Abstract
Background
Research from our laboratory, and that of other investigators, has demonstrated augmented levels of diacylglycerols (DAG) in the frontal cortex and plasma of subjects with Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI). We have extended these observations to investigate the frontal cortex of subjects with Parkinson’s disease (PD) and Lewy Body Disease (LBD), with and without coexisting pathologic features of AD.
Methods/Principal findings
Utilizing a high-resolution mass spectrometry analytical platform, we clearly demonstrate that DAG levels are significantly increased in the frontal cortex of subjects with PD, LBD with intermediate neocortical AD neuropathology, and in LBD with established neocortical AD neuropathology. In the case of the PD cohort, increases in cortical DAG levels were detected in cases with no neocortical pathology but were greater in subjects with neocortical pathology. These data suggest that DAG changes occur early in the disease processes and are amplified as cortical dysfunction becomes more established.
Conclusions
These findings suggest that altered DAG synthesis/metabolism is a common feature of neurodegenerative diseases, characterized by proteinopathy, that ultimately result in cognitive deficits. With regard to the mechanism responsible for these biochemical alterations, selective decrements in cortical levels of phosphatidylcholines in LBD and PD suggest that augmented degradation and/or decreased synthesis of these structural glycerophospholipids may contribute to increases in the pool size of free DAGs. The observed augmentation of DAG levels may be phospholipase-driven since neuroinflammation is a consistent feature of all disease cohorts. If this conclusion can be validated it would support utilizing DAG levels as a biomarker of the early disease process and the investigation of early intervention with anti-inflammatory agents.
Introduction
Extensive lipidomics evaluations of subjects with Alzheimer’s disease (AD) have demonstrated alterations in the levels of glycerophospholipids, sphingolipids, and diacylglycerols (DAG) in plasma and brain [1–3]. The most unique and consistent observation of these lipidomics studies is the elevation of DAG levels in AD frontal cortex [4–7] and mixed dementia temporal cortex [8]. Similarly DAG levels are augmented in the plasma of AD [9–11] and MCI [11] subjects, and appear to occur in a subset of patients encompassing 25 to 30% of MCI subjects and 40 to 45% of AD subjects [11].
Elevated DAG levels have also been reported from a lipidomics analysis of the visual cortex from 10 sporadic PD cases [12]. As is the case with MCI and AD, PD is characterized by a proteinopathy and usually proceeds to cognitive dysfunction [13]. While AD pathology involves amyloid plaques and neurofibrillary tangles, PD and Lewy body disease (LBD) are characterized by alpha-synuclein protein aggregates that form Lewy bodies [14–15]. As has been observed in AD, there are dramatic decrements in cortical cholinergic pathways [16–18] and cortical thinning [19–20], which presumably reflect processes of cognitive dysfunction in PD and LBD. However, post-mortem studies have demonstrated that many subjects have multiple proteinopathies [21] and that in the case of synucleinopathies, as in AD, there is a great deal of phenotypic heterogeneity [22–23] including various coexisting pathologic features of AD. Therefore, we extended our lipidomics evaluations of altered DAG metabolism in proteinopathies to PD and LBD subjects. Specifically, the cases were classified into 4 groups based on extensive neuropathological evaluations. These included a control group, a LBD group with early to intermediate-stage AD pathology (LBD-I-AD), a LBD group with established neocortical AD pathology (LBD-AD), and a PD group in which our investigation did not detect neocortical Lewy bodies or neurofibrillary tangles of AD.
Materials and methods
Study samples
Frontal cortex samples, bio banked at the Oregon Brain Bank, were from volunteer subjects who were evaluated at Oregon Health and Science University. IRB approval was from the OHSU Research Integrity Office (approval IRB1623). Postmortem samples were fully anonymized at the time of accession and informed consent was obtained before autopsy from the next of kin for all areas of research. Patients themselves were expired and did not provide consent. Frontal lobe tissue was flash frozen and stored at -80° C for biochemical studies. Tissue for morphologic evaluation was taken from all cortical lobes bilaterally, the anterior cingulate gyrus, hippocampus, amygdala, bilateral striatum and thalamus, midbrain, pons, medulla, and cerebellum as previously described [24]. In brief, sections from these regions were stained with hematoxalin and eosin with Luxol fast blue myelin stain. A modified Bielschowski silver impregnation method [25] as well as immunohistochemical staining for β-amyloid (Biolegend, San Diego, 4G8 antibody to β-amyloid amino acids 17–24) was used to identify diffuse and neuritic plaques in frontal and parietal cortices, hippocampus, basal ganglia, and cerebellum. Tau immunohistochemistry was performed on the hippocampus, frontal cortex, and occipital cortex using PHF1 antibody (a gift from Dr. Peter Davies, Albert Einstein College of Medicine, Manhasset, NY). The presence of Lewy bodies was determined by anti-alpha-synuclein immunohistochemistry using LB509 antibody (Covance, Princeton, NJ) performed on sections of midbrain, amygdala, hippocampus, and frontal cortex. Brain tissue was evaluated by a neuropathologist for features of neurodegenerative disease, which were identified and staged as previously reported and according to the NACC’s uniform data collection. Table 1 summarizes the patient demographics and neuropathologic features.
Table 1. Patient demographic and neuropathologic features of control, Lewy body disease with intermediate Alzheimer’s pathology (LBD-I-AD), Lewy body disease with Alzheimer’s pathology (LBD-AD), and Parkinson’s disease (PD) subjects.
PMI (post-mortem interval).
| Parameter | Controls | LBD-I-AD | LBD-AD | PD |
|---|---|---|---|---|
| N | 43 | 20 | 29 | 15 |
|
Age (Yrs ± SD) [Range] |
67.1 ± 8.4 [55–83] |
80.9 ± 10.5 [67–90] |
74.3 ± 8.8 [61–93] |
82.8 ± 9.5 [70–100] |
| Gender (% Males) | 60.5 | 65.0 | 62.1 | 73.3 |
|
PMI (Hrs ± SD) [Range] |
25.3 ± 16.9 [3–72] |
14.0 ± 15.1 [3–41] |
20.9 ± 27.18 [2–120] |
22.2 ± 25.4 [2–96] |
| Neocortical Plaques | Absent | Present | Present | Variable |
| Neocortical Neurofibrillary Tangles | Absent | III-IV | V-VI | Absent |
| Midbrain Lewy bodies | Absent | Present | Present | Present |
| Neocortical Lewy bodies | Absent | Present | Present | Absent |
Lipid extraction and analysis
Lipids were extracted from 20 to 40 mg of brain tissue with methyl-tert-butyl ether and methanol (1 mL methanol, 1 mL distilled water, and 2 mL methyl-tert-butyl ether) containing stabled isotope standards as described previously [5–7, 9, 11, 25–26]. Extracts were dried by centrifugal vacuum evaporation and dissolved in isopropanol: methanol: chloroform (4:2:1) containing 15 mM ammonium acetate. Constant infusion lipidomics (10 μL per min) were performed utilizing high-resolution (140,000; 0.3 to 3 ppm mass error) data acquisition on an orbitrap mass spectrometer (Thermo Q Exactive). Washes (500 μL) with methanol followed by hexane/ethyl acetate: chloroform (3:2:2), between samples, were used to minimize ghost effects.
