with a lower incidence of premenopausal breast cancer.
The inverse associations between current BMI and premenopausal breast cancer risk were no longer statistically significant after adjusting for body fatness indicators measured earlier in life.
No significant interaction between body fatness factors throughout the life course was found.
Women with the lowest birthweight, the highest somatotype at ages 5 and 10 and the highest BMI at age 18 and currently had a 72% lower incidence of premenopausal breast cancer than women with the opposite extreme of each of of these body fatness measures.
Introduction
There has been much interest in the relation between body fatness at different periods of life and the risk of breast cancer, especially premenopausal breast cancer. Observational studies have suggested that birthweight is positively associated with the risk of premenopausal breast cancer.1,2 Conversely, body fatness during childhood and adolescence is inversely related to the risk of premenopausal breast cancer3–9 as well as postmenopausal breast cancer,3,4,8,10,11 suggesting a long-term effect of body fatness at young age on breast cancer risk later in life. On the other hand, adult body fatness is inversely associated with the risk of premenopausal breast cancer,12 though positively associated with the risk of postmenopausal breast cancer.13–15 The mechanisms underlying these complex associations have not been elucidated.
A comprehensive understanding of the role of body fatness on the aetiology of breast cancer may need to account for the correlation and interaction of these factors throughout a woman's life course. As the role of body fatness for breast cancer risk changes during a woman's life course, it is of interest to determine the optimal weight at different stages of a woman's life that is associated with the lowest incidence of breast cancer, to target research for a better understanding of the mechanistic underpinning. In this study, we used data from Nurses’ Health Study II (NHSII) to systematically evaluate the potential independent as well as synergestic effect of body fatness at various periods of life from birth to ages 5, 10, 18 and throughout adult life on the risk of breast cancer among premenopausal women.
Methods
Study design and population
The NHSII is a prospective cohort study which was established in 1989, when 116 430 female registered nurses aged 25–42 years and living in one of the 14 US states responded to a baseline questionnaire about their medical histories, demographic factors and lifestyle factors. Questionnaires have been sent to NHSII participants biennially to update information on demographic, anthropometric and lifestyle factors as well as occurrence of diseases. This study was approved by the institutional review boards of the Harvard School of Public Health and the Brigham and Women's Hospital, Boston, MA.
Eligibility criteria and follow-up
The study population for the current study consists of 90 101 women participating in the NHSII who were premenopausal, free of cancer (except non-melanoma skin cancer) and reported birthweight in 1991. The study baseline was defined as 1991, since birthweight was first assessed in this year. Women were followed from completion of the 1991 questionnaire until the date of return of the 2009 questionnaire, a diagnosis of in situ or invasive breast cancer, death, reaching menopause or loss to follow-up, whichever occurred first.
Assessment of body fatness
In 1991, NHSII participants were asked to report their birthweight. Responses were requested in categories and options provided were < 2.5kg, 2.5 – 3.1kg, 3.2 – 3.8kg, 3.9 – 4.4kg, >=4.5kg and unknown. The validity of self-reported birthweight was evaluated in a sample of NHSII participants by comparing it with information provided by their mothers and abstracted from birth certificates; correlation coefficients between self-report and mothers’ recall, and between self-report and birth certificate were 0.75 and 0.74, respectively.16 For 3226 women whose birthweight information was missing and whose mother participated in the Nurses’ Mothers’ Cohort Study,17 birthweight data provided by the mother were used.
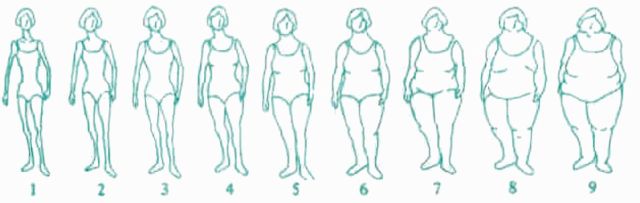
Somatotype at ages 5 and 10 years was assessed in 1989 by asking participants to choose from nine pictograms that which best depicted their figure outline at each age (Figure 1). In 1989, participants were also asked their weight at age 18 and adult height. Information on current weight was updated biennially through subsequent questionnaires from 1991 to 2007.
Figure 1.
Somatotype pictograms used to assess body fatness at ages 5 and 10 on the 1989 questionnaire of NHSII. Reprinted with permission from Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman SW, Mathysee SW (eds). The genetics of neurological and psychiatric disorders. Raven Press: New York; 1983. pp 115–20.
Assessment of breast cancer
On each biennial questionnaire, participants were asked whether they had been newly diagnosed with breast cancer during the previous 2 years and, if so, the date of diagnosis. Deaths were reported by family members or by the Postal Services in response to follow-up questionnaires. The National Death Index was also searched to investigate possible deaths of non-responders. When a case of breast cancer was reported, the participant (or next of kin for those who had died) was contacted to confirm the diagnosis and to obtain permission to obtain relevant hospital and pathology records. Medical records were obtained for 90% of cases in NHSII and pathology reports confirmed breast cancer in > 99% of women whose reports were reviewed. Because breast cancer was self-reported by the nurse participants with very high accuracy, cases for which medical records could not be obtained were included in the analyses. Only invasive incident cases of breast cancer were included in the analysis.
