Abstract
We compared lactate threshold (TLac) with non-invasive markers of an aerobic-anaerobic transition; namely, ventilatory (VT) and tissue saturation index (TSIT) thresholds. While identification of a breakpoint in blood lactate concentration ([BLa]) is common for determination of an aerobic-anaerobic transition, non-invasive measures, VT and NIRS, have also received attention as a means of determining this critical exercise intensity. We hypothesised that one or other of these non-invasive measures would have a strong association with TLac. Thirty-one (n=31) competitive male athletes (mean ± SD, age 29±9 yr, height 1.81±0.1 m, body mass 77.7±10.0 kg) performed graded incremental cycling to volitional exhaustion. Heart rate, TSI and gas exchange data were measured throughout and [BLa] was determined at fixed intervals. Threshold detection involved a segmented linear regression analysis minimising the squared sum of the residuals to determine TLac, TSIT and VT. Workload and HR at TLac, VT and TSIT were analysed using repeated measures ANOVA and correlation assessed using Pearson’s and interclass correlation coefficients. Thresholds at TSIT and TLac were not significantly different (255±35 vs. 249±30 W, P>0.05), suggesting that limitations in O2 delivery could be closely linked to an aerobic-anaerobic transition. However, poor correlation (r=0.55, ICC=0.54 and 95%LoA of +67 and −54 W) suggested other factors may exert an influence. Mean VT occurred at a significantly higher workload than TLac (271 ±35 vs 249±30 W, P<0.001). Consequently, VT proved less useful, giving an indication of when an aerobic-anaerobic transition had already occurred. In conclusion, non-invasive markers of the aerobic transition are not concurrent with TLac.
Keywords: Aerobic, anaerobic, muscle oxygenation, NIRS, ventilation
INTRODUCTION
The term “aerobic-anaerobic transition” was first used to describe an exercise intensity above which there is an exponential increase in blood lactate (BLa) concentration and associated changes in gas exchange variables (20, 21). Determination of the transition in athletes has become critical as a performance indicator, and for heart rate controlled training prescription. To date, physiological mechanisms underlying the transition are highly debated amongst researchers resulting in several definitions and methods of determination. While the identification of a breakpoint in [BLa] remains the most common method of determining the transition, reflecting the balance between lactate production and clearance, usage of non-invasive measures, such as gas exchange variables, and more recently near-infrared spectroscopy (NIRS), have received attention due to their potential for determining this critical exercise intensity.
Tissue saturation index (TSI) is the ratio of oxy-haemoglobin (HbO2) to total haemoglobin (tHb) and is commonly measured using NIRS. This ratio gives an indication of the balance between O2 delivery and consumption at a tissue specific level (3). While NIRS has been widely used in clinical scenarios, particularly in brain surgery to monitor cerebral oxygenation (3), the recent development and commercial availability of portable, user-friendly devices have led to growing applications in the field of sport performance (3). Athletes and coaches can now measure muscle specific TSI during exercise with relative ease. Exercise intensity increases the demand for O2 by the exercising musculature, and during a graded exercise test (GxT) the musculature becomes progressively deoxygenated as O2 delivery lags uptake. An exercise intensity is reached where the delivery of O2 by the circulation reaches a maximum and the tissue must therefore rely increasingly on anaerobic metabolism to sustain the effort. A TSI threshold (TSIT), detected using NIRS, could therefore potentially be used as a marker of a transition; however, there is still debate as to its efficacy.
As lactic acid accumulates in the blood it rapidly dissociates into lactate and H+ ions. The H+ ions are buffered by bicarbonate (HCO3−) forming carbonic acid (H2CO3) which is then converted to H2O and CO2 catalysed by carbonic anhydrase. This CO2 is termed non-metabolic CO2 and along with metabolic CO2 contributes to an increase in minute ventilation (VE) as detected by ventilatory chemoreceptors (14). It is possible to detect this increase in VE relative to VO2, and, therefore, infer the transition as an inflection point measured through gas exchange variables (1) known as a gas exchange or ventilatory threshold (VT). Some previous studies identified two ventilatory thresholds. VT1 can be identified as an inflection in VE/VO2 vs workload while VT2 can be identified using a plot of VE/VCO2 vs workload. Racinicas et al. (14) identified VT1 and VT2 and reported that muscle oxygenation in the Vastus Lateralis (VL) displayed a threshold at, or after, VT2. In the current study VT1 was the only threshold identified using gas exchange variables as it is more commonly used in published literature (6, 15, 18, 19, 22).