In positive ion ESI, the cations of choline plasmalogens, phosphatidylcholines, sphingomyelins, ceramides, galactosylceramides, lactosylceramides, and the ammonium adducts of diacylglycerols (DAG) were quantitated. The cation of bromocriptine was used to monitor for potential mass axis drift. In negative ion ESI, the anions of ethanolamine plasmalogens, sulfatides, phosphatidylserines, phosphatidylethanolamines, phosphatidylglycerols, phosphatidylinositols, and lysophosphatidylglycerols were monitored. The anion of bromocriptine was used to monitor for potential mass axis drift.
The masses and the MS2 validation data for critical lipids are summarized in Table 2.
Table 2. Exact masses and MS2 data for reported lipids.
| Lipid | Exact | Calc. Cation | ppm | Product | Energy | Calc. Cation | ppm |
| Cer d18:1/18:0 | 565.5434 | 566.5507 | 1.7 | d18:1 base | 25 | 264.26913 | 3.1 |
| Gal-Cer d18:1/24:1 | 809.6744 | 810.6817 | 2.5 | d18:1 base | 20 | 264.26913 | 3.1 |
| [MH-Gal] | 15 | 630.61832 | 0.36 | ||||
| Lac-Cer d18:1/18:1 | 887.6334 | 888.6407 | 2.1 | d18:1 base | 20 | 264.26913 | 3.4 |
| [MH-Lac] | 15 | 546.5245 | 1.8 | ||||
| Lipid | Exact | Calc. Anion | ppm | Product | Energy | Calc. Anion | ppm |
| Sulfatide d18:1/24:0 | 891.6469 | 890.6397 | 0.5 | HSO4 | 50 | 96.9595 | 1.1 |
| Sulfatide d18:1/24:1 | 889.6313 | 888.6240 | 1.7 | HSO4 | 50 | 96.9595 | 0.9 |
| Sulfatide d18:1/25:0 | 905.6626 | 904.6583 | 1.8 | HSO4 | 50 | 96.9595 | 1.2 |
| Sulfatide d18:1/26:1 | 917.6625 | 916.6553 | 1.8 | HSO4 | 50 | 96.9595 | 1.0 |
| Lipid | Exact | Calc. Cation | ppm | Product | Energy | Calc. Cation | ppm |
| DAG 34:1 (16:0/18:1) | 594.5223 | 612.5567 | 2.6 | [M-sn1] | 25 | 339.2894 | 0.88 |
| [M-sn2] | 25 | 313.2737 | 1.2 | ||||
| DAG 36:1 (18:0/18:1) | 622.5536 | 640.5880 | 2.1 | [M-sn1] | 25 | 339.2894 | 0.87 |
| [M-sn2] | 25 | 341.3050 | 0.58 | ||||
| DAG 36:2 (18:1/18:1) | 620.5379 | 638.5724 | 2.1 | [M-sn1] | 25 | 339.2894 | 0.92 |
| [M-sn2] | 25 | 339.2894 | 0.92 | ||||
| DAG 36:4 (16:0/20:4) | 616.5067 | 634.5411 | 3.0 | [M-sn1] | 25 | 361.2738 | 1.3 |
| [M-sn2] | 25 | 313.2738 | 1.5 | ||||
| DAG 38:6 (16:0/22:6) | 640.5066 | 658.5410 | 2.7 | [M-sn1] | 25 | 385.2737 | 0.77 |
| [M-sn2] | 25 | 313.2737 | 1.5 | ||||
| PlsC 32:0 | 717.5672 | 718.5745 | 2.1 | Phosphocholine | 25 | 184.0474 | 1.2 |
| PlsC 34:0 | 745.5981 | 746.6088 | 1.8 | Phosphocholine | 25 | 184.0474 | 1.1 |
| PtdC 34:5 | 751.5152 | 752.5225 | 2.7 | Phosphocholine | 25 | 184.0474 | 1.0 |
| PtdC 36:5 | 779.5465 | 780.5538 | 2.6 | Phosphocholine | 25 | 184.0474 | 0.8 |
| PtdC 38:5 | 807.5778 | 808.5851 | 3.4 | Phosphocholine | 25 | 184.0474 | 0.9 |
| PtdC 38:6 | 805.5621 | 806.5694 | 2.8 | Phosphocholine | 25 | 184.0474 | 1.1 |
| SM 22:1 | 784.6458 | 785.6531 | 2.6 | Phosphocholine | 25 | 184.0474 | 1.1 |
| SM 24:0 | 814.6928 | 815.7001 | 2.8 | Phosphocholine | 25 | 184.0474 | 1.2 |
| SM 24:2 | 810.6615 | 811.6688 | 2.5 | Phosphocholine | 25 | 184.0474 | 0.7 |
| SM 26:1 | 840.7085 | 841.7157 | 2.7 | Phosphocholine | 25 | 184.0474 | 1.1 |
| Lipid | Exact | Calc. Anion | ppm | Product | Energy | Calc. Antion | Ppm |
| PlsE 38:3 | 753.5672 | 752.5599 | 1.6 | sn1 18:1 | 25 | 281.2486 | 2.2 |
| sn2 20:2 | 25 | 307.2642 | 0.58 | ||||
| PlsE 38:6 | 747.5203 | 746.5165 | 1.8 | sn1 16:0 | 25 | 255.2329 | 0.96 |
| sn2 22:6 | 25 | 327.2329 | 0.53 | ||||
| PlsE 40:6 | 775.5516 | 774.5473 | 1.5 | sn1 18:0 | 25 | 283.2642 | 2.0 |
| sn2 20:6 | 25 | 327.2329 | 0.52 | ||||
| LPA 16:0 | 410.2433 | 409.2360 | 0.67 | P-Glycerol | 25 | 152.9953 | 2.1 |
| PtdG 32:0 | 722.5098 | 721.5025 | 1.7 | sn1 16:0 | 25 | 255.2329 | 0.65 |
| P-Glycerol | 25 | 152.9953 | 2.2 | ||||
| LPG 16:0 | 484.2801 | 483.2728 | 0.18 | 16:0 | 25 | 255.2329 | 0.72 |
| P-Glycerol | 25 | 152.9953 | 2.1 | ||||
| PtdS 36:1 | 789.5520 | 788.5447 | 1.3 | sn1 18:0 | 25 | 283.2642 | 2.2 |
| sn2 18:1 | 25 | 281.2486 | 2.0 | ||||
| PtdS 36:2 | 787.5364 | 786.5291 | 1.4 | sn1 18:1 | 25 | 281.2486 | 2.0 |
| sn2 18:1 | 25 | 281.2486 | 2.0 | ||||
| PtdS 38:3 | 813.5520 | 812.5447 | 2.7 | sn1 18:0 | 25 | 283.2642 | 2.1 |
| sn2 20:2 | 25 | 307.2642 | 0.61 |
Statistical analysis
R values (ratio of endogenous lipid peak area to the peak area of an appropriate internal standard) were calculated. R values were corrected for the wet weight of the tissue analyzed. Individual R values are presented in S1 Table. Data are presented as mean ± SEM. Data were analyzed with ANOVA, followed by the Dunnett’s test to compare groups.
