Abstract
Chronic supplementation of L-carnitine and carbohydrate has been reported to increase L-carnitine content in skeletal muscle and have positive influences on exercise variables and performance. This study investigated the acute intake of L-carnitine and carbohydrate on the exercise parameters of cycling. A total of 10 males (27.0 ± 4 years) completed two exercise sessions consisting of 40 min of cycling at 65% of VO2peak, followed by cycling to exhaustion at 85% of VO2peak. L-carnitine or a placebo was consumed 3 hours prior to exercise, and beverages consisting of 94 g of carbohydrate were consumed at both 2 hours, and 30 minutes prior to exercise. Repeated measures ANOVAs were used to compare respiratory exchange ratio (RER), blood lactate, and power output across experimental trials and time. A repeated measures t-test was used to analyze differences between conditions and time to exhaustion. RER was significantly lower (p=0.01) at baseline with L-carnitine ingestion (.83 ± .05) compared to the placebo ingestion (.86 ± .06). Blood lactate was significantly lower (p=0.02) after 10 minutes of cycling at 65% of VO2peak with ingestion of L-carnitine (35% change from baseline) compared to placebo ingestion (53% change from baseline). No differences were found for power output or time to exhaustion at 85% of VO2peak. Despite mentioned differences, acute intake of L-carnitine and carbohydrate does not appear to influence exercise parameters, likely due to a lack of sufficient change in the content of L-carnitine in skeletal muscle.
Keywords: Fuel utilization, supplementation, endurance exercise, time to exhaustion
INTRODUCTION
Endurance athletes of both recreational and professional caliber strive to improve performance. Supplementation is an avenue that some athletes use with the intention of impacting positively on performance. Not all endurance athletes refer to the use of supplements, but an estimated 20–30 billion dollars are spent on supplements each year in the United States and athletes of all levels are more likely to experiment with supplements compared to non-athletes (7, 15). The supplement L-carnitine is a multi-million-dollar industry every year, and the usage of L-carnitine is due to the role L-carnitine plays in fat oxidation (24). The primary function of L-carnitine is to shuttle activated fatty acyl molecules through the inner membrane of the mitochondria (5). A secondary function is to accept excess acetyl-CoA molecules in order to maintain the acetyl-CoA/CoA ratio in the mitochondrial matrix. When L-carnitine accepts an acetyl-CoA molecule, acetyl-carnitine is formed via the action of carnitine acetyltransferase and is moved out of the mitochondrial matrix (19).
Over the past 35 years, the impact of L-carnitine supplementation on exercise variables and performance has been investigated from both acute and chronic perspectives. The results of these studies have been inconsistent. For acute studies specifically, lower lactate levels (21, 29), increased power output (29), increased VO2peak (29), and increased time to exhaustion (8) have been reported, while other groups displayed no changes in physiological or performance variables (2, 4, 9, 17). In relation to chronic studies, increased VO2peak (11, 16) and decreased respiratory quotients (12, 32) have been presented, while many groups have reported no differences (3, 5, 6, 10, 13, 18, 23, 30). Despite some positive findings for acute and chronic studies, only three research groups performed muscle biopsies, and all three found no change in skeletal muscle content of L-carnitine, indicating no practical reason to supplement with L-carnitine alone (3, 4, 30).
The proposed mechanism of why supplementation of L-carnitine alone does not alter skeletal muscle content is due to the large concentration gradient of L-carnitine between the plasma and skeletal muscle (19). The concentration of L-carnitine in the plasma is 50–200 times less than in skeletal muscle (19, 26). To overcome the gradient, a Na+ dependent, high-affinity active transport process occurs (19). The transporter of L-carnitine across the sarcolemma is the organic cation transporter 2 (OCTN2) protein (26, 28). OCTN2 proteins are stimulated by extracellular Na+. A primary way to elevate the extracellular Na+ content is to increase the activity of the Na+/K+ pump (24). Insulin stimulates the activity of the Na+/K+ pump via translocation of the pump subunits from the intracellular location to the sarcolemma, similar to the response of GLUT4 transporters to insulin (27).