Several studies have previously investigated the relationship between lactate, ventilatory and NIRS derived markers of an aerobic-anaerobic transition in different combinations. Grassi et al. (8) reported significant correlation between onset of muscle deoxygenation and the onset of blood lactate accumulation (OBLA, a nominal [BLa] of 4mmol.L−1). Another study (6) reported a significant correlation between workload at TSIT and VT1; but reported no significant correlation between workload at TSIT and TLac; however, the technique used to determine TLac was not reported. Rao et al. (15) assessed the association between VT1 and NIRS derived thresholds in visceral organs rather than in exercising muscle. A moderate to strong correlations in O2 saturation in the kidney and brain with VT1 was reported, but wide limits of agreement were also identified. These authors also commented that the NIRS measured threshold slightly underestimated VT1. In recent years, several portable NIRS measurement devices have become available facilitating application of this non-invasive technique to athletic populations (4, 5, 19, 23). However, little published research is available to assess the efficacy of such devices as the majority of published studies have used laboratory based non-portable units.
Consequently, there are mixed opinions, and an ongoing debate, as to whether non-invasive markers of the aerobic-anaerobic transition are concurrent with more established blood derived measures. Certain ambiguity exists because of differing opinions on specific methods of transition detection and different variables associated with each measurement technique. The aim of the current study was to investigate if any association existed between TLac and non-invasive physiological markers of the aerobic-anaerobic transition, such as VT and NIRS assessed TSIT. The current study hypothesised that one or more of these non-invasive measures would have a strong association with TLac and thus occur at a non-significantly different exercise intensity.
METHODS
Participants
Thirty-one (n=31) competitive male cyclists and triathletes between the age of 18 and 46 participated in the current study (mean ± SD: age 29 ± 9 yr, height 1.81 ± 0.1 m, body mass 77.7 ± 10.0 kg). All participants were actively involved in their chosen sport and trained 5 to 7 times per week. Before agreeing to participate in the current study all participants were informed of the associated risks and requirements, both verbally and in writing. A 7-day period was allowed for participants to fully familiarise themselves with the risks and requirements associated with participation and make any queries known to investigators. Having agreed to participate, enlisted participants provided written informed consent. The Faculty of Health Sciences Research Ethics Committee in Trinity College Dublin granted ethical approval.
Participants were required to attend the laboratory on one occasion in a rested state, carbohydrate loaded, well hydrated and clean shaven. They were also required to abstain from both alcohol and caffeine in the 24 h prior to testing. All participants underwent a medical examination by a qualified medical doctor prior to testing. Any person presenting with cardiac abnormalities, respiratory difficulties, symptoms of cold or influenza, musculoskeletal injury that could impair performance, diabetes, hypertension, metabolic disorders or any other contra-indicatory symptoms were excluded. In addition, participants completed a medical questionnaire detailing previous personal and family health abnormalities, recent illness or injury, as well as details of recent travel and vaccinations.
Protocol
A GxT to volitional exhaustion was performed on an electromagnetically braked cycle ergometer (Lode Excalibur Sport; Groningen, The Netherlands). Participants’ initially identified a cycling position in which they were most comfortable by adjusting saddle height, saddle to handlebar distance and handlebar height. Participant’s feet were secured to the ergometer using their own cycling shoes with cleats and accompanying pedals. The protocol commenced with a 15-min warm-up at a workload of 120 Watt (W). The GxT began with a 3-min stationary phase followed by an active phase commencing at a workload of 100 W and subsequently increasing by 25 W every 3-min. During assessment participants maintained a constant self-selected cadence (permitted range was 5 rev.min−1) and the test was terminated when a participant was no longer able to maintain a constant cadence.
Heart rate (HR) data was recorded continuously by radio-telemetry using a Cosmed HR monitor (Cosmed, Rome, Italy). During the test, blood samples were collected from the middle finger of the right hand at the end of the second minute of each 3-min interval. The fingertip was cleaned to remove any sweat or blood and lanced using a long point sterile lancet (Braun, Melsungen, Germany). The blood sample was collected into a heparinised capillary tube (Brand, Wertheim, Germany) by holding the tube horizontal to the droplet and allowing transfer by capillary action. Subsequently, a 25μL aliquot of blood was drawn from the capillary tube using a YSI syringepet (YSI, OH, USA) and added into the chamber of a YSI 1500 Sport lactate analyser (YSI, OH, USA) for determination of [BLa] in mmol.L−1. The lactate analyser was calibrated to the manufacturer’s requirements (± 0.05 mmol.L−1) before each test using a standard solution (YSI, OH, USA) of known concentration (5 mmol.L−1).