Results
Diacylglycerols (DAG)
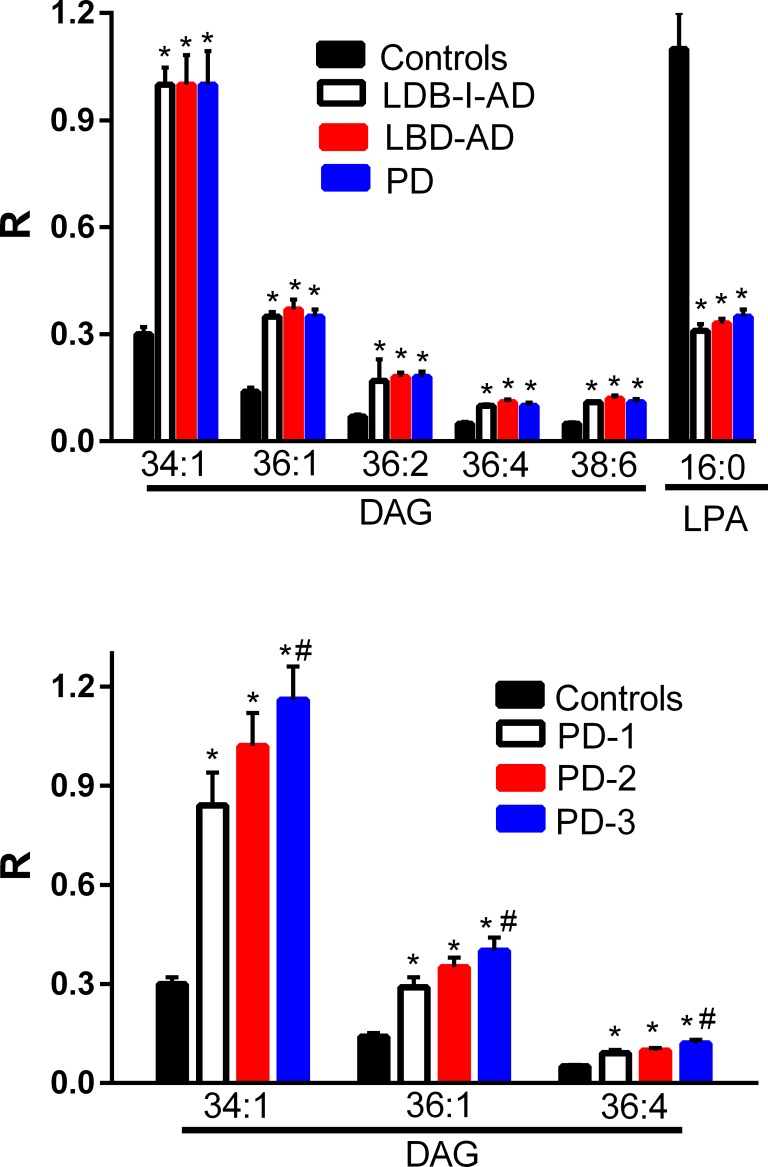
DAGs with both monounsaturated and polyunsaturated fatty acid side chains were significantly increased in the frontal cortex of the LBD-I-AD, LBD-AD, and PD groups (Fig 1). These data are similar to observations with AD frontal cortex [4–5]. Analysis of subgroups in the PD cohort revealed that DAGs were elevated (Fig 1) in subjects with no neocortical pathology (PD-1), subjects with sparse neocortical neuritic plaques (PD-2), and subjects with moderate to frequent neocortical neuritic plaques (PD-3). In addition there were greater DAG elevations in the cohort with the most severe cortical neuropathology (Fig 1).
Fig 1. Frontal cortex levels of diacylglycerols (DAG) and lysophosphatidic acid 16:0 (LPA) in control, Lewy body disease with intermediate Alzheimer’s disease (LBD-I-AD), Lewy body disease with Alzheimer’s disease (LBD-AD), and Parkinson’s disease (PD) tissues.
R = ratio of the peak area of the endogenous lipid to the peak area of the internal standard (mean ±SEM). Analysis of PD subgroups demonstrated that DAGs were augmented in the cortex of PD subjects with no neocortical pathology (PD-1, N = 5), subjects with sparse neocortical neuritic plaques (PD-2, N = 5), and subjects with moderate to frequent neocortical neuritic plaques (PD-3, N = 5). *, p < 0.01 vs. controls; #, p < 0.05 for PD-2 vs. PD3.
In contrast, the levels of lysophosphatidic acid 16:0, a precursor of DAGs, were significantly decreased in all 3 pathological groups (Fig 1). PD subgroup analysis did not reveal any significant differences in LPA levels between the PD-1, PD-2, or PD-3 subgroups.
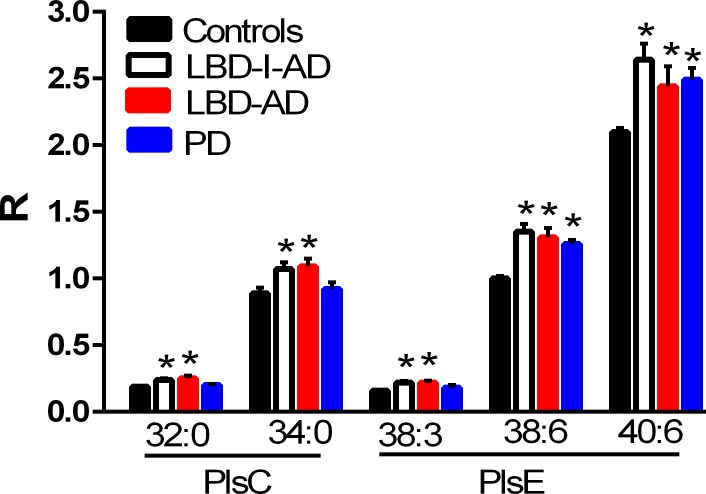
Plasmalogens
Both choline and ethanolamine plasmalogens were found to be increased in the frontal cortex of the LBD-I-AD and LBD-AD groups (Fig 2). Ethanolamine plasmalogens also tended to be augmented in PD frontal cortex (Fig 2). These data contrast with AD where plasmalogens tend to be decreased in the frontal cortex [4–5]. PD subgroup analysis did not reveal any significant differences in plasmalogen levels between the PD-1, PD-2, or PD-3 subgroups.
