Abstract
The purpose of the study was to compare smart apparel (SA) muscle activity measurements to surface electromyography (sEMG) muscle activity measurements during exercise, and determine if any systematic bias of the apparel exists. Thirty-five male participants (Ages 23.1±2.92 yrs, height 178±0.09 cm, mass 81.14±10.59 kg, body fat percentage 15.4±4.79%) provided informed consent for participation. During two separate exercise sessions, participants performed three sets of 12 bodyweight squats and pushups in both SA and sEMG. Conditions (sEMG or SA) were randomly counterbalanced. During sEMG, sensors were placed at selected anatomical locations on the right side. During SA, sEMG sensors were sewn into the fabric. Percent of maximal voluntary contractions (%MVC) were calculated. Paired t-test were used to analyze group mean differences in %MVC between conditions. Bland & Altman plots were created to determine any systematic bias. A Pearson’s product correlation was run to determine any association with intertrial variability and body fat percentage. The alpha levels were set a p<0.05. It was determined that there were no significant differences between %MVC in the SA and sEMG for three of the selected muscles (p>0.05). There was a significant difference in %MVC measured in the SA and sEMG for the RDELT (p=0.02). Specifically, the %MVC for the deltoid during pushups was 11.8% greater compared to sEMG. Intertrial differences were not significantly correlated with %BF (p>0.05). The apparel appears to be valid in the recreational population and appears to have no systematic bias.
Keywords: Electromyography, fitness, technology
INTRODUCTION
Having a sedentary lifestyle is a common link between obese individuals and those with cardiovascular disease (CVD). In the United States alone, more than 80 million adults live with CVD1. Approximately 35% of adults in the USA are classified obese (Body Mass Index [BMI] > 30 kg m−2) and about 15% reported a BMI > 35 kg m−2 (11). Each year, approximately 28 million adults die from of complications from being overweight and obese (4), which makes engaging in physical activity much more important.
It is well known that engaging in physical activity results in a reduction of all-cause mortality and is linked with a multitude of health benefits (15), including improved mood, reduced risk of stroke and heart attack, as well as reduced blood pressure. This is likely why physical activity has gained popularity. Currently, the American College of Sports Medicine (ACSM) recommends adults should get at least 150 minutes of moderate-intensity exercise per week. Exercise recommendations can be met through either 30–60 minutes of moderate-intensity exercise five days per week or 20–60 minutes of vigorous-intensity exercise three days per week (3). However, per the Center of Disease Control, more than 60 percent of American adults are not regularly physically active and 25 percent of all adults are not active at all (12). To help increase exercise participation and adherence, new exercise technology has been developed to promote physical activity.
Video games like the Nintendo Wii were designed for users to be active and have games specifically made for exercise (16). Various companies are also developing apps and wearable technology to help motivate people to start and continue exercising, such as the BodyMedia® FIT System (Jawbone, Pittsburgh, PA) (14). With the prevalence of people being more accustomed to using technology, there have been new breakthroughs in exercise technology with regards to its ability to facilitate weight loss.
Video games have been found to be an effective exercise tool as some require players to be active rather than sedentary. Adults using the Nintendo Wii were found to have mean increase in energy expenditure (7, 16). Maddison et al (9) found that there was a statistically significant (P=0.02) difference in BMI for the intervention group over 6 months and found a decrease in body fat (P=0.02) favoring those children who used active video games. While there was a small percentage change, the researchers noted that it was consistent with slowing weight gain compared to their growth in height as recommended in clinical guidelines. Results showed that the pervasiveness of technology in combination with the appeal of traditional video games can have implications for reducing sedentary behavior at various levels (9).
Wearable armband technology has also been developed to help adults with weight loss. Rogers et al (14) conducted a study that utilized a wearable armband that measured physical activity and energy expenditure. They used three groups: one with standard behavioral weight loss group meetings, a technology based group that utilized the armband and app, and an enhanced technology group that had the same materials as the previous group but they also received phone calls once a month from weight loss specialists. Results showed that after 6 months, weight loss occurred and energy expenditure in physical activity increased throughout all three groups (14). This is like the results of Barry et al that tested the SenseWearTM Armband (BodyMedia, Pittsburgh, PA, USA) who also found that use of armbands facilitated weight loss and increased energy expenditure (1). That study suggested that individuals who use self-monitoring strategies like the armband provided can help facilitate weight loss. Therefore, wearable technology appears to be effective with new devices are continually being developed.