Recently there has been renewed interest in the study of L-carnitine supplementation due to the action of insulin stimulating the Na+/K+ pump. Stephens et al. (25) reported increased L-carnitine content in skeletal muscle when L-carnitine was infused with a six-hour euglycemic hyperinsulinemic clamp. The content of L-carnitine in skeletal muscle increased by 15% from preinfusion to postinfusion. This increase in L-carnitine content was associated with a decrease in pyruvate dehydrogenase activity (30%) and muscle lactate (40%) during resting conditions, leading to more glycogen storage in skeletal muscle after an overnight fast. Due to the involved nature and decreased practicality of the euglycemic hyperinsulinemic clamp, Stephens et al. (26) investigated the influence of L-carnitine and carbohydrate ingestion on L-carnitine retention. Carbohydrate intake was hypothesized to stimulate the release of sufficient amounts of insulin to aide in L-carnitine uptake. Compared to the control trial, L-carnitine levels to decrease at a faster rate. The 24-hour urinary L-carnitine was 30% lower in the experimental trial. Stephens et al (26) concluded that the increased retention of L-carnitine likely occurred in skeletal muscle due to the action of insulin stimulating Na+ dependent transport across the sarcolemma.
Wall et al. (31) was the first research team to apply the concepts of supplementing L-carnitine and carbohydrate (26) to the exercise setting. Participants ingested L-carnitine and carbohydrate for a total of 24 weeks. L-carnitine content in skeletal muscle increased by 30% with supplementation. Following 30 minutes of low intensity exercise, muscle glycogen content was 35% greater, indicating increased fatty acid utilization. After 30 minutes of vigorous intensity exercise, skeletal muscle lactate content was 44% lower, and pyruvate dehydrogenase activity was 38% greater signaling better maintenance of the acetyl-CoA/CoA ratio, and therefore less lactate accumulation and decreased reliance on anaerobic metabolism (31).
Although research teams have provided new information regarding methods to increase L-carnitine content in skeletal muscle via an insulin response (25, 26, 31), little evidence of the effect on exercise parameters is available, specifically supplementation from an acute perspective. Acute intake is of importance due to the practical nature of a single day procedure compared to supplementing for many weeks. The purpose of the current investigation was to evaluate whether acute L-carnitine and carbohydrate intake alters respiratory exchange ratio (RER), blood lactate, power output, and time to exhaustion during 40 minutes of cycling at 65% of VO2peak, followed by cycling to exhaustion at 85% of VO2peak. It was hypothesized that if the current ingestion procedures lead to a change in skeletal muscle content of L-carnitine, it was expected that L-carnitine and carbohydrate consumption would lead to decreases RER and blood lactate, while increasing power output at 65% of VO2peak. It was also thought that power output and time to exhaustion at 85% of VO2peak would be increased with ingestion of L-carnitine and carbohydrate if indeed adequate L-carnitine uptake into skeletal muscle took place.
METHODS
Participants
A power analysis (G*Power, Heinrich-Heine-Universität Düsseldorf) was run to assist in determining sample size, and a total of ten moderately active males between the ages of 18–35 from the Springfield, Massachusetts area agreed to partake in the investigation. It was required of participants to have a minimum VO2peak of 45 ml· kg−1· min−1 in order to participate in the study. The value of 45 ml· kg−1· min−1 was used as the inclusion criteria because it would place participants at a minimum within the “good” category (60th percentile or above) for maximal oxygen consumption according to the American College of Sports Medicine standards for males between the ages of 20–29 (1). Participants were volunteers, and completed an informed consent and medical history questionnaire prior to participating in the study. All methods and procedures were reviewed and approved by the Institutional Review Board of Springfield College and conducted in accordance with the Declaration of Helsinki. See Table 1 for participant demographics.
Table 1.
Descriptive Statistics of Participants Represented as Mean and Standard Deviations (N = 10)
| Age | 27.00 ± 4.83 |
| VO2peak (ml· kg−1· min−1) | 50.90 ± 6.06 |
| Height (cm) | 177.00 ± 5.00 |
| Weight (kg) | 75.60 ± 8.51 |
| Body Fat (%) | 11.00 ± 5.06 |
| Endurance Exercise Participation (yrs) | 9.40 ± 6.00 |
| Exercise Sessions per Week (days) | 3.85 ± 2.06 |
| Duration of Exercise Sessions (min) | 57.25 ± 21.03 |
cm = centimeters, kg = kilograms, yrs = years, min = minutes
The current study was designed as a double-blind, randomized counterbalanced format. Data were obtained from all participants over the course of three testing sessions. All participants were familiarized with the procedures prior to partaking in the investigation. Participants served as their own control, so each condition was experienced by each participant.