Fig 2. Frontal cortex levels of plasmalogens in control, Lewy body disease with intermediate Alzheimer’s disease (LBD-I-AD), Lewy body disease with Alzheimer’s disease (LBD-AD), and Parkinson’s disease (PD) tissues.
PlsC, choline plasmalogens; PlsE, ethanolamine plasmalogen. R = ratio of the peak area of the endogenous plasmalogen to the peak area of the internal standard (mean ±SEM). *, p < 0.05 vs controls.
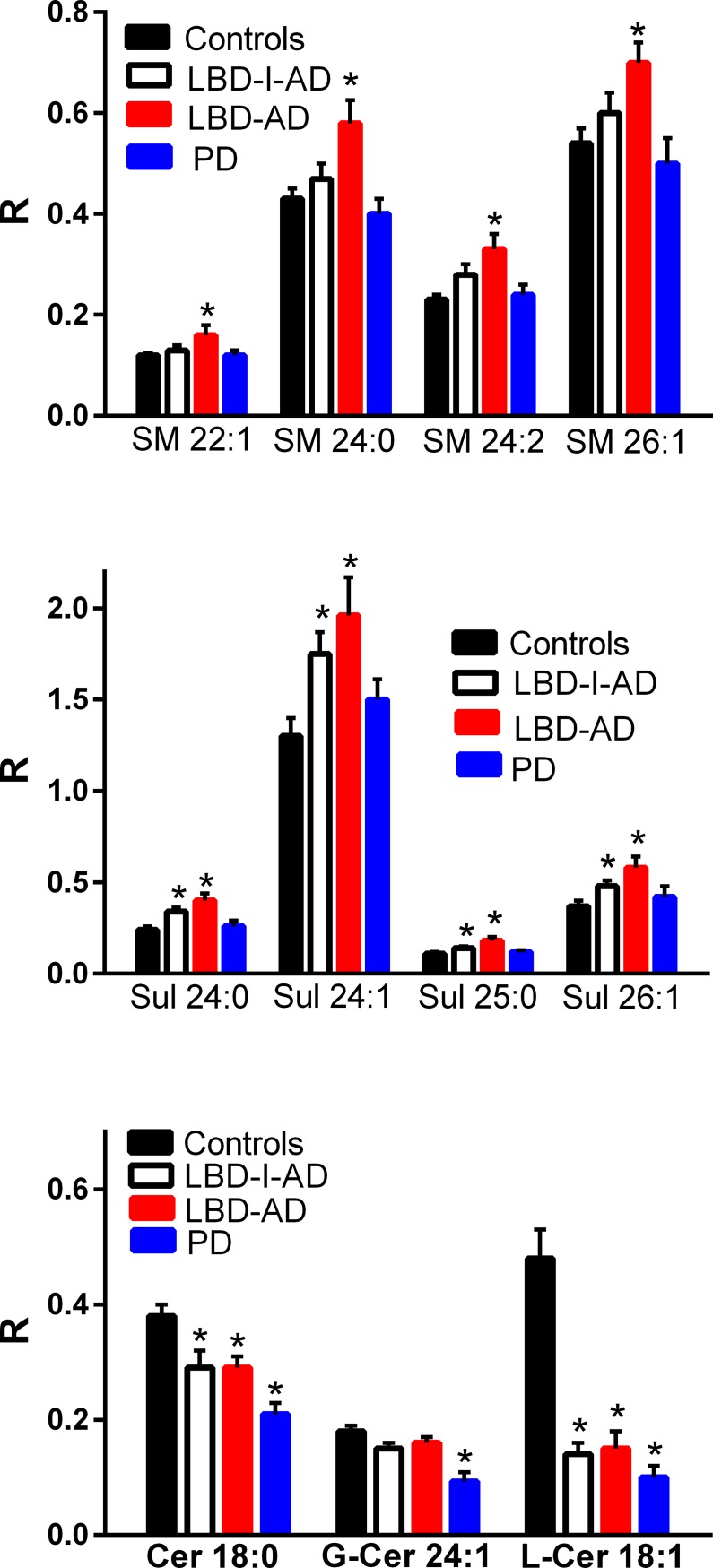
Sphingolipids
Several sphingomyelins were elevated in the frontal cortex but exclusively in the LBD-AD group while sulfatides were augmented in the frontal cortex of the LBD-I-AD and LBD-AD cohorts and not in the PD cohort (Fig 3). These data contrast with sulfatide decrements and unaltered sphingomyelin levels in AD cortex [4–5]. Ceramide metabolism also was altered, with decrements in ceramide d18:1 and lactosylceramide in all 3 disease groups while galactosyl/glucosyl-ceramide 24:1 was selectively decreased in the cortex of the PD cohort (Fig 3).
Fig 3. Frontal cortex levels of sphingolipids (SM, sphingomyelin; Sul, sulfatide, Cer, ceramide; G-Cer, galactosyceramide; L-Cer, lactosylceramide) in control, Lewy body disease with intermediate Alzheimer’s disease (LBD-I-AD), Lewy body disease with Alzheimer’s disease (LBD-AD), and Parkinson’s disease (PD) tissues.
R = ratio of the peak area of the endogenous sphingolipid to the peak area of the internal standard (mean ±SEM). *, p < 0.05. vs controls.