Assessment of other covariates
Information on other risk factors of breast cancer and potential confounders was assessed at baseline and/or throughout follow-up. Date of birth, age at menarche, family history of breast cancer [in mother or sister(s)], history of benign breast disease, parity, age at first birth, use of oral contraceptives, menopausal status, physical activity and alcohol consumption were assessed on the NHSII baseline questionnaire in 1989. Information on being born prematurely was collected in 1991. Data from subsequent questionnaires were used to biennially update information on current weight, history of benign breast disease, parity, age at first birth, use of oral contraceptives and menopausal status (from 1991 to 2007). Information on family history of breast cancer was updated in 1997, 2001 and 2005, physical activity in 1997 and 2005 and alcohol consumption in 1991, 1995, 1999, 2003 and 2007.
Statistical analysis
The incidence of breast cancer in relation to the various body fatness milestones throughout the life course was estimated using Cox proportional hazards models. To control as finely as possible for confounding by age, calendar time and any possible two-way interactions between these two time scales, we stratified the analysis jointly by age in months at start of follow-up and calendar year of the current questionnaire cycle. The time scale for the analysis was then measured as months since the start of the current questionnaire cycle, which is equivalent to age in months because of the way we structured the data and formulated the model for analysis.
Anthropometric measures included birthweight (< 5.5, 2.5kg, 2.5–3.1kg, 3.2–3.8kg, >=3.9kg), somatotype at ages 5 and 10 years (pictograms 1–9), BMI at age 18 (< 18.5, 18.5–19.9, 20–22.4, 22.5–24.9, 25–27.4 and ≥ 27.5 kg/m2) and current BMI (< 20, 20–22.4, 22.5–24.9, 25–27.4 and ≥ 27.5 kg/m2).
Missing data on each covariate were coded as indicator variables in the models. All exposure variables and covariates for which updated data are available were modelled in a time-varying manner using the most recent previous assessment. Four different covariate-adjusted models were considered: model I adjusted for age and early life factors (birthweight and premature birth); model II additionally adjusted for adult risk factors of breast cancer including family history of breast cancer, history of benign breast disease, age at menarche, parity, age at first birth, use of oral contraceptives, alcohol consumption, physical activity and body fatness factors earlier in life; model III additionally adjusted for other body fatness factors later in life; and model IV additionally adjusted for adult height.
These different models were selected to explore confounding and mediation. Since somatotype at age 5 and somatotype at age 10 were highly correlated (Spearman correlation coefficient = 0.81), these two factors were not adjusted for each other when one of the factors was assessed as the main exposure of interest. Since the effect of birthweight on breast cancer incidence is partially mediated by height,18 body fatness may be associated with height in children19–21 and height is an established risk factor of breast cancer,22,23 we included height only in model IV to investigate its potential mediating effect.
We assessed pair-wise interactions between the measures of body fatness throughout the life course in relation to breast cancer incidence by comparing a Cox proportional hazards model including only the main effect terms of indicator variables of each of the two body fatness variables with a model additionally including two-way interaction terms of indicator variables of the two body fatness variables, using a likelihood ratio test. Additional covariates were adjusted for in the analysis in two different covariate-adjusted models, to explore adjustment for confounding and improvement of model goodness-of-fit: model I only included covariates which occurred prior to both body fatness factors under evaluation; and model II additionally adjusted for all other risk factors of breast cancer.
To evaluate potential interaction of body fatness throughout the life course on the risk of premenopausal breast cancer, we calculated the hazard ratio associated with each pairwise combination of body fatness parameters throughout the life course. Hazard ratios were calculated using Cox proportional hazards models, including two-way interaction terms of the two corresponding body fatness parameters but no main effects, allowing calculation of hazard ratios for each combination of the categories of the two factors. The category with the largest sample size was selected as reference group. The analysis was adjusted for all assessed risk factors of breast cancer, including other body fatness factors.
The overall effect of being in the most favourable categories of body fatness throughout the life course, with respect to premenopausal breast cancer risk, was calculated as linear combination of β-values from a Cox proportional hazards model including all anthropometric variables assessed (no other covariates were included). The resulting value was exponentiated to generate the hazard ratio, and confidence intervals were calculated.
Results
A total of 1574 incident cases of premenopausal breast cancer were diagnosed during 1 133 893 patient-years of follow-up. A high Spearman correlation coefficient was found between somatotype at age 5 and somatotype at age 10 years (0.81) and somatotype at age 10 and BMI at age 18 (0.50) (Table 1). Low birthweight was associated with a reduced incidence of premenopausal breast cancer after adjusting for age and early life factors and other risk factors (HR = 0.74; 95% CI 0.58–0.95 comparing < 2.5kg with ≥ 3.9kg, P for trend < 0.001), and the association did not appreciably change after additional adjustment for body fatness later in life (Table 2). Relative to women who reported the leanest somatotype (pictogram 1), women who reported the heaviest somatotypes (pictograms 5–9) at ages 5 and 10 had a 43% (95% CI 27%-56%, P for trend < 0.0001) and 39% (95% CI 25%-51%, P for trend < 0.0001) lower incidence of premenopausal breast cancer, respectively, after adjusting for age and early life and adult risk factors for breast cancer. The association for both somatotype at age 5 and somatotype at age 10 were not changed substantially after additional adjustment for body fatness later in life and adult height. BMI at age 18 years ≥ 27.5 kg/m2 was associated with 33% (95% CI 5%-53%) lower incidence of premenopausal breast cancer relative to a BMI of < 18.5 kg/m2 (P for trend = 0.009) after adjusting for age and early life and adult risk factors. The association became weaker but remained significant after additional adjustment for body fatness factors later in life (P for trend = 0.02) but not after additional adjustment for height (P for trend = 0.06). Women with a high current BMI did not have a decreased incidence of breast cancer after adjusting for other early life and adult risk factors for breast cancer (HR = 0.84; 95% CI 0.67–1.06 comparing ≥ 27.5 kg/m2 with < 20 kg/m2, P for trend = 0.62) or after additionally adjusting for height (P for trend = 0.53).