Gas exchange variables including VE, VO2 and carbon dioxide production (VCO2), were measured on a breath by breath basis throughout the test, using a cardiopulmonary exercise testing unit (CPET) and an associated software package (Cosmed, Rome, Italy). Participants wore a face mask (Hans Rudolf, KA, USA) which was connected to the CPET unit. The metabolic unit was calibrated each day using ambient air and an α certified gas mixture containing 16% O2, 5% CO2 and 79% N2 (Cosmed, Rome, Italy). Volume calibration was performed using a 3L gas calibration syringe (Cosmed, Rome, Italy) Pressure calibration was performed by recording barometric pressure using a laboratory grade barometer. TSI data was measured continuously from the VL musculature of the left leg using a portable near infrared spectroscopy unit (MOXY, MN, USA) and associated software (Peripedal, IN, USA). TSI data assessed oxy-haemoglobin as a percentage of total haemoglobin within the tissue. Both oxy-and deoxy-haemoglobin are chromophores, groups of atoms within a molecule that absorb light differently at different wavelengths (9). Light emitted into the tissue at one location is detected at another and the amount of light absorbed is computed. Consequently, it is possible to make quantitative measurements of the concentrations of chromophores using the Beer-Lambert law via the following equation:
where I0 is the starting intensity, I1 is the intensity exiting the tissue, ɛ is the molar absorptivity, a property of the chromophore available in published literature, Pl is the path length and c is the concentration. NIRS technology uses LEDs as light emitters that fire in rapid sequence, the exiting light intensity is recorded simultaneously at a detector located a fixed distance from the emitter. In the current study an opaque black support strap was placed around the leg and over the apparatus to prevent intrusion of any unwanted ambient light during data recording.
Statistical Analysis
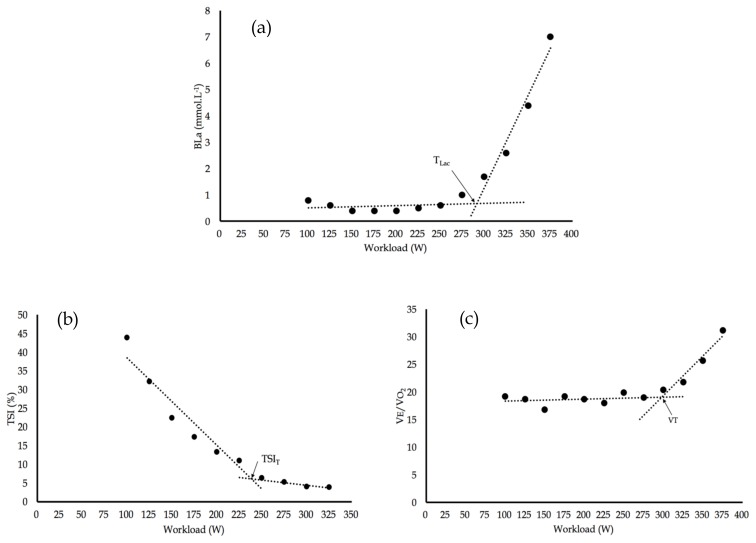
[BLa] was plotted as a function of workload. Mean ventilatory equivalent for oxygen (VE/VO2) and TSI data were determined during the final minute of each 3-min phase and subsequently plotted as a function of workload. Several methods have been devised to determine thresholds during the aerobic-anaerobic transition. In the current study, the method of threshold detection involved a segmented regression analysis. Two linear segments were plotted that minimised the squared sum of the residuals between the plotted points and best fit lines. The intersection of these the two linear segments was defined as the relevant breakpoint or threshold. This method was recently used by Driller et al. (5) to detect TLac, and proposed by Beaver et al. (1) to detect VT. This analysis procedure was used to identify TLac, VT and TSIT using commercially available software (SigmaPlot 13.0, Systat, IL, USA), see Figure 1. A linear best fit line was applied to each individual’s HR data set and HR at each computed threshold determined by extrapolation.
Figure 1.
(a) BLa vs workload displaying TLac (294 W), (b) TSI vs workload displaying TSIT (239 W) and (c) VEVO2 vs workload displaying VT (316 W).