Protocol
All testing sessions took place in the Human Performance Laboratory on the campus of Springfield College. Participants reported to the lab on three different occasions; first for initial testing, followed by two experimental trials. Participants reported at 7am for each testing session to control for diurnal variations and circadian rhythms. The first session included completion of the informed consent, medical history questionnaire, and the participant training/nutrition questionnaire. Height and mass were determined, and body composition was estimated via air displacement plethysmography (BOD POD, Cosmed, Rome, Italy). Participants then completed a maximal incremental exercise test on a Velotron cycle simulator (RacerMate, Inc. Seattle, WA) in order to determine VO2peak using a metabolic system (Physio-Dyne Max-II, AEI Technologies, Pittsburg, PA). Prior to the exercise test, participants were informed of the procedure and protocol. The seat on the cycle simulator was then fitted to the participant, specifically at 97% of greater trochanter height. The incremental test started with a power output of 95 watts, and increased by 35 watts every three minutes until conclusion of the test (20). Criteria for obtaining a valid VO2peak included: the point at which VO2 did not increase by more than 1.5 ml· kg−1· min−1 with an increase in work rate, an RER value greater than 1.20, or when the participant reached volitional exhaustion. The VO2peak value from the incremental exercise test was used to determine exercise intensities for both the L-carnitine and placebo trials.
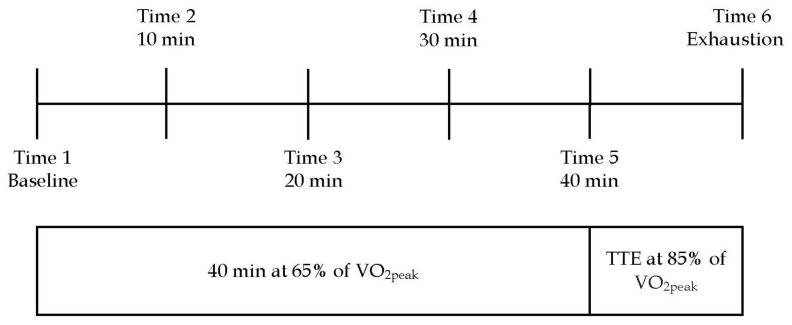
Participants were instructed to refrain from strenuous exercise for 24 hr prior to each of the experimental testing sessions. The experimental sessions were randomized to eliminate order effect. Participants were asked not to consume any alcohol or caffeine for 12 hr prior to each testing condition, and to adhere to the same diet 48 hr before each testing session. The exercise task consisted of performing 40 min of cycling at 65% of VO2peak, immediately followed by cycling at 85% of VO2peak until exhaustion. Criteria for exhaustion included: volitional fatigue, an RER value greater than 1.20, or a drop in RPMs below 50. Two different exercise intensities were administered in order to investigate the impact of supplementation on both physiological roles of L-carnitine (8, 31). The trials were held at least three days apart from each other to allow for adequate recovery time. See Figure 1 for a schematic representation of the exercise task.
Figure 1.
Schematic representation of the exercise task for both the L-carnitine and placebo conditions. Variables were measured at each 10-minute interval while cycling at 65% of VO2peak, and again when exhaustion occurred at 85% of VO2peak. *TTE = time to exhaustion.
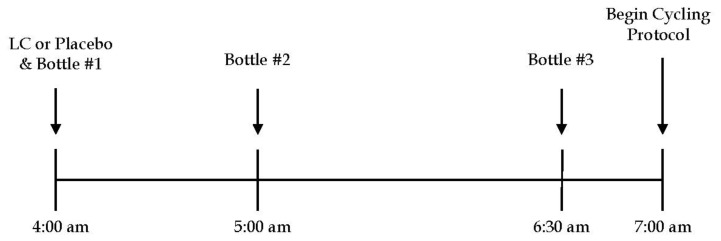
Ingestion Procedures Prior to both the L-carnitine and placebo trials, participants were provided solutions and capsules to ingest. The solutions were consumed after an overnight fast. Each participant was given three bottles, and capsules containing either 3 g of L-carnitine (Now Foods, Bloomingdale, IL) for the L-carnitine trial or flour for the placebo trial. Bottle one contained 200 ml of water and was consumed with the capsules of L-carnitine or flour 3 hr prior to the exercise session (4 am). Bottles two and three both contained 500 ml of solution with 94 g of carbohydrate in each bottle (Kool-Aid Fruit Punch Powder, Kraft Foods, Inc. Chicago, IL). The second bottle was consumed 2 hr prior to the exercise session (5 am). The third bottle was consumed 30 min prior to the exercise session (6:30 am). Sucrose was used as the carbohydrate source, which is a multiple transport carbohydrate. The ingestion procedures consisting of 94 g of carbohydrate, type of carbohydrate used, and the timing of ingestion followed the work of Stephens et al (26), which successfully lead to whole body L-carnitine retention in humans when the same ingestion procedures were used from an acute perspective. The importance of two bottles consisting of 94 g of carbohydrate is to stimulate a strong insulin response to assist in the uptake of L-carnitine into skeletal muscle. See Figure 2 for representation of the ingestion protocol.
Figure 2.