Table 1.
Spearman correlation coefficient between measures of body fatness throughout the life course, NHSII 1991–2009
| Birthweight | Somatotype at age 5 | Somatotype at age 10 | BMI at age 18 | Current BMIa | |
|---|---|---|---|---|---|
| Birthweight | 1.00 | 0.11 | 0.08 | 0.06 | 0.03 |
| Somatotype at age 5 | – | 1.00 | 0.81 | 0.39 | 0.19 |
| Somatotype at age 10 | – | – | 1.00 | 0.50 | 0.27 |
| BMI at age 18 | – | – | – | 1.00 | 0.46 |
| Current BMI | – | – | – | – | 1.00 |
aCurrent BMI is defined by the updated value assessed in the most recent previous biennial visit.
Table 2.
Hazard ratios and 95% confidence intervals for premenopausal breast cancer by measures of body fatness throughout the life course, NHSII 1991–2009
| No. of person-years | No. of cases | Covariate-adjusted | Covariate-adjusted | Covariate-adjusted | Covariate-adjusted | |
|---|---|---|---|---|---|---|
| HR (95% CI) Ia | HR (95% CI) IIb | HR (95% CI) IIIc | HR (95% CI) IVd | |||
| Birthweight | ||||||
| <2.5kg | 89826 | 115 | 0.74 (0.58–0.94) | 0.74 (0.58–0.95) | 0.69 (0.54–0.89) | 0.76 (0.59–0.98) |
| 2.5–3.1kg | 361094 | 462 | 0.75 (0.64–0.89) | 0.75 (0.64–0.88) | 0.71 (0.60–0.84) | 0.76 (0.64–0.90) |
| 3.2–3.8kg | 545342 | 770 | 0.83 (0.71–0.96) | 0.83 (0.71–0.96) | 0.80 (0.69–0.93) | 0.82 (0.71–0.96) |
| 3.9+kg | 137630 | 227 | 1.0 | 1.0 | 1.0 | 1.0 |
| P for trend | 1133893 | 1574 | < 0.001 | < 0.001 | < 0.0001 | 0.003 |
| Somatotype at age 5 | ||||||
| Somatotype 1 | 258356 | 394 | 1.0 | 1.0 | 1.0 | 1.0 |
| Somatotype 2 | 363726 | 510 | 0.93 (0.82–1.06) | 0.93 (0.82–1.07) | 0.94 (0.82–1.08) | 0.94 (0.82–1.07) |
| Somatotype 3 | 273662 | 369 | 0.87 (0.75–1.00) | 0.86 (0.75–1.00) | 0.90 (0.78–1.05) | 0.90 (0.77–1.04) |
| Somatotype 4 | 143438 | 195 | 0.85 (0.72–1.02) | 0.85 (0.72–1.02) | 0.93 (0.77–1.12) | 0.92 (0.76–1.10) |
| Somatotype 5–9 | 73549 | 69 | 0.58 (0.45–0.75) | 0.57 (0.44–0.73) | 0.64 (0.49–0.84) | 0.63 (0.48–0.82) |
| P for trend | 1112731 | 1537 | < 0.0001 | < 0.0001 | 0.01 | 0.006 |
| Somatotype at age 10 | ||||||
| Somatotype 1 | 197453 | 310 | 1.0 | 1.0 | 1.0 | 1.0 |
| Somatotype 2 | 349384 | 508 | 0.94 (0.82–1.09) | 0.94 (0.81–1.08) | 0.96 (0.83–1.11) | 0.96 (0.83–1.11) |
| Sometotype 3 | 261139 | 371 | 0.91 (0.78–1.05) | 0.90 (0.77–1.04) | 0.95 (0.81–1.12) | 0.95 (0.80–1.11) |
| Somatotype 4 | 180008 | 231 | 0.80 (0.68–0.95) | 0.79 (0.66–0.94) | 0.87 (0.72–1.05) | 0.87 (0.72–1.05) |
| Somatotype 5–9 | 132173 | 134 | 0.62 (0.51–0.77) | 0.61 (0.49–0.75) | 0.70 (0.56–0.88) | 0.69 (0.55–0.86) |
| P for trend | 1120155 | 1554 | < 0.0001 | < 0.0001 | 0.003 | 0.002 |
| BMI at age 18 (kg/m2) | ||||||
| < 18.5 | 162050 | 238 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18.5–19.9 | 280418 | 434 | 1.05 (0.89–1.22) | 1.09 (0.92–1.28) | 1.10 (0.93–1.29) | 1.12 (0.95–1.32) |
| 20–22.4 | 409172 | 571 | 0.93 (0.80–1.09) | 1.00 (0.85–1.17) | 1.01 (0.85–1.20) | 1.05 (0.88–1.24) |
| 22.5–24.9 | 161060 | 205 | 0.85 (0.70–1.02) | 0.94 (0.76–1.15) | 0.96 (0.77–1.19) | 1.00 (0.80–1.24) |
| 25–27.4 | 63661 | 68 | 0.71 (0.54–0.92) | 0.79 (0.59–1.06) | 0.81 (0.60–1.09) | 0.83 (0.62–1.13) |
| ≥ 27.5 | 49487 | 42 | 0.58 (0.41–0.80) | 0.67 (0.47–0.95) | 0.69 (0.48–0.99) | 0.72 (0.50–1.03) |
| P for trend | 1125846 | 1558 | < 0.0001 | 0.009 | 0.02 | 0.06 |
| Current BMI (kg/m2) e | ||||||
| < 20 | 65864 | 109 | 1.0 | 1.0 | 1.0 | 1.0 |