Smart apparel (SA) is one such newer form of wearable technology used in fitness. It combines electromyography (EMG) technology directly into the fabric of the clothing, which allows for measurement of muscle activity during exercise sessions. The suit syncs to an app on the user’s phone and provides real-time feedback about muscle activity patterns and effort measured as a percentage of the user’s maximal voluntary contraction (%MVC). This allows individuals to understand movement patterns and creates a mastery experience (successful accomplishment in a performance), which may increase self-efficacy during exercise (5) and possibly increase exercise adherence.
However, the technology of SA is relatively new and to our knowledge there has been little research to indicate the validity of the muscle activity measured in SA compared to surface EMG sensors. Additionally, to our knowledge, there is no data that demonstrates whether the product is systematically biased against different body types. Therefore, the purposes of the study were to compare SA muscle activity measurements to EMG muscle activity measurements during exercise, and to determine if there is any systematic bias of the apparel towards certain body types.
METHODS
Participants
A power analysis conducted with G*POWER 3.1 (Universitat Kiel, Germany) determined that 35 participants were needed in the present study for a power of 0.80, with an effect size of 0.5 and an α= 0.05. Participant demographics are presented in Table 1. Participants were recreationally active males age 18–44 years who strength trained at least 30 minutes per day, three times per week for the past six months. They were free of any musculoskeletal injuries within the past six months and any cardiovascular or uncontrolled metabolic disease in the past year. All participants provided written informed consent prior to participation and the Institutional Review Board at California State University, San Bernardino approved the protocol.
Table 1.
Participant demographics.
| Age | 23.1±2.92 yrs |
| Height | 178±0.09 cm |
| Mass | 81.14±10.59 kg |
| Body fat percentage | 15.4±4.79 % |
Protocol
Research personnel randomized order of exercise sessions using a random numbers generator to either performing the exercise session with the EMG first or SA first using blocked randomization to minimize a training effect. Subjects were required to abstain from caffeine and exercise 24 hours prior to testing and alcohol 48 hours prior. Experimentation was comprised of two exercise sessions lasting approximately one hour each. Sessions were performed 24 to 48 hours apart. Participants were asked to not perform any exercise between the two testing sessions so fatigue does not influence muscle activity measurements of the second exercise session.
After completing the informed consent and health history questionnaire, height to the nearest 0.01 cm and weight to the nearest 0.1 kg were measured using a calibrated stadiometer and scale, respectively. Body composition was subsequently measured using a bioelectrical impedance analyzer (Omron HBF-306; Omron Healthcare Inc., USA). Subsequently, during both exercise sessions, all participants performed the same standardized warm-up which lasted approximately three to five minutes.
Once the warmup and maximal voluntary contractions (MVC) were completed (described below), participants performed body weight squats and pushups while muscle activity was measured by either the SA or EMG. Squats were performed with arms extended straight in front and feet hip-to-shoulder width apart. Participants were asked to bend their knees until their thighs were approximately parallel to the ground, then push through their heels and extend their knees until their legs were straight. Pushups were performed in a plank position with arms extended. Participants were asked to lower down and bend their elbows back at a 45-degree angle until their upper arms were approximately parallel to the ground, and then extend their elbows until they returned to a plank position with arms extended. Each exercise was performed for three sets of 12–15 repetitions; however, only the first and second sets were included in the statistical analysis to avoid fatigue during the third set from potentially affecting %MVC measurements.
EMG
Standard skin preparation procedures were used prior to application of the surface EMG sensors (Delsys Trigno Wireless; Delsys Inc., Natwick, MA, USA). Each EMG sensor was placed on the belly of the following muscles on the right side of the body: lateral head of the deltoid, mid pectoralis major, rectus femoris, biceps femoris. Participants then performed the warm-up prior to performing the MVCs for each muscle group. The first MVC performed on the pectoralis major was measured while the participants lay on a flat bench with a loaded bar, which they were unable to move. They were instructed to push against the bar vertically as if they were trying to perform a bench press for three seconds. The MVC for the lateral deltoid was done with the participant standing laterally to a wall with their arm down at their side and the back of their hand placed against it. They were instructed to try and lift their arm, as if they are performing a lateral raise against a wall. The MVC for the rectus femoris was performed with the participant sitting on a massage table. They were instructed to kick out as if performing a leg extension while a member of the research team pushed against their ankle as resistance. The MVC for the biceps femoris was performed with the participants laying prone on a massage table. Participants then attempted to curl their leg as if performing a leg curl with a member of the research team pulled at their ankle resisting the motion. Each MVC was performed three times for each muscle with one minute of rest between MVCs. After the MVCs, participants performed the exercises.