Schematic representation of the ingestion procedures administered prior to the exercise task for both the L-carnitine (LC) and Placebo conditions. Bottle #1 = 200 ml of water; Bottle #2 = 94 g of CHO in 500 ml of water; Bottle #3 = 94 g of CHO dissolved in 500 ml of water.
Variables Measured : RER, blood lactate, and power output were recorded at baseline (time point 1), 10 min at 65% of VO2peak (time point 2), 20 min at 65% of VO2peak (time point 3), 30 min at 65% of VO2peak (time point 4), 40 min at 65% of VO2peak (time point 5), and when the participant reached exhaustion when exercising at 85% of VO2peak (time point 6). RER was recorded via the metabolic system, power output was recorded via the Velotron cycle simulator, and blood lactate was recorded via capillary blood drawn from the fingertips of participants (Accusport Lactate Analyzer, Sports Resource Group, Hawthorne, NY). The time to exhaustion variable was recorded only when the participant reached exhaustion at 85% of VO2peak.
Statistical Analysis
The dependent variables included RER, blood lactate, power output, and time to exhaustion. A total of three 2 × 6 repeated measures analysis of variances were used to assess differences in RER, blood lactate, and power output across experimental trials and time. The independent variables included condition and time. The two levels of the first independent variable (condition) were L-carnitine and placebo conditions. The six levels of the second independent variable (time) were described in the previous section. The Mauchly’s Test of Sphericity was used to determine if the basic assumption of homogeneity of variance was violated. If the basic assumption of homogeneity of variance was violated, the Greenhouse-Geisser Statistic was used. If a significant interaction was found, a simple effects test was conducted to determine where differences existed. A repeated measures t-test was used to assess differences in time to exhaustion when cycling at 85% of VO2peak between the L-carnitine and placebo conditions. SPSS was used to run all statistical analyses (IBM Corp, Version 22), and p<0.05 was accepted as a significant difference for the statistical analyses of the investigation.
RESULTS
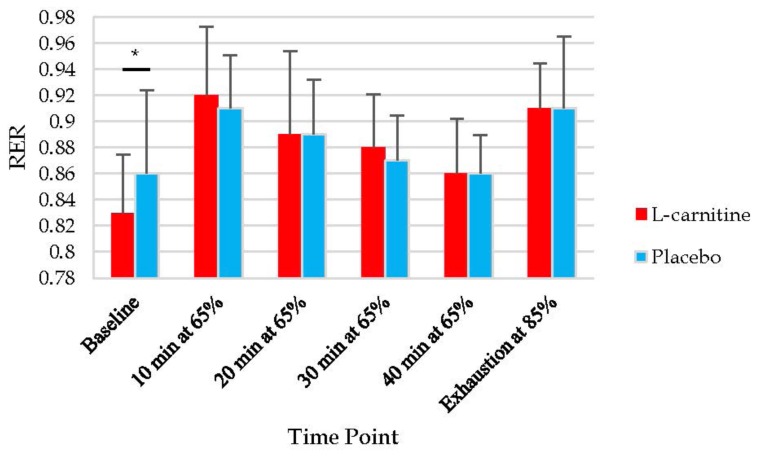
Descriptive statistics for all variables included in the repeated measures ANOVAs can be found in Table 2. A significant interaction was found between condition and time for RER (p=0.01). RER was significantly lower at baseline during the L-carnitine condition when compared to the placebo condition. The mean RER at baseline during the L-carnitine condition was 0.83 ± 0.05, and the mean RER at baseline during the placebo condition was 0.86 ± 0.06. At all other time points there were no significant differences for RER (Figure 3).
Table 2.
Descriptive statistics of mean values and standard deviations for exercise variables at all time pints for L-carnitine and placebo conditions.
| Time 1 | Time 2 | Time 3 | Time 4 | Time 5 | Time 6 | |
|---|---|---|---|---|---|---|
| RER | ||||||
| L-carnitine | 0.83±0.05* | 0.92±0.05 | 0.89±0.04 | 0.88±0.04 | 0.86±0.04 | 0.91±0.05 |
| Placebo | 0.86±0.05 | 0.91±0.04 | 0.89±0.04 | 0.87±0.03 | 0.88±0.03 | 0.91±0.05 |
| Blood Lactate | ||||||
| L-carnitine | N/A | 35±49* | 55±100 | 59±77 | 67±74 | 162±114 |
| Placebo | N/A | 53±44 | 55±64 | 52±37 | 58±63 | 117±57 |
| Power Output | ||||||
| L-carnitine | N/A | 159±23 | 155±20 | 154±20 | 153±20 | 208±31 |
| Placebo | N/A | 157±22 | 155±21 | 151±20 | 150±19 | 205±28 |
Units: blood lactate, percent change from baseline; power output, watts. Time 1, baseline; Time 2, 10 min at 65% of VO2peak; Time 3, 20 min at 65% of VO2peak; Time 4, 30 min at 65% of VO2peak; Time 5, 40 min at 65% of VO2peak; Time 6, time to exhaustion at 85% of VO2peak.
p<0.05 for differences between L-carnitine and Placebo conditions.