| 20–22.4 | 209205 | 271 | 0.75 (0.60–0.94) | 0.78 (0.62–0.99) | 0.78 (0.62–0.99) | 0.78 (0.62–0.99) |
| 22.5–24.9 | 238623 | 367 | 0.86 (0.69–1.06) | 0.91 (0.73–1.14) | 0.91 (0.73–1.14) | 0.91 (0.73–1.14) |
| 25–27.4 | 197505 | 283 | 0.79 (0.63–0.99) | 0.87 (0.69–1.10) | 0.87 (0.69–1.10) | 0.87 (0.69–1.09) |
| ≥ 27.5 | 422696 | 544 | 0.70 (0.57–0.86) | 0.84 (0.67–1.06) | 0.84 (0.67–1.06) | 0.83 (0.66–1.05) |
| P for trend | 1133893 | 1574 | 0.003 | 0.62 | 0.62 | 0.53 |
aAdjusted for age (continuous), premature birth (< 38 weeks, ≥ 38 weeks) and birthweight (except in the analysis of birthweight).
bAdditionally adjusted for family history of breast cancer (yes, no), history of benign breast disease (yes, no), age at menarche (≤ 10 years, 11 years, 12 years, 13 years, 14 years, ≥ 15 years), interaction between parity (0, 1, 2, 3, 4, ≥ 5) and age at first birth (< 24 years, 25–30 years, > 30 years) with nulliparous women as reference, use of oral contraceptives (never, past and < 5 years, past and ≥ 5 years, current and < 5 years, current and 5–9 years, current and ≥ 10 years), alcohol consumption (never, < 7.5 g/day, 7.5–14 g/day, 15–29 g/day, ≥ 30 g/day), physical activity (< 3 mets/week, 4–8 mets/day, 9–17 mets/day, 18–26 mets/day, 27–41 mets/day, ≥ 42 mets/day) and body fatness factors earlier in life (somatotype or BMI). Since somatotype at age 5 and somatotype at age 10 were highly correlated (Spearman correlation coefficient = 0.81), these two factors were not adjusted for each other when one of the factors was assessed as the main exposure of interest.
cAdditionally adjusted for body fatness factors (somatotype or BMI) later in life.
dAdditionally adjusted for height (continuous in inches).
eCurrent BMI is defined by the updated value assessed in the most recent previous biennial visit.
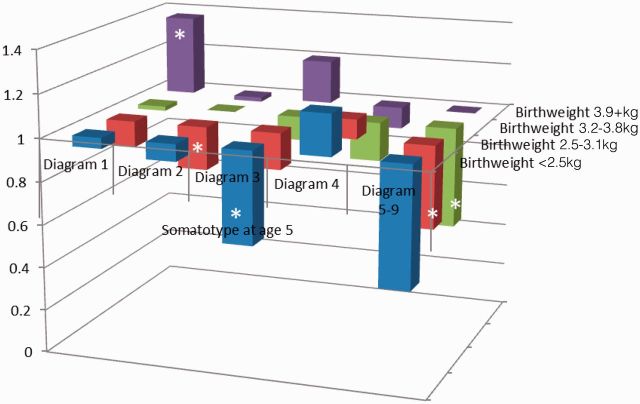
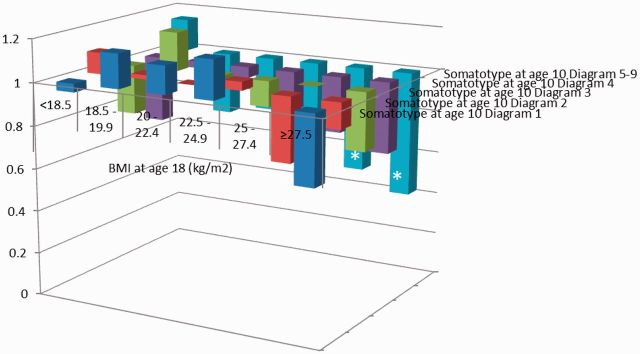
The likelihood ratio tests did not reveal any significant interactions between body fatness measures throughout the life course. Adjustment for risk factors of breast cancer including other body fatness variables did not alter the results. When hazard ratios for premenopausal breast cancer incidence were cross-classified by two body fatness indicators, the lowest incidence of premenopausal breast cancer was found for women with the lowest birthweight and the highest body fatness in childhood, adolescence and early adulthood. When birthweight and body fatness at age 5 were considered, women with a birthweight < 2.5kg and childhood somatotypes pictograms 5–9 had the lowest incidence of premenopausal breast cancer, whereas women with a birthweight of ≥ 3.9kg and somatotype pictogram 1 had the highest incidence relative to women with a birthweight of 3.2–3.8kg and somatotype 2 (Figure 2.1). Similar trends were observed with cross-classifications of birthweight with body fatness in adolescence, at age 18, and in early adulthood. When childhood somatotypes at age 10 were cross-classified with BMI at age 18, the incidence of premenopausal breast cancer among women with the highest body fatness at both time points was about half of the incidence of women with somatotype 2 and BMI at age 18 of 20–22.4 kg/m2 (Figure 2.2). Similar associations were observed for the cross-classification involving childhood body fatness at age 5 and current BMI.