The workload and HR at TLac, VT and TSIT were analysed using a one-way repeated measure analysis of variance (ANOVA). Tukey post-hoc multiple comparison tests quantified detected significant differences. Pearson’s product moment correlation coefficients (r) were computed between threshold variables to determine association; coefficients of determination (r2) were also computed. Bland-Altman analysis, with calculation of upper and lower 95%LoA, was performed to establish agreement between measures. For all statistical analysis a value of P < 0.05 inferred significance. All statistical analysis and graphical presentation of data were performed using Prism 6 (GraphPad, CA, USA).
RESULTS
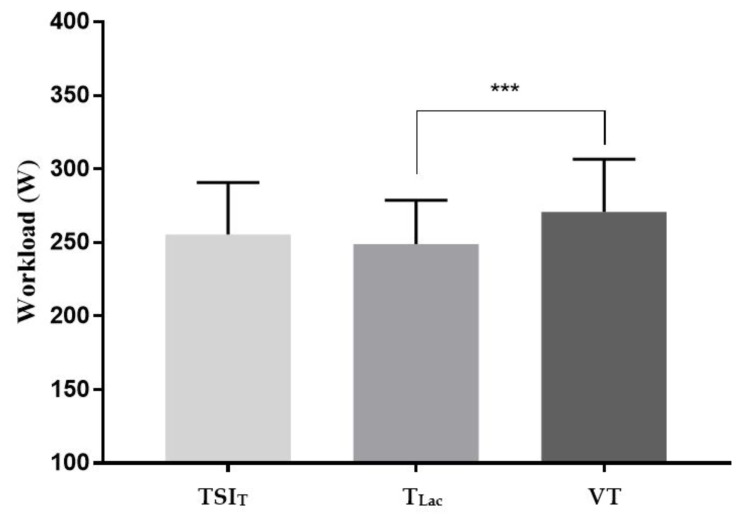
Thirty-one male individuals (n= 31) participated in the current study. Mean and standard deviation of GxT performance indicators are displayed in Table 1. Mean data for workload at computed thresholds are depicted in Figure 2. A repeated measures ANOVA revealed that mean workloads at computed thresholds were significantly different (F = 4.73, P ≤ 0.001). Tukey post-hoc tests quantified that load at VT was significantly higher than at TLac (P ≤ 0.001).
Table 1.
Mean GxT performance indicators, n=31.
| BLa at TLac (mmol.L−1) | Workload at OBLA (W) | Pmax (W) | VO2max (mL.kg−1.min−1) | HRmax (beats.min−1) | |
|---|---|---|---|---|---|
| Mean | 2.4 | 308 | 349 | 62.5 | 183 |
| SD | 1.0 | 33 | 31 | 8.1 | 11 |
Figure 2.
Mean workload at each computed threshold. *** denotes significant difference of P ≤ 0.001. Bars denote SD, n=31.
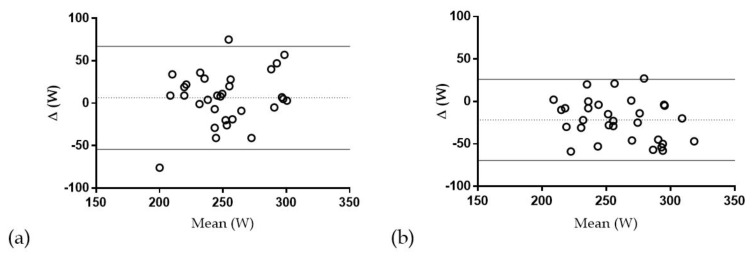
The coefficient of determination (r2) in the comparison of VT with TLac was 0.55, revealing that only a moderate proportion of the variance in TLac was attributable to VT. In addition, only moderate reproducibility was evident in the comparison of VT and TLac (ICC = 0.56). The wide limits of agreement in the comparison of VT vs TLac (+70 to −26 W) indicated moderate threshold concurrency, see Table 2 and Figure 3.
Table 2.
Correlation and association analysis of computed thresholds, n=31.
| r | ICC | Mean bias (W) | 95% LoA (W) | |
|---|---|---|---|---|
| TSIT vs TLac | 0.56 | 0.54 | 6.387 | +67 to −54 |
| TSIT vs VT | 0.41 | 0.36 | −15.35 | +60 to −91 |
| VT vs TLac | 0.74 | 0.56 | −21.74 | +70 to −26 |
Figure 3.
(a) Bland-Altman plot displaying mean bias with upper and lower 95% LoA for (a) TSIT vs TLac; (b) VT vs TLac; n=31.