Smart Apparel
During the SA sessions, MVCs were completed as part of the apparel calibration, which were the same for each participant. The suit itself is a set of a compression shirt and shorts made of 75% Nylon and 25% Lycra® Spandex that are lined with EMG sensors designed to measure muscle activity. The shirt measures upper-body muscle groups, including: pectorals, biceps, triceps, deltoids, latissimus dorsi and trapezius muscles. The shorts measure lower-body muscle groups, including: quadriceps, hamstrings and gluteal muscles. The suit has an application that instructs users on how to perform each MVC, with each MVC lasting about five seconds. For the MVC of the deltoids, the participant extended and raised their arms to the side with thumbs pointing down, while contracting maximally. For the pectorals, the participant pressed their hands together in front of their chest, and drove elbows down and towards each other. For the MVC of the quads, the participant sat and extended their leg straight ahead and contracted their quadriceps muscle maximally. And for the hamstrings, the participant laid on the floor, bent their knees at 90 degrees, extended their leg and squeezed the hamstring of the opposite leg still on the ground, while continuing to lift their hips off the ground. After calibration, participants performed the same exercises as described above.
Statistical Analysis
The EMG data was collected via EMGworks Acquisition software (Delsys Inc., Natwick, MA, USA). It was full-wave rectified, and then the root mean square of each repetition in each exercise set was calculated for each respective muscle (RPEC and RDELT during pushups, RQUAD and RHAM during squats). The data was then normalized against the MVC for the respective muscles to produce a percentage of MVC (%MVC), which is a measure of effort during exercise. A paired t-test was then used to determine differences in %MVC measurements between conditions with alpha level being set at p = 0.05. A Bland & Altman analysis was used to determine any systematic bias of the apparel, and a Pearson’s product correlation was used to determine an association between intertrial variability and %BF.
RESULTS
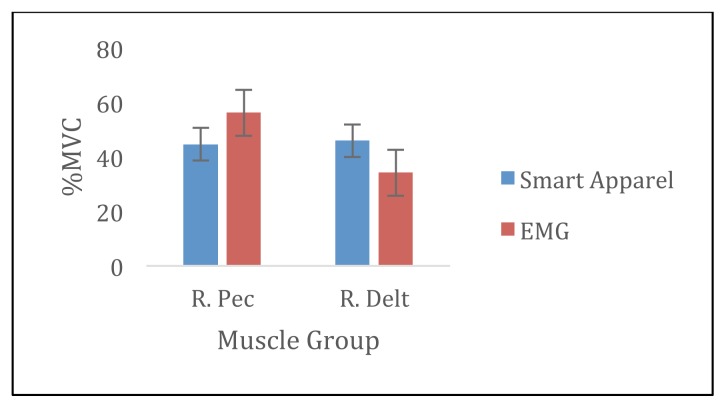
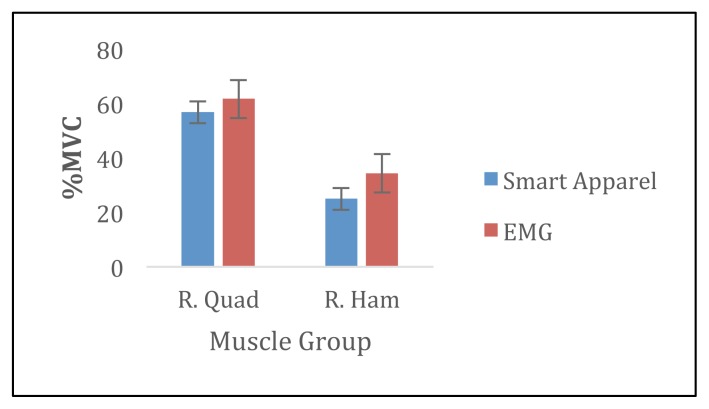
There were no significant differences between %MVC in the SA and the EMG for the RPEC, RQUAD, and RHAM muscles (p> 0.05). There was a significant different in %MVC measured in the SA and the EMG for the RDELT (p= 0.02). Specifically, the %MVC measured during pushups was 11.8% greater compared to %MVC measured in EMG (Figures 1 and 2).
Figure 1.
Average %MVC during push-ups for smart apparel and EMG.
Figure 2.
Average %MVC during squats for smart apparel and EMG.