Figure 2.1.
Hazard ratios of premenopausal breast cancer by birthweight and somatotype at age 5, NHS II 1991–2009.
*Hazard ratio compared with the reference group (birthweight of 3.2–3.8kg and diagram (2) is statistically significant at alpha = 0.05.The analysis was adjusted for age, premature birth, age at menarche, height, current BMI, BMI at age 18, family history of breast cancer, history of benign breast disease, parity and age at first birth, oral contraceptive use, alcohol consumption and physical activity.
Figure 2.2.
Hazard ratios of premenopausal breast cancer by somatotype at age 10 and BMI at age 18, NHS II 1991–2009.
*Hazard ratio compared with the reference group (somatotype at age 10 diagram 2 and BMI at age 18 of 20–22.4 kg/m2) is statistically significant at alpha = 0.05.The analysis was adjusted for age, birthweight, premature birth, age at menarche, height, current BMI, family history of breast cancer, history of benign breast disease, parity, age at first birth, oral contraceptive use, alcohol consumption and physical activities.The incidence rate is not estimable for the group with somatotype at age 10 ranked as 1 and BMI at age 18 of 25–27.4 kg/m2 due to sparse sample size.
The multivariate Cox proportional hazards model for hazard ratios of premenopausal breast cancer including all body fatness factors is shown in Table 3. The hazard ratio of premenopausal breast cancer associated with selected lifetime trajectories of body fatness factors are summarized in Table 4. Considering all body fatness measures throughout the life course, women with a birthweight of < 2.5kg, childhood somatotypes 5–9 at ages 5 and 10 and a BMI at age 18 and throughout adulthood of 27.5 kg/m2 had a 72% (95% CI 54%-83%) lower incidence of premenopausal breast cancer compared with women with a birthweight of 3.9+kg, childhood somatotype 1 at ages 5 and 10, a BMI at age 18 of < 18.5 kg/m2 and an adult BMI of < 20 kg/m2.
Table 3.
Covariate-adjusted Cox proportional hazards model for hazard ratios of premenopausal breast cancer with all body fatness factors included
| DF | Parameter estimate | Standard error | P-value | IRR | 95% CI | |
|---|---|---|---|---|---|---|
| Birthweight | ||||||
| 3.2–3.8kg | 1 | −0.1838 | 0.0766 | 0.020 | 0.83 | (0.72–0.97) |
| 2.5–3.1kg | 1 | −0.3003 | 0.0826 | < 0.001 | 0.74 | (0.63–0.87) |
| <2.5kg | 1 | −0.3083 | 0.1171 | < 0.010 | 0.74 | (0.58–0.92) |
| Somatotype at age 5 | ||||||
| Diagram 2 | 1 | −0.0886 | 0.0955 | 0.350 | 0.92 | (0.76–1.10) |
| Diagram 3 | 1 | −0.0933 | 0.1170 | 0.430 | 0.91 | (0.72–1.15) |
| Diagram 4 | 1 | 0.0426 | 0.1341 | 0.750 | 1.04 | (0.80–1.36) |
| Diagrams 5–9 | 1 | −0.2035 | 0.1792 | 0.260 | 0.82 | (0.57–1.16) |
| Somatotype at age 10 | ||||||
| Diagram 2 | 1 | 0.0083 | 0.1031 | 0.940 | 1.01 | (0.82–1.23) |
| Diagram 3 | 1 | 0.0296 | 0.1273 | 0.820 | 1.03 | (0.80–1.32) |
| Diagram 4 | 1 | −0.0933 | 0.1411 | 0.510 | 0.91 | (0.69–1.20) |
| Diagrams 5–9 | 1 | −0.2359 | 0.1613 | 0.140 | 0.79 | (0.58–1.08) |
| BMI at age 18 (kg/m2) | ||||||
| 18.5–19.9 | 1 | 0.0778 | 0.0838 | 0.350 | 1.08 | (0.92–1.27) |
| 20–22.4 | 1 | −0.0151 | 0.0860 | 0.860 | 0.99 | (0.83–1.17) |
| 22.5–24.9 | 1 | −0.0868 | 0.1092 | 0.420 | 0.92 | (0.74–1.14) |
| 25–27.4 | 1 | −0.2378 | 0.1520 | 0.120 | 0.79 | (0.59–1.06) |
| ≥ 27.5 | 1 | −0.4079 | 0.1838 | 0.030 | 0.67 | (0.46–0.95) |
| Current BMI (kg/m2) | ||||||
| 20–22.4 | 1 | −0.2372 | 0.1164 | 0.040 | 0.79 | (0.63–0.99) |
| 22.5–24.9 | 1 | −0.0503 | 0.1134 | 0.660 | 0.95 | (0.76–1.19) |
| 25–27.4 | 1 | −0.0907 | 0.1180 | 0.440 | 0.91 | (0.73–1.15) |
| ≥ 27.5 | 1 | −0.1161 | 0.1142 | 0.310 | 0.89 | (0.71–1.11) |
Table 4.