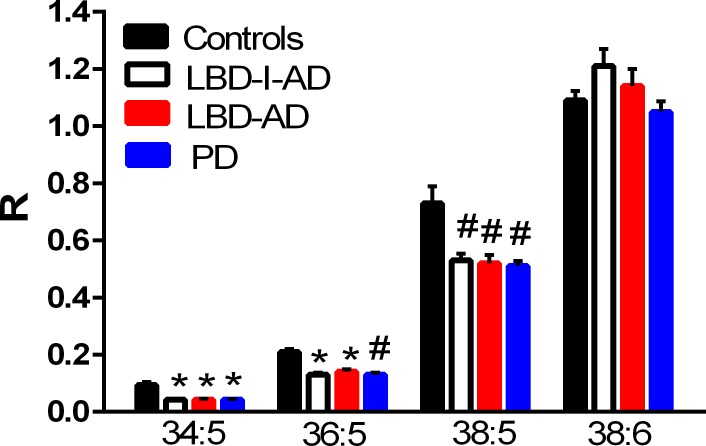
Phosphatidyl (diacyl) glycerophospholipids
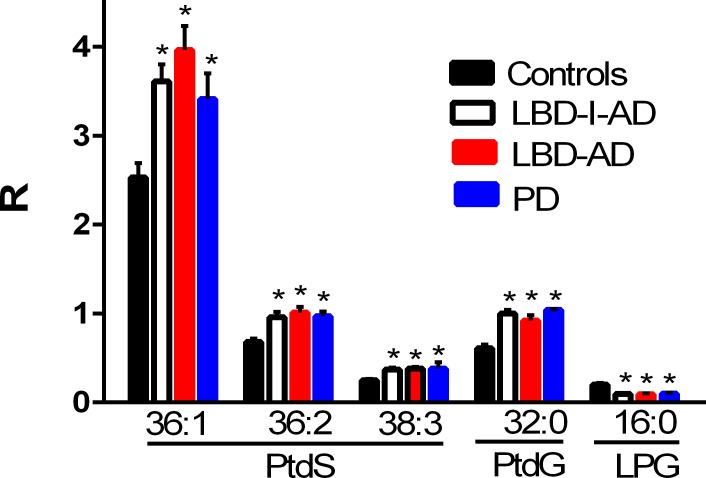
Phosphatidylcholines with 20:5 substitutions were decreased in all 3 neuropathological groups (Fig 4). This included phosphatidylcholines (PC) PC 34:5, PC 36:5, and PC 38:5 (Fig 4). In contrast, phosphatidylserines and phosphatylylglycerols were augmented in the frontal cortex of all 3 neuropathological groups (Fig 5). In concert with augmented phosphatidylglycerol levels, decrements in the levels of lysophosphatidylglycerols were measured (Fig 5). PD subgroup analysis did not reveal any significant differences in diacyl glycerophospholipid levels between the PD-1, PD-2, or PD-3 subgroups.
Fig 4. Decreased frontal cortex levels of 20:5-containing phosphatidylcholines (PtdC) in Lewy body disease with intermediate Alzheimer’s disease (LBD-I-AD), Lewy body disease with Alzheimer’s disease (LBD-AD), and Parkinson’s disease (PD) tissues.
R = ratio of the peak area of the endogenous PtdC to the peak area of the internal standard (mean ±SEM). *, p < 0.01; #, p < 0.05 vs controls.
Fig 5. Frontal cortex levels of phosphatidylserines (PtdS), phosphatidylglycerol 32:0 (PtdG), and lysophosphatidylglycerol 16:0 (LPG) in control, Lewy body disease with intermediate Alzheimer’s disease (LBD-I-AD), Lewy body disease with Alzheimer’s disease (LBD-AD), and Parkinson’s disease (PD) tissues.
R = ratio of the peak area of the endogenous lipid to the peak area of the internal standard (mean ±SEM). *, p < 0.01 vs controls.
Discussion
With the emergence of cognitive dysfunction in elderly patients, the practice of medicine is complicated in that mixed pathologies are present in many individuals. In addition, there is a variable penetrance of these pathologies resulting in a large phenotypic variation [27–28]. This heterogeneity limits the ability of physicians to provide a clinical diagnosis that would allow for earlier therapeutic intervention. In addition the co-morbidities of multiple ongoing pathologies complicates our ability to define potential points of intervention for new therapeutic strategies.
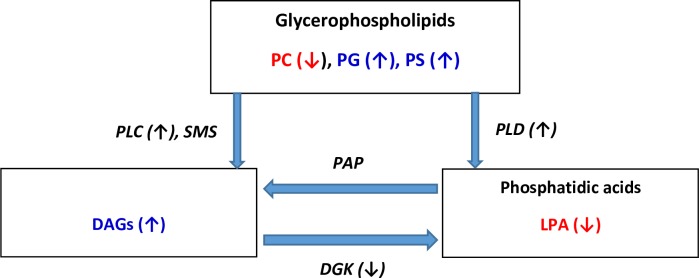
Disruption of lipid homeostasis appears to be a common biochemical feature of the investigated proteinopathies and may contribute to the neuronal dysfunction responsible for cognitive decline. The observed increases in DAG levels in the frontal cortex of AD, PD, and LBD subjects are of key interest since DAGs are essential for the synthesis of structural glycerophospholipids, for energy metabolism, and act as mediators of signal transduction, including nuclear signaling. DAGs are essential second messengers in the nuclear lipid signaling pathway with levels being tightly controlled by DAG kinase (Fig 6) which terminates DAG signaling via conversion of DAGs to phosphatidic acids [29–30]. Our observations of elevated DAGs and lower levels of lysophosphatidic acid suggest that there may be a dysfunction in the DAG kinase regulation of DAG steady–state levels in proteinopathies. This conjecture may be of import since disruption of DAG kinase function has be speculated to contribute to both neuroinflammation [31] and amyloid deposition [32]. The microRNA, miR-34a, a negative regulator of DAG kinase [33], is up-regulated in frontal cortex in AD [34] suggesting that metabolism of DAGs by DAG kinase may be decreased in AD brain. Unfortunately DAG kinase (10 isoforms) has not been characterized in synucleinopathies.
Fig 6. Abbreviated schematic presentation of DAG metabolism.
LPA, lysophosphatidic acid; PC, phosphatidylcholine; PG, phosphatidylglycerol; PS, phosphatidylserine; PLC, phospholipase C; PLD, phospholipase D; SMS, sphingomyelin synthase; DGK, diacylglycerol kinase. Red indicates lowered levels and blue indicates increased levels observed in our study.
The other major metabolic route, to regulate DAG levels, is their utilization in the synthesis of diacylglycerophospholipids (Fig 6). In this case, both choline (EC 2.7.8.2) and ethanolamine (EC 2.7.8.1) phosphotransferase have been measured in human brain [35] and in isolated synaptosomes [36], supporting the essential roles of these enzymes in neuronal function. Metabolism of glycerophospholipids to DAGs involves phospholipase C and phospholipase D (PLD) [6, 37]. However, the augmented levels of phosphatidylserines, phosphatidylglycerols and plasmalogens, along with unaltered phosphatidylethanolamine and phosphatidylinositol levels observed in our study, does not suggest that global alterations in these metabolic pathways contribute to the elevated DAG pools. The specific decreases in phosphatidylcholine levels suggests that this glycerophospholipid pool is selectively metabolized to DAGs or that there is a selective decrease in choline phosphotransferase activity in synucleinopathies that results in decreased phosphatidylcholine synthesis.