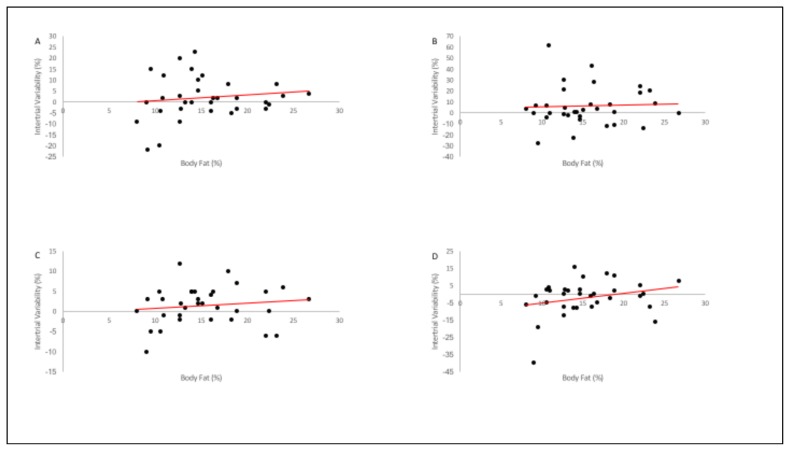
Inspection of individual differences demonstrates the influence of participants’ biological variation on the intertrial reliability of SA (measurement of %MVC from one set to the next). A Pearson correlation analysis of SA intertrial differences (set1 – set2) and %BF determined that there was no significant association (p> 0.05) between the variables (Table 2) although, it appears that certain muscle groups recorded greater variability between sets in participants with lower %BF (Figure 3).
Table 2.
Correlation analysis of intertrial differences and %BF.
| RPEC | RDELT | RQUAD | RHAM | ||
|---|---|---|---|---|---|
| Body Fat | Pearson Correlation | .126 | .047 | .125 | .265 |
| Sig. (2-tailed) | .483 | .797 | .490 | .136 |
Figure 3.
Correlation plots of intertrial variability and %BF for A) RPEC, B) RDELT, C) RQUAD, and D) RHAM
Although there were no significant mean intertrial (set1 – set2) differences between trials for the men for RPEC, RQUAD, and RHAM muscle groups (p> 0.05), there was a trend towards significant intertrial differences for RDELT (p = 0.052). Additionally, individual set-to-set values were notable and variable. The range in %MVC differences between set one and set two of exercise during the SA exercise session varied from -22% to 23% in the RPEC, −28% to 62% in RDELT, −28% to 12% in RQUAD, and −40% to 16% in RHAM.
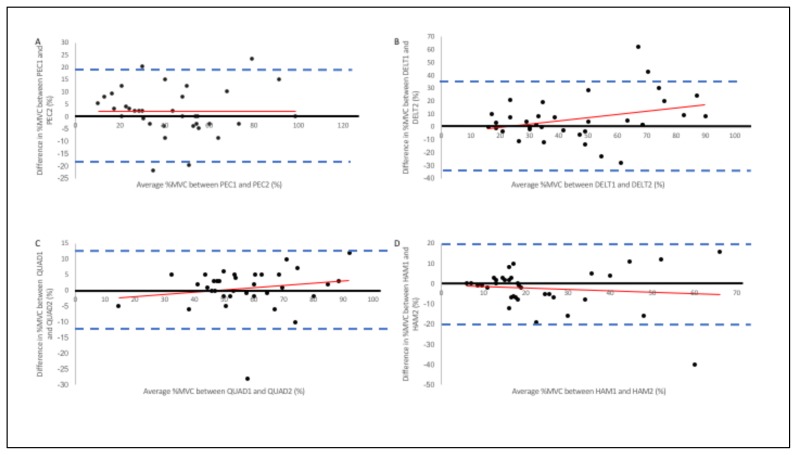
Results from the Bland and Altman analyses for %MVC are shown in Figure 4. The figure reveals the magnitude of the 95% confidence interval and that there was no systematic bias in individual %MVC measurement at the extremes of the respective %MVC distributions (Figure 4). This was consistent for all four muscle groups measured.
Figure 4.