Figure 3.
Changes in RER for both the L-carnitine and placebo conditions. Values are expressed as means and standard deviations. Percentages represent percent of VO2peak. * p<0.05 for differences between L-carnitine and placebo conditions
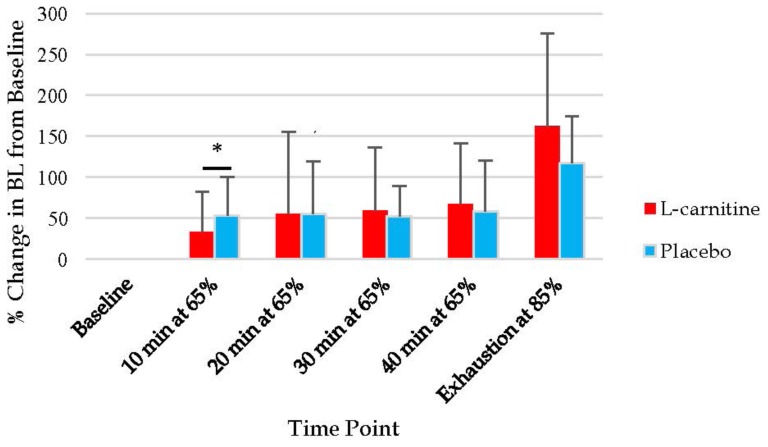
A significant interaction was found between condition and time for blood lactate (p=0.02). Blood lactate was significantly lower after 10 min of cycling at 65% of VO2peak during the L-carnitine condition when compared to the placebo condition. The mean blood lactate concentration at time point 2 for the L-carnitine condition was 35% ± 11% greater than baseline, while in the placebo condition blood lactate levels were 53% ± 31% greater than baseline. Blood lactate levels did not differ significantly at any other time points between the conditions (Figure 4).
Figure 4.
Changes in blood lactate (BL) represented as a percent change from baseline for both the L-carnitine and placebo conditions. Values are expressed as means and standard deviations. Percentages represent percent of VO2peak.* p<0.05 for differences between L-carnitine and placebo conditions.
No significant interaction was found between condition and time for power output (p=0.66). As well, no main effect was found for power output between conditions (p=0.39). However, a main effect for time was found (p=0.00). The post hoc analysis revealed expected physiological responses to the exercise task.
A repeated measures t-test was used to assess the differences in time to exhaustion when cycling at 85% of VO2peak. The mean time to exhaustion for the L-carnitine condition was 8:53 ± 6:07 min. The mean time to exhaustion for the placebo condition was 10:10 ± 6:59. No significant difference existed between conditions (p=0.41).
DISCUSSION
Only recently has there been evidence that L-carnitine content in skeletal muscle can be increased when supplementation is coincided with a strong insulin response (25, 31). Although the increase of L-carnitine did improve performance and exercise variables when supplementing for 24 weeks (31), doing so for that length of time is not practical due to the high load of carbohydrate intake each day. The purpose of the current research was to investigate the more realistic nature of acute intake of L-carnitine and carbohydrate in the exercise setting.
Previous research by Stephens et al (25) indicated alterations of resting fuel metabolism and increases in the content of L-carnitine in skeletal muscle via the use of a six hour euglycemic hyperinsulinemic insulin clamp. A proposed increase in muscle fat oxidation was suggested due to an increase in glycogen storage, indicating an inhibition of carbohydrate oxidation from an increase in L-carnitine mediated fat oxidation. In the current investigation, baseline RER values were lower when acute L-carnitine and carbohydrate intake occurred, indicating a possible increase in fatty acid oxidation during the resting state. Although the decrease in RER is in accordance with previous research, the methods for ingestion in the current investigation occurred via oral ingestion, not the euglycemic hyperinsulinemic clamp (25). The lower RER value in the current study may be due to an increase in the L-carnitine content in skeletal muscle, and if indeed the content of L-carnitine increased, the proposed mechanism for L-carnitine uptake is thought to occur via a strong insulin response stimulating the activity of the Na+/K+ pump, increasing extracellular Na+ concentration. Active transport of L-carnitine into skeletal muscle is Na+ dependent, therefore increasing the amount of Na+ in the extra-cellular location could stimulate L-carnitine transport into skeletal muscle (24, 26–28).