Although workload at TSIT was not significantly different from any other computed threshold, the coefficient of determination (r2) in the comparison of TSIT with TLac was 0.31, indicating that only small to moderate proportion of the variance in TLac was attributable to TSIT. The computed ICC of TSIT with TLac was 0.54. The wide limits of agreement in the comparison of TSIT vs TLac (+67 to −54 W) also indicated moderate threshold concurrency, see Table 2 and Figure 3.
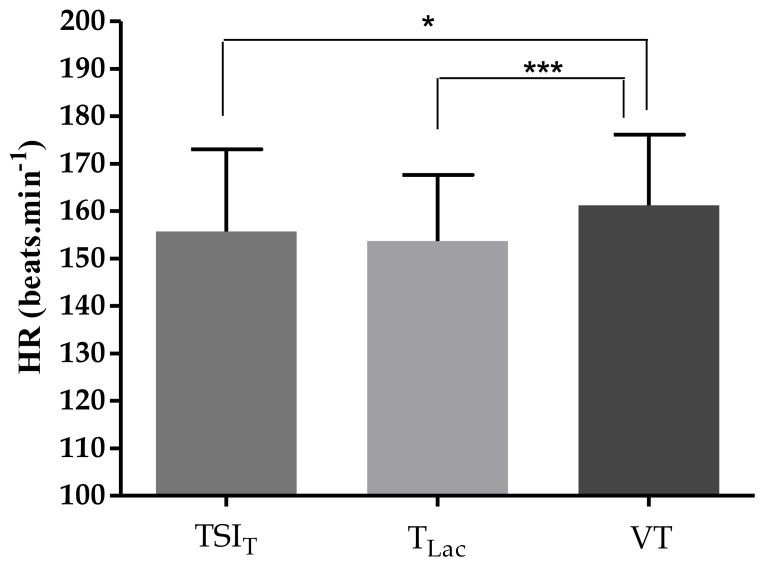
Mean HR at each computed threshold displayed a similar pattern of results to workload at each threshold, see Figure 4. Analysis revealed that HR data at computed thresholds were significantly different (F = 8.06, P ≤ 0.001). Tukey post-hoc analysis revealed that HR at VT was significantly higher than both TSIT (P < 0.05) and TLac (P < 0.001). Upper and lower 95% LoA and mean bias for HR data at computed threshold comparing TSIT and TLac were 18 to −22 and +2 beats.min−1, respectively, and comparing VT and TLac were 24 to −8 and +8 beats.min−1, respectively.
Figure 4.
Mean HR at each assessed threshold. * denotes significantly different to VT, * infers P < 0.05 and *** infers P < 0.001. Bars denote SD, n=31.
DISCUSSION
The aerobic-anaerobic transition is a concept used by physiologists, coaches and athletes to attain training benefits through specifically designed training programmes and for monitoring an athlete’s performance during training and racing. Ever since it was first described there has been an ongoing debate as to the specific mechanisms underlying it, and the most appropriate and accurate way to pinpoint it. It is now widely accepted that measuring [BLa] during incremental exercise is the ‘gold standard’ method, however, this is an invasive measure. Accordingly, non-invasive measures, such as VT and NIRS derived TSI, have been developed.
The aim of the current study was to establish if correlation and association existed between invasive and non-invasive measures. Several key findings were made: TSIT occurred at a non-significantly different (P > 0.05) mean workload to TLac. However, VT occurred at a significantly higher workload than both TLac and TSIT. There was a small to moderate correlation in the comparisons of TSIT with TLac, and a slightly stronger correlation in comparisons of VT with TLac.
Studies in the past have compared different methods of pinpointing the aerobic-anaerobic transition with many contrasting outcomes. The methodology and specific variables compared differ across studies making it difficult to draw comparisons and conclusions. Soller et al. (18) described NIRS as a method of estimating [H+] and concluded that TLac was significantly higher than VT1 and NIRS [H+] threshold. Zhang et al. (23) identified two inflexions in muscle oxygenation during incremental exercise. A significant correlation was identified between the first inflexion and VT1. Consequently, they concluded that NIRS could be used to detect TLac; however, this was potentially an errant conclusion as they did not show any comparisons to [BLa] or TLac. van der Zwward et al. (19) concluded that during incremental exercise the oxygenation threshold was reproducible and coincided with VT1. However, they also documented that VT1 recorded higher reproducibility than NIRS derived oxygenation threshold and therefore favoured it in the context of determining an aerobic-anaerobic transition. In their opinion, the unknown influence of adipose tissue thickness on absorbance may have led to this conclusion. The finding reported by Grassi et al. (8) that the onset of muscle deoxygenation was correlated with OBLA was not supported by the current study. The workload at OBLA (308±33 W) was significantly higher (P<0.001) than all other computed thresholds in the current study, poorly correlated with TSIT (r=0.5) and VT (r=0.54) and moderately correlated with TLac (r=0.82). The current study used the same method, namely a segmented linear regression model to detect TSIT, TLac and VT, and although several methods of threshold detection currently exist, it was decided that using the same mathematical procedure for threshold detection would eliminate potential sources of bias.