Other metabolic sources of DAG include de novo synthesis from the glycolytic pathway and from sphingolipid metabolism (Fig 6). Since glucose hypometabolism appears to predominate in most neurodegenerative disorders [38] and plasma glucose levels are generally unaltered in AD [6], this is less likely to contribute to the reported increases in plasma and brain DAG levels. With regard to sphingolipid metabolism, the synthesis of sphingomyelins which involves the transfer of a phosphocholine head group to a ceramide (EC 2.7.8.27; sphingomyelin synthase) results in the generation of DAGs [36]. The augmented sphingomyelin levels observed in the LDB-AD group in our study and the prior study of PD [12] suggest that this metabolic pathway also may be a contributing factor to augmented DAG levels in the frontal cortex in late-stage synucleinopathies. This contrasts with studies of AD where sphingomyelin levels were found to be unaltered in the frontal cortex [4–5]. While altered sphingolipid metabolism has been hypothesized to be involved in the induction of PD [39], the inconsistent findings of altered sphingolipid metabolites between laboratories remain to be resolved before firm conclusions can be drawn [12, 40–41].
A role of inflammation in the augmentation of augmented plasma DAGs in MCI and AD, and of neuroinflammation in the augmentation of brain levels of DAGs may be important since inflammation is known to result in induction of PLD [42–44]. In dementias brain microglia presumably are involved in the induction PLD and other neuroinflammatory pathways [45–51]. Similarly, peripheral inflammation may be responsible for elevated levels of DAGs in AD [7, 9, 10, 52] and MCI [7, 9] plasma. In contrast individuals possessing normal cognition until death at greater than 85 years of age did not demonstrate elevations in DAGs [5, 6]. However, at autopsy these subjects demonstrated significant AD neuropathology and have been coined non-demented AD neuropathology (NDAN). These data suggest that neuritic plaques and tangles or Lewy bodies do not initiate the observed alterations in DAG levels but may exacerbate the ongoing biochemical changes. The early detection of neuroinflammation and elevated DAG levels in MCI, AD, PD, and LBD suggest that early anti-inflammatory interventions may ultimately be neuroprotective and block cognitive decline. This conclusion is supported by the observations that individuals utilizing repeated NSAID use have a reduced incidence of AD while established AD patients do not benefit for NSAID therapy [52, 53].
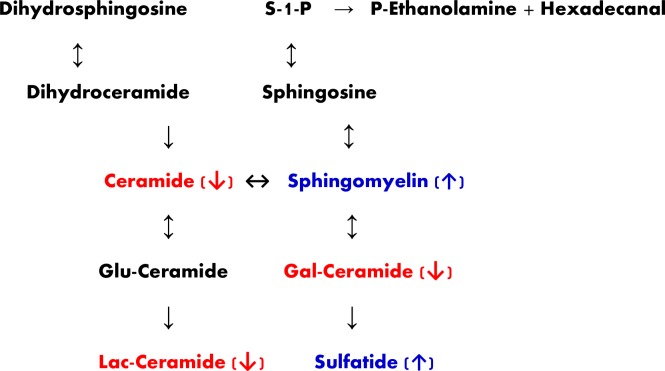
Recently, heterozygous mutations in glucocerebrosidase GBA1, which catalyzes the hydrolysis of glucosyl ceramides, have been shown to be the most common genetic risk factor for PD and LBD [54–57]. The underlying mechanism that produces this risk is unknown and both gain-of-function mechanisms involving abnormal GBA1 accumulation and loss-of-function mechanisms that purport decreased GBA1 activity have been hypothesized as mechanisms [58]. The lack of a consistent phenotype of PD or LBD in patients with GBA1 mutations, viewed as one argument against a loss-of-function mechanism, may simply indicate the complexity of multifactorial disease in which other factors interact with both GBA1 and alpha-synuclein to produce the pathologic findings and clinical symptoms of disease. Similarly, mutations in LRRK2, the chief cause of hereditary PD, have been modeled in mice and shown to increase ceramide levels in brain [59]. Glucocerebrosidase deficiency in PD and LBD would be expected to increase glucocerebroside content of brain tissue. Secondary effects of this might include increases in ceramide and its constituent sphingolipids, of which our current work demonstrates increases in both sphingomyelins and sulfatides that are not present in AD alone but are seen specifically when neocortical Lewy bodies are present. In addition, our non-biased analytical platform revealed decrements in cortical ceramide levels as previously reported [60]. We also detected decrements in galactosylceramide 24:1 and/or gluclosylceramide 24:1 in PD cortex, which cannot be differentiated by direct infusion lipidomics. However, previous studies have not detected alterations in cortical glucosylceramides in PD [61], therefore we assume the monitored decreases were in galactosylceramide (Fig 7). Large decrements in lactosylceramides were monitored in all 3 neuropathological groups further supporting complex alterations in sphingolipid metabolism in these disorders (Fig 7).
Fig 7. Abbreviated schematic presentation of sphingolipid metabolism.
Gal, galactosyl; Glu, glucosyl; Lac, lactosyl; S-1-P, sphingosine-1-phosphate. Red indicates lowered levels and blue indicates increased levels observed in our study.
Previously, we determined plasmalogens, ether phospholipids that are abundant in brain tissue and hypothesized to protect against reactive oxygen species [61], to be decreased in AD [62–63]; our current study demonstrates this not to be the case, however, in cerebral cortex affected by PD and LBD. As LBD and PD feature less global cerebral cortical damage and atrophy than AD, this might be anticipated. However, surprisingly, we found plasmalogens and especially those containing ethanolamine are increased in PD and LBD cortex compared to even controls.
Conclusions
Accumulation of DAGs in AD cortex appears to be a consistent finding among a number of lipidomics laboratories. We have extended these findings to include PD, LBD with intermediate AD pathology, and LBD with AD pathology. Considering the tight control of the homeostatic regulation of DAG levels and the multiple roles of these neutral lipids, continued research into their potential role of DAG dysfunction in neurodegenerative changes resulting in cognitive decline is imperative. In addition, it is imperative to determine if plasma DAG levels are elevated in PD and LBD subjects, as they are in MCI and AD patients. If this is the case plasma DAG levels may represent a useful biomarker for future neurodegeneration and thereby provide a rationale for early NSAID intervention to block or slow the disease processes invoked by neuroinflammation. Early intervention is critical to the success of any anti-inflammatory approach [52].