Despite lower RER values at rest with L-carnitine and carbohydrate ingestion in the current investigation, RER values did not differ between conditions during any time point of the exercise task. A lack of differences indicates one of two occurrences. First, L-carnitine content in skeletal muscle may not have been influenced with acute L-carnitine intake, and all indications point toward this outcome. Second, even if skeletal muscle L-carnitine content increased enough to influence resting RER, it may not have been sufficient enough of an increase in the muscle pool to alter fuel metabolism during exercise. Regardless of the occurrence, RER values during the exercise task were not influenced with acute intake, which indicates no changes in the substrate being utilized.
Along with RER, blood lactate levels differed between conditions at one time point. After 10 minutes of cycling at 65% of VO2peak, blood lactate was significantly lower when L-carnitine and carbohydrate were consumed prior to exercise. Despite the difference in blood lactate between the conditions at 10 minutes into exercise, there were no other differences at any time point of the exercise task. If the skeletal muscle L-carnitine content had increased with acute intake, it would be expected that blood lactate would be lower at all time points, not just 10 minutes into the exercise task (31). There are two proposed mechanisms for reduced blood lactate following alterations in the pool of L-carnitine in skeletal muscle. With more L-carnitine in the muscle, the amount of acetyl-CoA production from β-oxidation may increase, leading to more citrate being produced. The increased levels of citrate could cause a down regulation of carbohydrate flux because of product inhibition of critical enzymes used within glucose oxidation including pyruvate dehydrogenase, phosphofructokinase, and hexokinase, leading to less lactate production (26). A secondary role of L-carnitine is to maintain the acetyl-CoA/CoA ratio during intense exercise. Balancing the ratio allows the activity of the enzyme pyruvate dehydrogenase to be maintained because acetyl-CoA production will match acetyl-CoA entrance into the TCA cycle, allowing more pyruvate to be shuttled into the mitochondria without being reduced to lactate (30, 31).
Despite lower blood lactate after 10 minutes of cycling at 65% of VO2peak in the L-carnitine condition, subsequent differences were not observed for RER at the same time point. Lack of differences in RER when blood lactate was lower indicates that the mechanism for lower blood lactate was not due to an increase in fatty acid oxidation. It is possible that the blood lactate levels were lower because of the secondary role of L-carnitine balancing the acetyl-CoA/CoA ratio allowing pyruvate to be converted into acetyl-CoA instead of lactate (31), but this is unlikely due to the moderate intensity level when blood lactate was evaluated. If the skeletal muscle content of L-carnitine would have increased by a sufficient amount, it is expected that blood lactate would be lower when compared to the placebo condition throughout the entire exercise bout, not just at a single time point. Lack of differences between conditions indicates that the L-carnitine content in skeletal muscle was not changed by a sufficient amount to produce differences in blood lactate that would be considered beneficial to an entire exercise bout.
Power output was similar between both the L-carnitine and placebo conditions. Previously, Wall et al (31) reported power output to be increased by 11% after chronic supplementation of L-carnitine and carbohydrate. The proposed reason for the increase in power output was due to a 21% increase in skeletal muscle L-carnitine content. Wall et al (31) concluded that the increased L-carnitine content allowed for better buffering of excess acetyl-CoA molecules contributing to lower lactate levels. Lower lactate levels allowed the participants to maintain a higher workload without the adverse effects of decreasing pH. In the present study, no differences in power output between trials indicates a lack of sufficient change in skeletal muscle content of L-carnitine. Therefore, no positive effects of excess acetyl-CoA buffering would occur, causing lactate levels to be the same between conditions.
When evaluating the demographics of the participants, it is evident that there was a wide range in terms of number of days of aerobic based exercise sessions per week as well as the typical duration of those sessions. Most participants included in the study participated in aerobic exercise sessions of 45 min or more. One participant was a competitive cyclist who trained for at least 90 min or more per session. Another participant had an average of 30 min for typical aerobic exercise sessions, the lowest average duration of all the participants. It is important to note that this participant performed a significant amount of high intensity interval training, instead of standard aerobic based training. High intensity interval training yields similar adaptations to standard aerobic training (14). Based on this information, it is likely that the duration of the experimental trials in the currently study were not impacted by differences in the aerobic training practices of the participants. Despite wide ranges in the training history of participants, all were above the criteria of a VO2peak of 45 ml· kg−1· min−1, indicating at least a moderate level of cardiorespiratory fitness or higher. Previous research has shown that untrained sedentary individuals were able to cycle below lactate threshold at 61.6% of VO2max (22). Subjects in the currently study, being at least moderately trained, should have been able to maintain 40 min at 65% of VO2peak while staying below lactate threshold, which was supported by collected data.