Hazard ratio of premenopausal breast cancer associated with selected trajectories of lifetime body fatness factors
| Birthweight | Somatotype at age 5 | Somatotype at age 10 | BMI at age 18 (kg/m2) | Current BMI (kg/m2) | Hazard ratio | 95% CI |
|---|---|---|---|---|---|---|
| >=3.9kg | Diagram 1 | Diagram 1 | < 18.5 | < 20 | 1.00 | – |
| <2.5kg | Diagram 5–9 | Diagram 5–9 | ≥ 27.5 | ≥27.5 | 0.28 | (0.17–0.46) |
| 2.5–3.1kg | Diagram 4 | Diagram 4 | 25–27.4 | 25–27.4 | 0.51 | (0.33–0.77) |
| 3.2–3.8kg | Diagram 3 | Diagram 3 | 22.5–24.9 | 22.5–24.9 | 0.68 | (0.48–0.96) |
| 3.2–3.8kg | Diagram 2 | Diagram 2 | 20–22.4 | 20–22.4 | 0.60 | (0.43–0.83) |
| 3.2–3.8kg | Diagram 2 | Diagram 2 | 18.5–19.9 | 20–22.4 | 0.65 | (0.47–0.92) |
Discussion
In this large premenopausal cohort of the NHSII, we observed the lowest incidence of breast cancer among women in the lowest birthweight category and the highest categories of childhood, adolescent and early adult body fatness. We did not observe any multiplicative interaction between the various measures of body fatness assessed, but we found a statistically significant decrease in premenopausal breast cancer risk among women with low birthweight and high body fatness in childhood through early adulthood, as well as a statistically significant increase in premenopausal breast cancer incidence among women with high birthweight and low body fatness in childhood through early adulthood. Furthermore, when body fatness indicators throughout the life course were assessed simultaneously, women with the lowest birthweight, and highest body fatness throughout childhood and adulthood had the lowest incidence of breast cancer.
Results from this study are consistent with previous reports on higher birthweight associated with a higher risk of premenopausal breast cancer1,2 and higher body fatness at young ages linked with a lower risk of premenopausal breast cancer.3–9,11,12 However, no previous study has considered the linear combination of body fatness measures throughout the life course on breast cancer risk, to quantify the maximum protective effect body size throughout the life course can have for the risk of breast cancer. Given the highly complex effect of individual body fatness measures at various points of a woman's life course, this analysis provides estimation of the overall effect of all body fatness measures combined. Nonetheless, it is worth noting that the optimal body fatness curve for breast cancer prevention does not necessarily coincide with the optimal body fatness curve for prevention of most other chronic diseases.
Additional adjustment for body fatness factors later in life as well as other risk factors of breast cancer, including adult height, did not materially change the positive association of premenopausal breast cancer with birthweight. This suggests that the potential effect of birthweight on the risk of premenopausal breast cancer is not likely mediated through altering body fatness, reproductive factors or other risk factors later in life. Similarly, the significant negative association of premenopausal breast cancer with somatotype at age 5, somatotype at age 10 and BMI at age 18, after adjusting for all other body fatness factors and risk factors, suggested an independent effect of body fatness from childhood and adolescence to age 18 years on the risk of premenopausal breast cancer. This is not likely confounded by body fatness earlier in life or mediated by altering reproductive factors, body fatness or other measured risk factors later in life. On the other hand, the association of current BMI with premenopausal breast cancer incidence was attenuated after adjusting for body fatness earlier in life (except birthweight), suggesting potential confounding by these risk factors. Since current BMI and body fatness in childhood and early adulthood are correlated, and these earlier body fatness measures are upstream of current BMI in the causal pathway, it is likely that current BMI does not have an effect on premenopausal breast cancer independent of these earlier body fatness measures.