Supporting information
(XLSX)
Acknowledgments
We thank the participants of the aging study and their families for their valuable contribution to aging research. This research was funded by Lincoln Memorial University and by NIA-AG08017.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funding sources had no influence on data collection or interpretation. This work was funded by Lincoln Memorial University and the NIA (AG08017) to RLW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wood PL. Lipidomics of Alzheimer's disease: current status. Alzheimers Res Ther. 2012; 4:5 doi: 10.1186/alzrt103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood PL, Wood JA. Critical assessment of the status of Alzheimer’s disease Biomarkers. J. Parkinson’s disease & Alzheimer’s disease 2014; 1:4. [Google Scholar]
- 3.Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, et al. Circulating plasmalogen levels and Alzheimer Disease Assessment Scale-Cognitive scores in Alzheimer patients. J Psychiatry Neurosci. 2010; 35:59–62. doi: 10.1503/jpn.090059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012; 287:2678–88. doi: 10.1074/jbc.M111.274142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr. 2015; 27:270–8. doi: 10.1017/neu.2015.18 . [DOI] [PubMed] [Google Scholar]
- 6.Wood PL, Medicherla S, Sheikh N, Terry B, Phillipps A, Kaye JA, et al. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer's Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer's Disease. J Alzheimers Dis. 2015; 48:537–46. doi: 10.3233/JAD-150336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood PL, Locke VA, Herling P, Passaro A, Vigna GB, Volpato S, et al. Targeted lipidomics distinguishes patient subgroups in mild cognitive impairment (MCI) and late onset Alzheimer's disease (LOAD). BBA Clin. 2015; 5:25–8. doi: 10.1016/j.bbacli.2015.11.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam SM, Wang Y, Duan X, Wenk MR, Kalaria RN, Chen CP, et al. (2014) Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol Aging. 35:2369–81. doi: 10.1016/j.neurobiolaging.2014.02.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood PL, Phillipps A, Woltjer RL, Kaye JA, Quinn JF. Increased lysophosphatidylethanolamine and diacylglycerol levels in Alzheimer’s disease plasma. JSM Alzheimer’s Disease and Related Dementia 2014; 1:1001. [Google Scholar]
- 10.González-Domínguez R, García-Barrera T, Gómez-Ariza JL. (2015) Application of a novel metabolomic approach based on atmospheric pressure photoionization mass spectrometry using flow injection analysis for the study of Alzheimer's disease. Talanta. 131:480–9. doi: 10.1016/j.talanta.2014.07.075 . [DOI] [PubMed] [Google Scholar]
- 11.Wood PL, Medicherla S, Sheikh N, Terry B, Phillipps A, Kaye JA, et al. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer's Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer's Disease. J Alzheimers Dis. 2015; 48:537–46. doi: 10.3233/JAD-150336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, et al. Lipid pathway alterations in Parkinson's disease primary visual cortex. PLoS One. 2011; 6:e17299 doi: 10.1371/journal.pone.0017299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgrove J, Alty JE, Jamieson S. Cognitive impairment in Parkinson's disease. Postgrad Med J. 2015; 91:212–20. doi: 10.1136/postgradmedj-2015-133247 . [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Paik JH, Shin DW, Kim HS, Park CS, Kang JH. What is the Clinical Significance of Cerebrospinal Fluid Biomarkers in Parkinson's disease? Is the Significance Diagnostic or Prognostic? Exp Neurobiol. 2014; 23:352–64. doi: 10.5607/en.2014.23.4.352 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017; 16:55–65. doi: 10.1016/S1474-4422(16)30291-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry EK, McKeith I, Thompson P, Marshall E, Kerwin J, Jabeen S,. et al. Topography, extent, and clinical relevance of neurochemical deficits in dementia of Lewy body type, Parkinson's disease, and Alzheimer's disease. Ann N Y Acad Sci. 1991; 640:197–202. [DOI] [PubMed] [Google Scholar]
- 17.Mukaetova-Ladinska EB, Andras A, Milne J, Abdel-All Z, Borr I, Jaros E, et al. Synaptic proteins and choline acetyltransferase loss in visual cortex in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2013; 72:53–60. doi: 10.1097/NEN.0b013e31827c5710 . [DOI] [PubMed] [Google Scholar]
- 18.Liu AK, Chang RC, Pearce RK, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer's and Parkinson's disease. Acta Neuropathol. 2015; 129:527–40. doi: 10.1007/s00401-015-1392-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012; 83:188–94. doi: 10.1136/jnnp-2011-300828 . [DOI] [PubMed] [Google Scholar]
- 20.Blanc F, Colloby SJ, Philippi N, de Pétigny X, Jung B, Demuynck C, et al. Cortical Thickness in Dementia with Lewy Bodies and Alzheimer's Disease: A Comparison of Prodromal and Dementia Stages. PLoS One. 2015; 10:e0127396 doi: 10.1371/journal.pone.0127396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015; 129:729–48. doi: 10.1007/s00401-015-1406-3 . [DOI] [PubMed] [Google Scholar]
- 22.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012; 79:1944–50. doi: 10.1212/WNL.0b013e3182735e9a . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tagliafierro L, Glenn OC, Zamora ME, Beach TG, Woltjer RL, Lutz MW, et al. Genetic analysis of α-synuclein 3' untranslated region and its corresponding microRNAs in relation to Parkinson's compared to dementia with Lewy bodies. Alzheimers Dement. 2017. [Epub ahead of print] doi: 10.1016/j.jalz.2017.03.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, et al. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013; 81:977–83. doi: 10.1212/WNL.0b013e3182a43e45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downing AE. Neuropathological histotechnology In Laboratory methods in histotechnology, Armed Forces Institute of Pathology; (1992), pp. 88–89. Prophet EB, Mills B, Arrington JB, Leslie H, Sobin MD (Editors). [Google Scholar]
- 26.Wood PL. Non-targeted lipidomics utilizing constant infusion high resolution ESI mass spectrometry In Springer Protocols, Neuromethods: Lipidomics; 2017; 125: 13–19. Wood PL (ed). ISSN 0893-2336.and ISSN 1940-6045 (electronic). [Google Scholar]
- 27.Fujishiro H, Nakamura S, Sato K, Iseki E. Prodromal dementia with Lewy bodies. Geriatr Gerontol Int. 2015;15:817–26. doi: 10.1111/ggi.12466 . [DOI] [PubMed] [Google Scholar]
- 28.Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014; 6:46 doi: 10.1186/alzrt274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evangelisti C, Bortul R, Tabellini G, Papa V, Cocco L, Martelli AM. Nuclear expression of diacylglycerol kinases: possible involvement in DNA replication. Eur J Histochem. 2006; 50:9–13. . [PubMed] [Google Scholar]