The primary finding of the current research is that the acute intake of L-carnitine and carbohydrate that follows the specific protocol used in this investigation does not appear to impact the pool of L-carnitine in skeletal muscle enough to experience positive changes in exercise variables during a bout of both moderate and vigorous intensity cycling. The supplemental patterns used in this investigation were based on the procedures that lead to whole body L-carnitine retention (26). These same procedures did not impact positively on exercise parameters most likely due to a lack of sufficient accumulation of L-carnitine in skeletal muscle. Therefore, acute supplementation of L-carnitine and carbohydrate is not recommended be used as a means of improving cycling performance when using the supplemental protocol from the current work. For individuals looking to improve exercise variables and performance via the use of L-carnitine supplementation, chronic L-carnitine and carbohydrate intake is a better method to increase the content of L-carnitine in skeletal muscle and to influence performance (31). Future research should investigate shorter duration supplemental periods of L-carnitine and carbohydrate, while also looking at accumulation in skeletal muscle via biopsies. Identifying a shorter supplementation period would increase the practicality as well as adherence to supplemental protocols.
ACKNOWLEDGEMENTS
No funding was provided to contribute to the completion of this manuscript. The authors report no conflicts of interest. The authors appreciate the time the participants dedicated to the study and adhering to the early morning ingestion procedures. The authors would also like to thank Dr. Elizabeth O’Neill for her contributions to the project.
REFERENCES
- 1.ACSM. ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 2.Abramowicz WN, Galloway SD. Effects of acute versus chronic L-carnitine L-tartrate supplementation on metabolic responses to steady state exercise in males and females. Int J Sport Nutr Exerc Metab. 2005;15(4):386–400. doi: 10.1123/ijsnem.15.4.386. [DOI] [PubMed] [Google Scholar]
- 3.Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, Fink WJ. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int J Sport Nutr. 1994;4(3):280–288. doi: 10.1123/ijsn.4.3.280. [DOI] [PubMed] [Google Scholar]
- 4.Brass EP, Hoppel CL, Hiatt WR. Effect of intravenous L-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin Pharmacol Ther. 1994;55(6):681–692. doi: 10.1038/clpt.1994.85. [DOI] [PubMed] [Google Scholar]
- 5.Broad EM, Maughan RJ, Galloway SD. Effects of four weeks L-carnitine L-tartrate ingestion on substrate utilization during prolonged exercise. Int J Sport Nutr Exerc Metab. 2005;15(6):665–679. doi: 10.1123/ijsnem.15.6.665. [DOI] [PubMed] [Google Scholar]
- 6.Broad EM, Maughan RJ, Galloway SD. Carbohydrate, protein, and fat metabolism during exercise after oral carnitine supplementation in humans. Int J Sport Nutr Exerc Metab. 2008;18(6):567–584. doi: 10.1123/ijsnem.18.6.567. [DOI] [PubMed] [Google Scholar]
- 7.Cassileth BR, Heitzer M, Wesa K. The public health impact of herbs and nutritional supplements. Pharm Biol. 2009;47(8):761–767. doi: 10.1080/13880200902991581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha YS, Choi SK, Suh H, Lee SN, Cho D, Li K. Effects of carnitine coingested caffeine on carnitine metabolism and endurance capacity in athletes. J Nutr Sci Vitaminol (Tokyo) 2001;47(6):378–384. doi: 10.3177/jnsv.47.378. [DOI] [PubMed] [Google Scholar]
- 9.Colombani P, Wenk C, Kunz I, Krahenbuhl S, Kuhnt M, Arnold M, Frey-Rindova P, Frey W, Langhans W. Effects of L-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: a double-blind crossover field study. Eur J Appl Physiol Occup Physiol. 1996;73(5):434–439. doi: 10.1007/BF00334420. [DOI] [PubMed] [Google Scholar]
- 10.Decombaz J, Deriaz O, Acheson K, Gmuender B, Jequier E. Effect of L-carnitine on submaximal exercise metabolism after depletion of muscle glycogen. Med Sci Sports Exerc. 1993;25(6):733–740. [PubMed] [Google Scholar]
- 11.Dragan GI, Vasiliu A, Georgescu E, Dumas I. Studies concerning chronic and acute effects of L-carnitine on some biological parameters in elite athletes. Physiologie. 1987;24(1):23–28. [PubMed] [Google Scholar]