A recent study by Driller et al. (5) used a commercially available ‘wearable lactate threshold sensor’ and reported good agreement between it and other traditional markers of TLac. However, the study reported by Driller et al. (5) focused on the gastrocnemius musculature which would not be considered a primary force generating muscle group during cycling. The VL musculature was used in the current study as it has been shown, through EMG analysis, to be a primary force generating muscle during the crank cycle (11). Vastii musculature are primarily active during the pedal down-stroke with peak activity recorded around 55°, in contrast, the gastrocnemius musculature is only recruited for a short period after 90°, peaking around 107° (17).
In the current study, during the GxT the only variable which participants had control over was cycling cadence and it was a study requirement to maintain cadence within a 5 rev.min−1 range between 75 and 95 rev.min−1. Gotshall et al. (7) investigated the effect of 3 different pedal cadences during 200 W cycling on haemodynamic responses. Their data suggested that increasing the cadence resulted in a greater metabolic cost with the haemodynamic response favouring higher cadence. It was proposed that the action of the skeletal muscle pump improved across cadence resulting in increased muscle blood flow and cardiac output. Accordingly, an analysis was performed (data not shown) to determine if variation within this range (75 to 95 rev.min−1) had any effect on the workload and TSI at which each threshold occurred. No obvious pattern was uncovered, consequently, we concluded that the narrow cadence range used in the current study minimised any significant effect of cadence on the haemodynamic response and thus had little impact on transition detection.
The pattern of results obtained in the current study can be explained by a simple model assessing supply, demand and consumption of oxygen in the exercising musculature from a cellular and tissue level. Exercising at low intensities, such as, during the early stages of a GxT, relies primarily on the recruitment of slow oxidative aerobic fibers. During this phase there is no increase in [BLa] as slow oxidative fibers do not produce excessive amounts of lactate, are consumers of lactate and, consequently, the rate of lactate production equals the rate of lactate clearance. Under such conditions the supply of O2 equals demand through appropriate increases in cardiac output and local vasodilation. As exercise intensity progressively increases, O2 consumption increases and this can be seen through a steady decline in TSI as the musculature becomes deoxygenated. This decline in TSI eventually reaches a breakpoint and plateaus, indicative of O2 delivery reaching a maximum. As a result of this deoxygenation and an imbalance of O2 delivery relative to uptake and consumption, additional recruitment of anaerobic fibers occurs. Firstly, fast oxidative glycolytic fibers and eventually fast fatigable fibers which are potent producers of lactic acid. There is a resultant imbalance in the rates of lactate production and clearance, and, consequently [BLa] increases giving rise to TLac. The fact that TSIT does not occur at a significantly different workload to TLac could potentially indicate that the limiting of O2 delivery occurs in unison with, or is closely related to, the increase in [BLa]. However, the poor correlation and wide 95% LoA identified in the current study between TSIT and TLac indicates that other factors may also influence this process. Unlike TLac and VT, which are reflective of whole body status, TSIT is not a whole-body measure and uses shallow light penetration to sample a very small volume of tissue under investigation. In addition, while VL is a primary force producing muscle during cycling, other involved muscle groups may influence production of lactate and therefore non-metabolic CO2 and thus have an influence on the comparison of NIRS derived threshold with whole body measures.
The increase in [BLa] has a knock-on effect on ventilation and gas exchange variables. Lactic acid has a pKa of 3.87 and rapidly dissociates in body fluids (22). The resulting H+ ions are buffered by bicarbonate to form carbonic acid which dissociates into CO2 and H2O. Therefore, in addition to metabolically produced CO2, this non-metabolic production of CO2 is detected by ventilatory chemoreceptors. Changes in VE as a function of VO2 remain steady and linear during the aerobic phase as there is no non-metabolic production of CO2. During the anaerobic phase production of non-metabolic CO2 increases as H+ ions dissociated from lactic acid are buffered. This increase in CO2 production is manifested as an increase in VE but not in VO2. VE and VO2 become desynchronised giving rise to a detectable threshold in VE/VO2 data; namely, VT. In the current study, the workload at which VT occurred was significantly higher than the workload at TLac. This can be explained in the context of the underlying physiological mechanisms involved. Increases in lactic acid occur at the level of the exercising muscle. There is a time-lag before the resultant increase in non-metabolic CO2 can be detected by central chemoreceptors. The blood must travel through the circulatory system, diffuse across the blood brain barrier and be detected by central chemoreceptors before a change in ventilation can take place. Although VT was easily detectable, VT occurs after an appreciable increase the production of lactate. In endurance based activities, the athlete aims to limit the effects of fatigue and remain in an aerobic zone of energy production. Therefore, in this context, VT may not be as useful an indicator of the aerobic-anaerobic transition, since it appears significantly after the non-linear increase in blood lactate occurs.