Bland & Altman Plots for A) RPEC, B) RDELT, C) RQUAD, and D) RHAM. The vertical axes represent the difference in %MVC between the first and second %MVC measurements from SA (smart apparel). The horizontal axes represent the mathematical average for each participants %MVC measurements. The solid dark line represents the ideal lack of difference between the two %MVC trials. The dashed lines denote the upper (+2SD) and lower (−2SD) limits of agreement
DISCUSSION
The primary purpose of this investigation was to determine the validity of SA in measuring muscle activity patterns during exercise and determine if any systematic bias within the product existed. To our knowledge, we are among the first to partially validate SA and determine that intertrial differences are not significantly correlated with %BF, indicating that SA can be a useful tool for individuals engaged in physical activity.
Confirming our hypothesis, there were no significant differences between SA and sEMG muscle activity measurements during exercise for the RPEC, RQUAD, and RHAM muscle groups (p > 0.05). However, there was a significant difference in the measurements for the RDELT muscle group (p = 0.02). This particular finding was surprising since the other muscle groups SA measurements appeared to measure consistently with sEMG measurements. However, it is likely that the location of the sensor contributed to the variability measured. Additionally, RDELT measurements occurred during push-ups. Therefore, it is possible that the sensor lost contact with the skin during the movement as a result of the shoulder flexion during the movement, affecting the muscle activity measurements (13).
The intraindividual set-to-set variability is something of importance that should be discussed in regards to SA. This variability reflects the ability of the apparel to measure muscle activity from one set of an exercise to the next. In the present study, the set-to-set variability was not significantly different for all muscle groups (p > 0.05), although RDELT trended towards significance (p = 0.052). This indicates that the measurements from SA between the first two sets of exercise were fairly consistent. This is somewhat inconsistent with previous research (10) as it was determined that there was a significant change in sEMG amplitude and median frequency (p < 0.01) because of fatiguing exercise. However, in the present study, participants were not conducting what would be considered fatiguing exercise which may explain the dichotomous results.
Also, since many different individuals will have the opportunity to wear and use the apparel, it is of interest to users to know if %BF can affect the readings of the apparel. Since abnormal amounts of fat can sometimes attenuate the signal measured by sEMG (2), there was a possibility that the EMG sensors in the apparel would have difficulty recording muscle activity in participants with more %BF. However, the correlation analysis determined that there was no significant association between intertrial variability (set1 – set2) and %BF for each muscle group (p > 0.05). In fact, the R2 value for the RPEC, RDELT, RQUAD and RHAM were 0.02, 0.002, 0.02, and 0.07, respectively, indicating that very little variability in the SA measurements were accounted for by %BF.
It should also be noted that the range in intertrial (set1 – set2) measurements from SA were vast. For RPEC, the difference in measurements ranged from −22% to 23%. For RDELT, the differences ranged from −28% to 62%. For RQUAD, the differences ranged from −28% to 12%. And for RHAM, the differences ranged from −40% to 16%. It is possible that fatigue played a factor in the difference in measurements because of the exercise itself (8, 10). However, participants recruited indicated that they regularly participated in physical activity and were familiar with the exercises performed in the protocol. Additionally, the movements used body weight as the resistance and contained a rest period of up to three minutes. Therefore, we believe that the variability in measurements was not likely due to fatigue, but due to placement of the sensors within the suit and their ability to maintain contact with the skin during exercise. Also, because the order of condition was randomly selected, we believe that there was no learning effect that contributed to the variability measured between the SA trials.
While the range in intertrial measurements may not be significantly different, they may be clinically significant. The apparel has the potential to be used in the rehabilitation setting since it can report muscle activity firing patterns like sEMG (6). During rehabilitation, one of the goals is to ensure correct muscle firing patterns to reduce the likelihood of re-injury. The differences seen between the trials could potentially impact clinician recommendations during each rehabilitation session. We recommend that clinicians ensure proper fitting apparel be used for patients to ensure that sensor contact is consistent throughout the exercise session to reduce the chance of highly variable muscle activity measurements between the trials.
Bland & Altman analysis revealed that there was no systematic bias in individual %MVC estimation at the extremes of the respective %MV distributions. By assessing the scatter of the plots, it is clear that at the ends of the mean %MVC distributions, the scatter of the differences is not wider nor more narrow compared to each other. Figure 4b appears to be the exception as there is wider scatter as the mean %MVC increases. While unexpected, we again attribute the pattern due to construction of the apparel and sensor placement as well as the change in sensor position as a result of the pushups activity. Losing contact with the skin is a source of error when using sEMG (13) and therefore is likely the cause of this exception for RDELT when interpreting the Bland & Altman analysis.