- 30.Trubiani O, Guarnieri S, Diomede F, Mariggiò MA, Merciaro I, Morabito C, et al. Nuclear translocation of PKCα isoenzyme is involved in neurogenic commitment of human neural crest-derived periodontal ligament stem cells. Cell Signal. 2016; 28:1631–41. doi: 10.1016/j.cellsig.2016.07.012 . [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya R, Tanaka T, Hozumi Y, Nakano T, Okada M, Topham MK, et al. Downregulation of diacylglycerol kinase ζ enhances activation of cytokine-induced NF-κB signaling pathway. Biochim Biophys Acta. 2015; 1853:361–9. doi: 10.1016/j.bbamcr.2014.11.011 . [DOI] [PubMed] [Google Scholar]
- 32.Tanabe F, Nakajima T, Ito M. Involvement of diacylglycerol produced by phospholipase D activation in Aβ-induced reduction of sAPPα secretion in SH-SY5Y neuroblastoma cells. Biochem Biophys Res Commun. 2014; 446:933–9. doi: 10.1016/j.bbrc.2014.03.038 . [DOI] [PubMed] [Google Scholar]
- 33.Shin J, Xie D, Zhong XP. MicroRNA-34a enhances T cell activation by targeting diacylglycerol kinase ζ. PLoS One. 2013; 8:e77983 doi: 10.1371/journal.pone.0077983 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodall EF, Heath PR, Bandmann O, Kirby J, Shaw PJ. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci. 2013; 7:178 doi: 10.3389/fncel.2013.00178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horibata Y, Hirabayashi Y. Identification and characterization of human ethanolaminephosphotransferase1. J Lipid Res. 2007; 48:503–8. doi: 10.1194/jlr.C600019-JLR200 . [DOI] [PubMed] [Google Scholar]
- 36.Strosznajder J, Radominska-Pyrek A, Horrocks LA. Choline and ethanolamine glycerophospholipid synthesis in isolated synaptosomes of rat brain. Biochim Biophys Acta. 1979; 574:48–56. . [DOI] [PubMed] [Google Scholar]
- 37.Carrasco S, Mérida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007; 32:27–36. doi: 10.1016/j.tibs.2006.11.004 . [DOI] [PubMed] [Google Scholar]
- 38.Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J Neurosci Res. 2017;. [DOI] [PubMed] [Google Scholar]
- 39.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: Potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008; 275:5767–73. doi: 10.1111/j.1742-4658.2008.06709.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbott SK, Li H, Muñoz SS, Knoch B, Batterham M, Murphy KE, et al. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov Disord. 2014; 29:518–26. . [DOI] [PubMed] [Google Scholar]
- 41.Boutin M, Sun Y, Shacka JJ, Auray-Blais C. Tandem Mass Spectrometry Multiplex Analysis of Glucosylceramide and Galactosylceramide Isoforms in Brain Tissues at Different Stages of Parkinson Disease. Anal Chem. 2016; 88:1856–63. doi: 10.1021/acs.analchem.5b04227 . [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Frohman MA. Mammalian phospholipase D physiological and pathological roles. Acta Physiol (Oxf). 2012; 204:219–26. doi: 10.1111/j.1748-1716.2011.02298.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein J. Functions and pathophysiological roles of phospholipase D in the brain. J Neurochem. 2005; 94:1473–87. doi: 10.1111/j.1471-4159.2005.03315.x . [DOI] [PubMed] [Google Scholar]
- 44.Frohman MA. The phospholipase D superfamily as therapeutic targets. Trends Pharmacol Sci. 2015; 36:137–44. doi: 10.1016/j.tips.2015.01.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood P.L. (2003) Microglia: Roles of microglia in chronic neurodegenerative diseases IN Neuroinflammation. Mechanisms and Management, 2nd edition Wood P.L. (ed.). Humana Press, Totowa, NJ: pg. 3–27. ISBN 978-1-4684-9720-5 ISBN 978-1-59259-297-5 (eBook). [Google Scholar]
- 46.Streit WJ, Xue QS. Microglia in dementia with Lewy bodies. Brain Behav Immun. 2016; 55:191–201. doi: 10.1016/j.bbi.2015.10.012 . [DOI] [PubMed] [Google Scholar]
- 47.Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, et al. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun. 2015; 3:32 doi: 10.1186/s40478-015-0209-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat Disord. 2012; 18 Suppl 1:S207–9. doi: 10.1016/S1353-8020(11)70064-5 . [DOI] [PubMed] [Google Scholar]
- 49.Mrak RE, Griffin WS. Common inflammatory mechanisms in Lewy body disease and Alzheimer disease. J Neuropathol Exp Neurol. 2007; 66:683–6. doi: 10.1097/nen.0b013e31812503e1 . [DOI] [PubMed] [Google Scholar]
- 50.Zhang QS, Heng Y, Yuan YH, Chen NH. Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation. Toxicol Lett. 2017; 265:30–37. doi: 10.1016/j.toxlet.2016.11.002 . [DOI] [PubMed] [Google Scholar]
- 51.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003; 106:518–26. doi: 10.1007/s00401-003-0766-2 . [DOI] [PubMed] [Google Scholar]
- 52.Lichtenstein MP, Carriba P, Masgrau R, Pujol A, Galea E. Staging anti-inflammatory therapy in Alzheimer's disease. Front Aging Neurosci. 2010; 2:142 doi: 10.3389/fnagi.2010.00142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imbimbo BP, Solfrizzi V, Panza F. Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment? Front Aging Neurosci. 2010; 2 pii 19. doi: 10.3389/fnagi.2010.00019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004; 41:937–40. doi: 10.1136/jmg.2004.024455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004; 81:70–3. . [DOI] [PubMed] [Google Scholar]
- 56.Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008; 65:379–82 doi: 10.1001/archneurol.2007.68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009; 361:1651–61. doi: 10.1056/NEJMoa0901281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aflaki E, Westbroek W, Sidransky E. The Complicated Relationship between Gaucher Disease and Parkinsonism: Insights from a Rare Disease. Neuron. 2017; 93:737–746. doi: 10.1016/j.neuron.2017.01.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrazza R, Cogo S, Melrose H, Bubacco L, Greggio E, Guella G, et al. LRRK2 deficiency impacts ceramide metabolism in brain. Biochem Biophys Res Commun. 2016; 478:1141–6. doi: 10.1016/j.bbrc.2016.08.082 . [DOI] [PubMed] [Google Scholar]
- 60.Abbott SK, Li H, Muñoz SS, Knoch B, Batterham M, Murphy KE, et al. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov Disord. 2014; 29:518–26. . [DOI] [PubMed] [Google Scholar]
- 61.Boutin M, Sun Y, Shacka JJ, Auray-Blais C. Tandem Mass Spectrometry Multiplex Analysis of Glucosylceramide and Galactosylceramide Isoforms in Brain Tissues at Different Stages of Parkinson Disease. Anal Chem. 2016; 88:1856–63. doi: 10.1021/acs.analchem.5b04227 . [DOI] [PubMed] [Google Scholar]
- 62.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006; 1763:1511–26. doi: 10.1016/j.bbamcr.2006.08.038 . [DOI] [PubMed] [Google Scholar]
- 63.Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr. 2015; 27:270–8. doi: 10.1017/neu.2015.18 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.