Results from this study did not replicate findings from a previous analysis within the Nurses’ Health Study and NHSII combined, suggesting a statistically significant interaction between body fatness at ages 10–20 years and birthweight (< vs > = 3.9kg) in their association with the risk of breast cancer;11 however, the previous analysis combined pre- and postmenopausal breast cancer. Whereas we also found high childhood and early adulthood body fatness associated with a decreased breast cancer incidence after stratifying the analysis by categories of birthweight, the associations were not restricted to lower birthweight categories. The discrepancy in the results may be due to the restriction of the current study to premenopausal women. Although the association with somatotype does not differ by menopausal status,11 our previous studies have suggested that birthweight is more strongly associated with premenopausal than postmenopausal breast cancer,1,2 suggesting different roles of birthweight in the aetiology of premenopausal vs postmenopausal breast cancer, which may include modifying the effect of childhood body fatness. Few other studies had sufficient statistical power to assess potential interaction of multiple body fatness factors during women's life course on the risk of breast cancer. Among studies which simultaneously assessed two or more lifetime anthropometric factors, premenopausal breast cancer risk was inversely associated with body fatness in childhood,24–29 adolescence 28–30 and early adulthood 12,27–28,30–35 in some but not other studies.36–40
The mechanisms underlying the link between birthweight and breast cancer have not been completely elucidated, but several factors have been proposed to contribute to this association.1 Besides the possible role of intrauterine exposure to growth hormones, in particular insulin-like growth factor 2 (IGF-2), IGF-1, growth factor and estrogen, epigenomic imprinting may also be important.2 The puzzling inverse association between premenopausal breast cancer and several body fatness indices beore the menopause has been traced to hormonal and metabolic factors. Body fatness during childhood has been associated with slower adolescent growth, whereas peak height growth velocity as a measure of adolescent growth was associated with an increased risk of premenopausal breast cancer.41 On the other hand, obesity in pre-adolescent and adolescent girls is also related to higher insulin42 and androgen levels.43 Women with a high BMI in early adulthood have lower levels of sex hormone-binding globulin (SHBG), follicular estradiol, luteal estradiol and progesterone, and higher levels of free testosterone than normal weight women.44,45 Increased insulin signalling, decreased SHBG and increased endogenous levels of free estrogen, androgen and testosterone have been associated with a higher risk of premenopausal breast cancer.46,47 Endogenous progesterone has been hypothesized to be associated with a higher risk of breast cancer, but no consistent evidence has been provided.47–50 Though these hormonal links are not directly supporting all findings from the current study, they suggest independent pathways for body mass at different times throughout the life course to affect the breast cancer risk potentially through altering women's hormonal profile.
Our study comprises a large sample size and long duration of follow-up, permitting assessment of the aetiology of diseases such as breast cancer which has a long latency period. The comprehensive assessment of various lifetime measures of body fatness and risk factors for breast cancer provides a unique opportunity to evaluate the role of body fatness in relation to breast cancer throughout the life course, while accounting for various potential confounding variables. Since information on birthweight, childhood and adolescent somatotype and weight at age 18 was collected through recall, some misclassification is likely but, due to the prospective nature of the study design and analysis, is likely non-differential and attenuates some of the associations.
In conclusion, based on 18 years of follow-up among premenopausal women in the NHSII, we observed the lowest incidence of premenopausal breast cancer among women in the lowest birthweight category and the highest categories of childhood, adolescent and early adulthood weight. Elucidating hormonal, metabolic and other factors associated with body fatness at different stages throughout the life course may help shed light on the mechanistic underpinnings of breast cancer initiation and promotion.
Funding
This work was supported by Public Health Service grant R01 CA114326 from the National Cancer Institute, National Institutes of Health (to K.B.M.). The Nurses’ Health Study II is supported by Public Health Service grant UM1 CA176726 from the National Cancer Institute, National Institutes of Health.
Conflict of interest: None.
References
- 1. Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer 2006;119:2007–25. [DOI] [PubMed] [Google Scholar]
- 2. Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol 2007;8:1088–100. [DOI] [PubMed] [Google Scholar]
- 3. Magnusson C, Baron J, Persson I, et al. Body size in different periods of life and breast cancer risk in post-menopausal women . Int J Cancer 1998;76:29–34. [DOI] [PubMed] [Google Scholar]
- 4. Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer 1999;85:2400–09. [DOI] [PubMed] [Google Scholar]
- 5. Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1121–27. [PubMed] [Google Scholar]
- 6. Baer HJ, Colditz GA, Rosner B, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res 2005;7:R314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verla-Tebit E, Chang-Claude J. Body fatness factors and the risk of premenopausal breast cancer in Germany. Eur J Cancer Prev 2005;14:419–26. [DOI] [PubMed] [Google Scholar]
- 8. Palmer JR, Adams-Campbell LL, Boggs DA, et al. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 2007;16:1795–802. [DOI] [PubMed] [Google Scholar]
- 9. Oh H, Boeke CE, Tamimi RM, et al. The interaction between early life body size and physical activity on risk of breast cancer. Int J Cancer 2015;137:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bardia A, Vachon CM, Olson JE, et al. Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2008;17:374–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 2010;171:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med 2006;166:2395–402. [DOI] [PubMed] [Google Scholar]