This study is not without limitations that should be addressed. The sample population was only male. Therefore, generalizations to the female population should be limited as they were not tested in the present study. Surface EMG is only reliable if proper skin preparation techniques were followed for each participant. A set of guidelines was provided to each participant to increase the validity of the sEMG measurements, however, it is unclear if each participant followed the guidelines as requested. Fatigue may have also impacted the measurements from each method. However, participants were required to abstain from exercise between each of the testing sessions so fatigue did not play a major factor. Also, participants were experienced weight lifters and in the present study only performed body weight exercises. Therefore, fatigue may not have played a significant role as the exercises performed were not of great intensity. Our participants were within the normal range for %BF, and therefore, generalizations to individuals outside the normal ranges should be carefully considered, as we have no data as to the efficacy of SA in overweight and obese individuals.
The present study sought to investigate the intraindividual variations and validity of SA in the recreational, exercising population, as well as determine if any systematic bias exists within the apparel. It was determined that SA appears to be valid in the trained population and has no systematic bias. Additionally, the trial-to-trial variability is not significantly associated with body composition of the user. Future research should include investigating the validity of the apparel in the female population, determining if SA can be a useful tool in weight loss regimens, as well as determining how to prevent loss of skin contact by the sensors.
REFERENCES
- 1.Barry VW, McClain AC, Shuger S, Sui X, Hardin JW, Hand GA, Wilcox S, Blair SN. Using a technology-based intervention to promote weight loss in sedentary overweight or obese adults: a randomized controlled trial study design. Diabetes Metab Syndr Obes. 2011;4:67–77. doi: 10.2147/DMSO.S14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Luca CJ. The use of surface electromyography in biomechanics. JAppl Biomech. 1997;13(2):135–163. [Google Scholar]
- 3.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I, Nieman DC, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 4.Circulation. U.S. National Library of Medicine; 2013. Heart Disease and Stroke Statistics--2013 Update: A Report from the American Heart Association. Web. 15 Feb. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson D. How personal trainers can use self-efficacy theory to enhance exercise behavior in beginning exercisers. Strength and Conditioning Journal. 2010;32(3):67–71. [Google Scholar]
- 6.Kleissen RFM, Buurke JH, Harlaar J, Zilvold G. Electromyography in the biomechanical analysis of human movement and its clinical application. Gait Posture. 1998;8(2):143–158. doi: 10.1016/s0966-6362(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.Lanningham-Foster L, Foster RC, McCrady SK, Jensen TB, Mitre N, Levine JA. Activity promoting games and increased energy expenditure. J Pediatr. 2009;154:819–823. doi: 10.1016/j.jpeds.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linssen WH, Stegeman DF, Joosten EM, van’t Hof MA, Binkhorst RA, Notermans SL. Variability and interrelationships of surface EMG parameters during local muscle fatigue. Muscle Nerve. 1993;16(8):849–856. doi: 10.1002/mus.880160808. [DOI] [PubMed] [Google Scholar]
- 9.Maddison R, Foley L, Mhurchu CN, Jiang Y, Jull A, Prapavessis H, Hohepa M, Rodgers A. Effects of active video games on body composition: a randomized controlled trial. Am J Clin Nutr. 2011;94(1):156–163. doi: 10.3945/ajcn.110.009142. [DOI] [PubMed] [Google Scholar]
- 10.Masuda K, Masuda T, Sadoyama T, Inaki M, Katsuta S. Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol. 1999;9(1):39–46. doi: 10.1016/s1050-6411(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Physical Activity and Health: A Report of the Surgeon General. Centers for Disease Control and Prevention; n.d. Web. 07 June 2017. [Google Scholar]
- 13.Reaz MBI, Hussain MS, Mohd-Yasin F. Techniques of EMG signal analysis: detection, processing, classification, and applications. Biol Preced Online. 2006;8(1):11–35. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers RJ, Lang W, Barone Gibbs B, Davis KK, Burke LE, Kovacs SJ, Portzer LA, Jakicic JM. Applying a technology-based system for weight loss in adults with obesity. Obes Sci Practice. 2016;(2):3–12. doi: 10.1002/osp4.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Current Sports Med Rep. 2013;12(4):215–17. doi: 10.1249/JSR.0b013e31829a68cf. [DOI] [PubMed] [Google Scholar]
- 16.White K, Schofield G, Kilding AE. Energy expended by boys playing active video games. J Sci Med Sport. 2011;14(2):130–134. doi: 10.1016/j.jsams.2010.07.005. [DOI] [PubMed] [Google Scholar]