- 12.Gorostiaga EM, Maurer CA, Eclache JP. Decrease in respiratory quotient during exercise following L-carnitine supplementation. Int J Sports Med. 1989;10(3):169–174. doi: 10.1055/s-2007-1024895. [DOI] [PubMed] [Google Scholar]
- 13.Greig C, Finch KM, Jones DA, Cooper M, Sargeant AJ, Forte CA. The effect of oral supplementation with L-carnitine on maximum and submaximum exercise capacity. Eur J Appl Physiol Occup Physiol. 1987;56(4):457–460. doi: 10.1007/BF00417775. [DOI] [PubMed] [Google Scholar]
- 14.Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 15.Knapik JJ, Steelman RA, Hoedebecke SS, Austin KG, Farina EK, Lieberman HR. Prevalence of dietary supplement use by athletes: systematic review and meta-analysis. Sports Med. 2016;46(1):103–123. doi: 10.1007/s40279-015-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marconi C, Sassi G, Carpinelli A, Cerretelli P. Effects of L-carnitine loading on the aerobic and anaerobic performance of endurance athletes. Eur J Appl Physiol Occup Physiol. 1985;54(2):131–135. doi: 10.1007/BF02335919. [DOI] [PubMed] [Google Scholar]
- 17.Natali A, Santoro D, Brandi LS, Faraggiana D, Ciociaro D, Pecori N, Buzzigoli G, Ferrannini E. Effects of acute hypercarnitinemia during increased fatty substrate oxidation in man. Metabolism. 1993;42(5):594–600. doi: 10.1016/0026-0495(93)90218-d. [DOI] [PubMed] [Google Scholar]
- 18.Oyono-Enguelle S, Freund H, Ott C, Gartner M, Heitz A, Marbach J, Maccari F, Frey A, Bigot H, Bach AC. Prolonged submaximal exercise and L-carnitine in humans. Eur J Appl Physiol Occup Physiol. 1988;58(1–2):53–61. doi: 10.1007/BF00636603. [DOI] [PubMed] [Google Scholar]
- 19.Pekala J, Patkowska-Sokola B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S, Librowski T. L-carnitine--metabolic functions and meaning in humans life. Curr Drug Metab. 2011;12(7):667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 20.Romer LM, McConnell AK, Jones DA. Inspiratory muscle fatigue in trained cyclists: effects of inspiratory muscle training. Med Sci Sports Exerc. 2002;34(5):785–792. doi: 10.1097/00005768-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Siliprandi N, Di Lisa F, Pieralisi G, Ripari P, Maccari F, Menabo R, Giamberardino MA, Vecchiet L. Metabolic changes induced by maximal exercise in human subjects following L-carnitine administration. Biochim Biophys Acta. 1990;1034(1):17–21. doi: 10.1016/0304-4165(90)90147-o. [DOI] [PubMed] [Google Scholar]
- 22.Simon J, Young JL, Blood DK, Segal KR, Case RB, Gutin B. Plasma lactate and ventilation thresholds in trained and untrained cyclists. J Appl Physiol. 1986;60(3):777–781. doi: 10.1152/jappl.1986.60.3.777. [DOI] [PubMed] [Google Scholar]
- 23.Soop M, Bjorkman O, Cederblad G, Hagenfeldt L, Wahren J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J Appl Physiol. 1988;64(6):2394–2399. doi: 10.1152/jappl.1988.64.6.2394. [DOI] [PubMed] [Google Scholar]
- 24.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581(Pt 2):431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab. 2006;91(12):5013–5018. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- 26.Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments L-carnitine retention in humans. J Appl Physiol. 2007;102(3):1065–1070. doi: 10.1152/japplphysiol.01011.2006. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney G, Klip A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol Cell Biochem. 1998;182(1–2):121–133. [PubMed] [Google Scholar]
- 28.Tamai I, Ohashi R, Nezu Ji, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 29.Vecchiet L, Di Lisa F, Pieralisi G, Ripari P, Menabo R, Giamberardino MA, Siliprandi N. Influence of L-carnitine administration on maximal physical exercise. Eur J Appl Physiol Occup Physiol. 1990;61(5–6):486–490. doi: 10.1007/BF00236072. [DOI] [PubMed] [Google Scholar]
- 30.Vukovich MD, Costill DL, Fink WJ. Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise. Med Sci Sports Exerc. 1994;26(9):1122–1129. [PubMed] [Google Scholar]
- 31.Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol. 2011;589(Pt 4):963–973. doi: 10.1113/jphysiol.2010.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyss V, Ganzit GP, Rienzi A. Effects of L-carnitine administration on VO2max and the aerobic-anaerobic threshold in normoxia and acute hypoxia. Eur J Appl Physiol Occup Physiol. 1990;60(1):1–6. doi: 10.1007/BF00572178. [DOI] [PubMed] [Google Scholar]