Endurance based activities require athletes to sustain a relatively high exercise intensity for extended period of times. Athletes training for endurance events frequently maintain exercise HR in designated individualised zones based on laboratory based GxT to maximise their adaptive responses. Results of the current study imply that usage of [BLa] correlated HR zones remain the most effective method of training personalisation. The current wide limits of agreement depicted for TSIT vs TLac and VT vs TLac (Figure 3) suggest that non-invasive measures could potentially lead to inaccuracies for threshold identification on an individual basis. The wide limits of agreement observed following analysis of HR data at each detected threshold confirm this concern. Therefore, we would caution that usage of non-invasive threshold assessment could potentially inaccurately determine critical exercise intensities to effectively attain the desired training benefits.
The use of NIRS technology to detect critical exercise intensities has several limitations which have been highlighted in the past. Firstly, as indicated by van der Zwaard et al. (19) variations in adipose tissue thickness between participants can have an effect on the amplitude of NIRS signals. Adipose tissue thickness in the path of light penetration and detection was not measured and therefore its influence on the amplitude of NIRS signals could not be quantified. There are mixed consensuses regarding the specific placement of NIRS devices over the musculature. Mizuno et al. (12) and Koga et al. (10) stated that differences exists in the NIRS signal between the proximal and distal ends of the VL during static knee extension and cycling, respectively. However, Kime et al. (9) and Boone et al. (2) reported no difference in NIRS signal between the proximal and distal portions of the VL during cycling ramp exercise. The MOXY NIRS unit used in the current study is relatively new to the consumer market and a small body of research exists on its use in laboratory based environments. Little is known about the exact specifications and algorithms which MOXY uses to measure TSI/SmO2. Previous studies using NIRS as a means of measuring haemodynamic responses to exercise have used either a laboratory based (Hammatsu NIRO-200NX) or wireless portable unit (Artinins Portomon system) units. One problem encountered during the current study was the fact that MOXY reports data as ‘SmO2’ whereas studies using other systems report data as TSI, HHb, O2Hb or variations of the same. Following a review of the literature it was revealed that TSI and SmO2 are in fact the same and give an indication of the ratio of HbO2 to total Hb mass (tHb) in the musculature of interest expressed as a percentage. Cornachione et al. (4) stated that ‘SmO2, which has also been referred to the tissue oxygenation index and tissue saturation index, is considered a measure of the O2 saturation within the capillaries of subdermal skeletal muscle. As such, changes in SmO2 during exercise are a direct reflection of the balance between the availability of oxygenated blood and the use of O2 by the active muscles’. Therefore, TSI was used in the current study as it is a more universal measure, and in the future, it would be easier to compare apparatus and studies if the majority report data as TSI. A useful piece of research would be to compare the current NIRS unit to a laboratory standard NIRS unit, or to other portable NIRS units that have been used extensively in the scientific research to date. It would also prove useful to carry out a test retest study to assess the reliability of these various devices and methods of threshold detection.
In conclusion, the current study evaluated association of VT and TSIT with TLac during graded incremental exercise. No significant differences were observed for either workload or heart rate data at TSIT compared to TLac, possibly suggesting that limitations in O2 delivery may be closely linked to the aerobic-anaerobic transition. However, weak correlation between the two thresholds suggests that other factors may also have an influence on this process. VT occurred at a significantly higher workload and HR to TLac which would prove less useful to an athletic population as it gives an indication of when an aerobic-anaerobic transition has occurred rather than when it is occurring. Therefore, it would appear that neither TSIT nor VT are suitable non-invasive determinant of the aerobic-anaerobic transition when both are simultaneously compared to the invasive measure of TLac.