- 13. Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev 1993;15:110–32. [DOI] [PubMed] [Google Scholar]
- 14. Magnusson CM, Roddam AW, Pike MC, et al. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer 2005;93:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Eliassen AH, Tamimi RM, et al. Adult body size and physical activity in relation to risk of breast cancer according to tumor androgen receptor status. Cancer Epidemiol Biomarkers Prev 2015;24:962–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troy LM, Michels KB, Hunter DJ, et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol 1996;25:122–27. [DOI] [PubMed] [Google Scholar]
- 17. Michels KB, Willett WC, Graubard BI, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes (Lond) 2007;31:1078–85. [DOI] [PubMed] [Google Scholar]
- 18. Michels KB, Xue F, Terry KL, Willett WC. Longitudinal study of birthweight and the incidence of breast cancer in adulthood. Carcinogenesis 2006;27:2464–68 [DOI] [PubMed] [Google Scholar]
- 19. Freedman DS, Khan LK, Mei Z, Dietz WH, Srinivasan SR, Berenson GS. Relation of childhood height to obesity among adults: the Bogalusa Heart Study. Pediatrics 2002;109:e23. [DOI] [PubMed] [Google Scholar]
- 20. Buchan IE, Bundred PE, Kitchiner DJ, Cole TJ. Body mass index has risen more steeply in tall than in short 3-year olds: serial cross-sectional surveys 1988–2003. Int J Obes (Lond) 2007;31:23–29. [DOI] [PubMed] [Google Scholar]
- 21. Ralt D. The muscle-fat duel or why obese children are taller? BMC Pediatr 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willett WC, Tamimi R, Hankinson SE, Hazra A, Eliassen AH, Colditz GA. Nongenetic factors in the causation of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK. (eds). Diseases of the Breast. 5th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 23. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- 24. De Stavola BL, dos Santos Silva I, McCormack V, Hardy RJ, Kuh DJ, Wadsworth ME. Childhood growth and breast cancer. Am J Epidemiol 2004;159:671–82. [DOI] [PubMed] [Google Scholar]
- 25. Brinton LA, Swanson CA. Height and weight at various ages and risk of breast cancer. Ann Epidemiol 1992;2:597–609. [DOI] [PubMed] [Google Scholar]
- 26. Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1121–27. [PubMed] [Google Scholar]
- 27. Swerdlow AJ, De Stavola BL, Floderus B, et al. Risk factors for breast cancer at young ages in twins: an international population-based study. J Natl Cancer Inst 2002;94:1238–46. [DOI] [PubMed] [Google Scholar]
- 28. Sangaramoorthy M, Phipps AI, Horn-Ross PL, Koo J, John EM. Early life factors and breast cancer risk in Hispanic women: the role of adolescent body size. Cancer Epidemiol Biomarkers Prev 2011;20:2572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol 2011;174:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bandera EV, Chandran U, Zirpoli G, et al. Body size in early life and breast cancer risk in African American and European American women. Cancer Causes Control 2013;24:2231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le Marchand L, Kolonel LN, Myers BC, Mi MP. Birth characteristics of premenopausal women with breast cancer. Br J Cancer 1988;57:437–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. John EM, Sangaramoorthy M, Hines LM, et al. Body size throughout adult life influences postmenopausal breast cancer risk among hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomarkers Prev 2015;24:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai M, Minami Y, Kuriyama S, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer 2010;103:1443–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peacock SL, White E, Daling JR, Voigt LF, Malone KE. Relation between obesity and breast cancer in young women. Am J Epidemiol 1999;149:339–46. [DOI] [PubMed] [Google Scholar]
- 35. Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1121–27. [PubMed] [Google Scholar]
- 36. Sanderson M, Shu XO, Jin F, et al. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer 2002;86:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayberry RM. Age-specific patterns of association between breast cancer and risk factors in black women, ages 20 to 39 and 40 to 54. Ann Epidemiol 1994;4:205–13. [DOI] [PubMed] [Google Scholar]
- 38. Hirose K, Tajima K, Hamajima N, et al. Association of family history and other risk factors with breast cancer risk among Japanese premenopausal and postmenopausal women. Cancer Causes Control 2001;12:349–58. [DOI] [PubMed] [Google Scholar]
- 39. Shu XO, Jin F, Dai Q, et al. Association of body size and fat distribution with risk of breast cancer among Chinese women. Int J Cancer 2001;94:449–55. [DOI] [PubMed] [Google Scholar]
- 40. Wenten M, Gilliland FD, Baumgartner K, Samet JM. Associations of weight, weight change, and body mass with breast cancer risk in Hispanic and non-Hispanic white women. Ann Epidemiol 2002;12:435–34. [DOI] [PubMed] [Google Scholar]
- 41. Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer 1999;85:2400–09 [DOI] [PubMed] [Google Scholar]
- 42. Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol 1995;269:E118–26. [DOI] [PubMed] [Google Scholar]
- 43. Baer HJ, Colditz GA, Willett WC, et al. Adiposity and sex hormones in girls. Cancer Epidemiol Biomarkers Prev 2007;16:1880–88. [DOI] [PubMed] [Google Scholar]
- 44. Tworoger SS, Eliassen AH, Missmer SA, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev 2006;15:2494–501. [DOI] [PubMed] [Google Scholar]
- 45. Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst 1996;88:756–58. [DOI] [PubMed] [Google Scholar]
- 46. Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr 2007;86:s823–35. [DOI] [PubMed] [Google Scholar]
- 47. Endogenous Hormones and Breast Cancer Collaborative Group: Key TJ, Appleby PN, Reeves GK, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 2013;14:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev 1994;18:79–85. [PubMed] [Google Scholar]
- 49. Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer 1997;75:1075–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women . J Natl Cancer Inst 2004;96:1856–65. [DOI] [PubMed] [Google Scholar]