REFERENCES
- 1.Beaver W, Wasserman K, Whipp B. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 2.Boone J, Koppo K, Barstow T, Bouckaert J. Pattern of deoxy [Hb+ Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol. 2009;105:851–859. doi: 10.1007/s00421-008-0969-2. [DOI] [PubMed] [Google Scholar]
- 3.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornachione K, McLaren J, Heil D. Use of a wireless NIRS monitor to track changes in muscle oxygenation for laboratory-based Nordic skiing test protocol. Science and Skiing. 2014;VI:369–376. [Google Scholar]
- 5.Driller M, Borges N, Plews D. Evaluating a new wearable lactate threshold sensor in recreational to highly trained cyclists. Sports Eng. 2016;19:229–235. [Google Scholar]
- 6.Fujimoto S, Yoshikawa T, Tateishi Y, Lixin W, Hara T, Mimura T, Hirata K. Evaluation of muscle oxygenation during exercise by NIRS in normal subjects; significance of the NIRS threshold. Jap J Aniol. 2007;25:25–30. [Google Scholar]
- 7.Gottshall R, Bauer T, Fahrner S. Cycling cadence alters exercise haemodynamics. Int J Sports Med. 1996;1996;17:17–21. doi: 10.1055/s-2007-972802. [DOI] [PubMed] [Google Scholar]
- 8.Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P. Blood lactate accumulation and muscle deoxygenation during incremental exercise. J Appl Physiol. 1999;87:348–355. doi: 10.1152/jappl.1999.87.1.348. [DOI] [PubMed] [Google Scholar]
- 9.Kime R, Im J, Moser D, Lin Y, Nioka S, Katsumura T, Chance B. Reduced heterogeneity of muscle deoxygenation during heavy bicycle exercise. Med Sci Sports Exerc. 2005;37:412–417. doi: 10.1249/01.mss.0000155401.81284.76. [DOI] [PubMed] [Google Scholar]
- 10.Koga S, Poole C, Ferreira L, Whipp B, Kondo N, Saitoh T, Barstow T. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol. 2007;103:2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Caldwell G. Muscle coordination in cycling: effect of surface incline and posture. J Appl Physiol. 1998;85:927–934. doi: 10.1152/jappl.1998.85.3.927. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno M, Tokizawa K, Iwakawa T, Muraoka I. Inflection points of cardiovascular responses and oxygenation are correlated in the distal but not the proximal portions of muscle during incremental exercise. J Appl Physiol. 2004;97:867–873. doi: 10.1152/japplphysiol.00213.2004. [DOI] [PubMed] [Google Scholar]
- 13.Murkin J, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 14.Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol. 2014;5:142. doi: 10.3389/fphys.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao P, Danduran M, Loomba R, Dixon J, Hoffman G. Near-infrared spectroscopic monitoring during cardiopulmonary exercise testing detects anaerobic threshold. Pediatr Cardiol. 2012;33:791–796. doi: 10.1007/s00246-012-0217-8. [DOI] [PubMed] [Google Scholar]
- 16.Robergs R, Ghiasvand F, Parke D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol-Reg Integ Comp Physiol. 2004;28:502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 17.Ryan M, Gregor R. EMG profiles of lower extremity muscles during cycling at constant workload and cadence. J Electromyogr Kinesiol. 1992;2:69–80. doi: 10.1016/1050-6411(92)90018-E. [DOI] [PubMed] [Google Scholar]
- 18.Soller B, Yang Y, Lee S, Wilson C, Hagan R. Non-invasive determination of exercise-induced hydrogen ion threshold through direct optical measurement. J Appl Physiol. 2008;104:837–844. doi: 10.1152/japplphysiol.00849.2007. [DOI] [PubMed] [Google Scholar]
- 19.van der Zwaard S, Jaspers R, Blokland I, Achterberg C, Visser J, Anne R, de Koning J. Oxygenation threshold derived from near-infrared spectroscopy: Reliability and its relationship with the first ventilatory threshold. PloS one. 2016;11:e0162914. doi: 10.1371/journal.pone.0162914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman K, McIlroy M. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol. 1964;14:844–852. doi: 10.1016/0002-9149(64)90012-8. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman K, Whipp B, Koyl S, Beaver W. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35:236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 22.Whipp B. Physiological mechanisms dissociating pulmonary CO2 and O2 exchange dynamics during exercise in humans. Exp Physiol. 2007;92:347–355. doi: 10.1113/expphysiol.2006.034363. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Wang B, Nie Q, Luo Q, Gong H. Portable muscle oxygenation monitor based on near infrared spectroscopy. Front Optoelectron. 2009;2:248–252. [Google Scholar]