Zhang H., Zhang T.T., Liu H., Shi D.Y., Wang M., Bie X.M., Li X.G., and Zhang X.S. Thioredoxin-Mediated ROS Homeostasis Explains Natural Variation in Plant Regeneration.
The authors have acknowledged that errors were made in the creation of some of the figures for this article. A detailed description of the figure corrections is shown below. Both original and corrected figures are provided for ease of comparison.
In the original Figure 3, B and D, the gel blot images were overly contrasted and tightly cropped. The top image in Figure 3B and images in Figure 3D were combined from two gels. In the corrected Figure 3, B and D, the images are appropriately cropped and provided without any manipulations of contrast. Three white lines have been inserted to separate the top image in Figure 3B and images in Figure 3D. The legend of the figure has been changed accordingly for clarity.
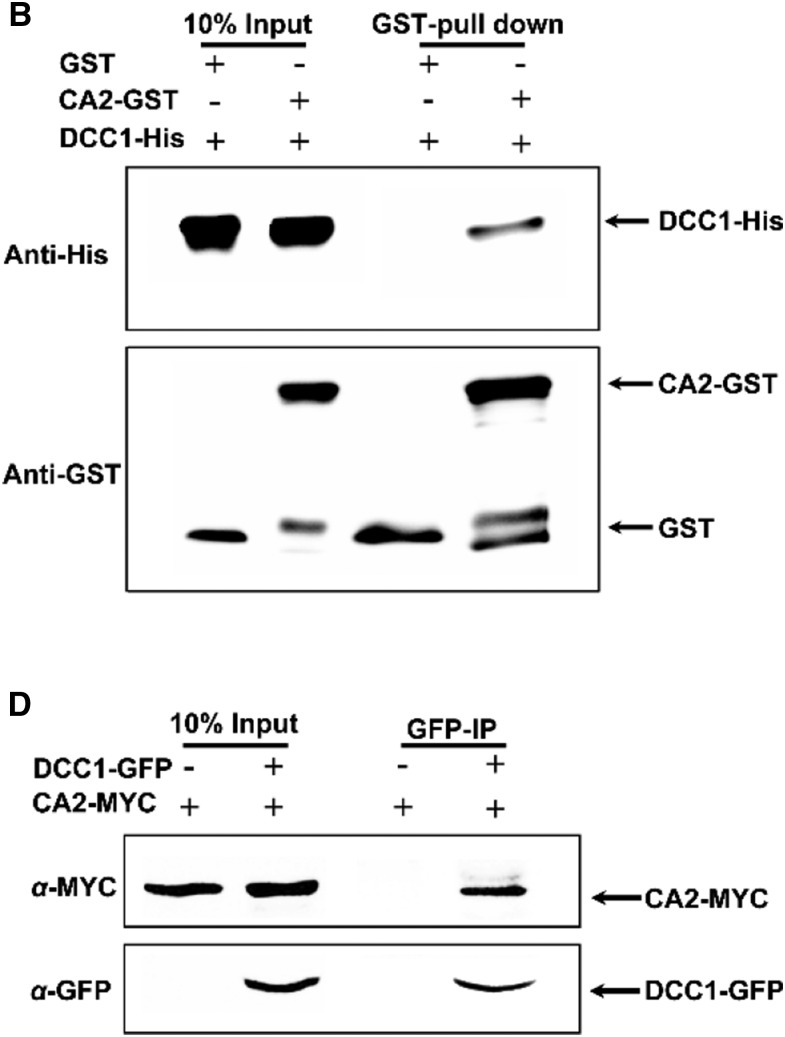
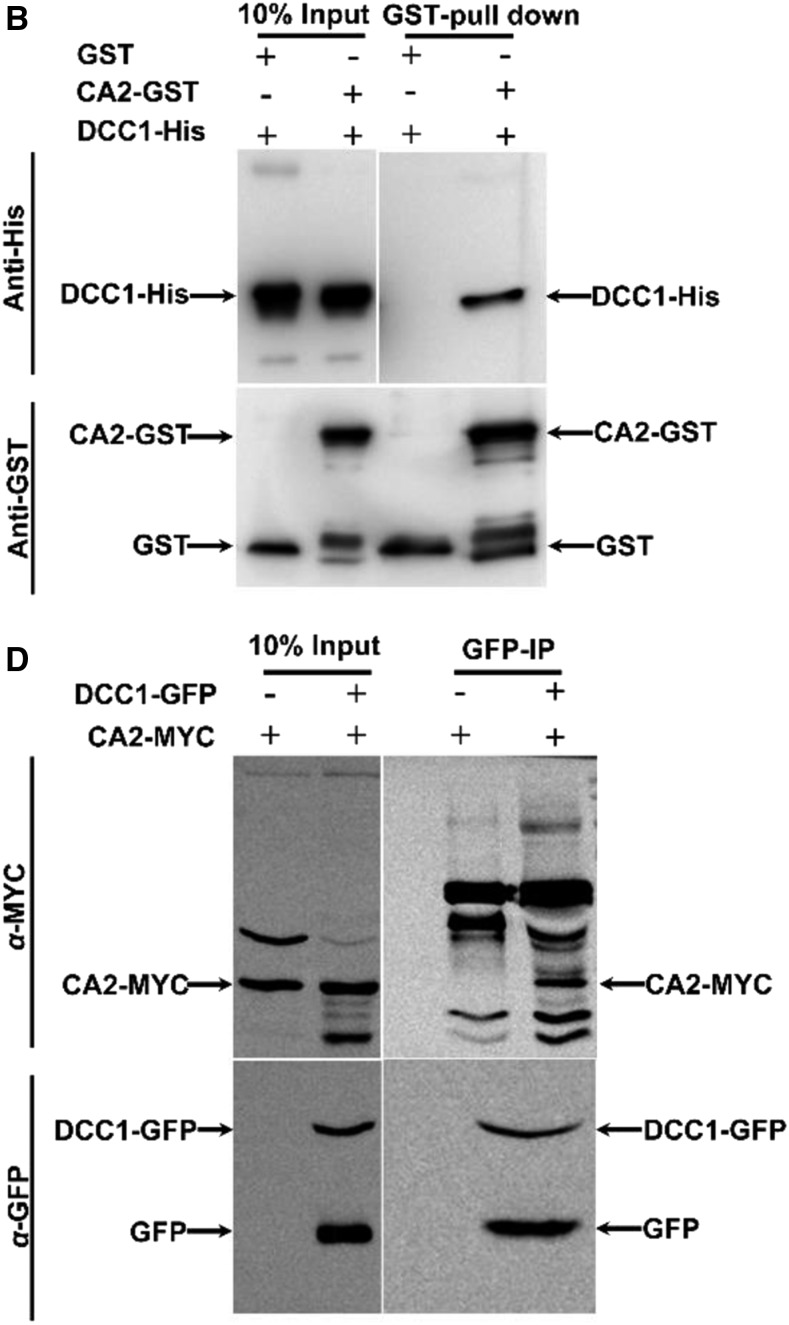
Figure 3.
Original: DCC1 interacts with CA2. B, Interaction between DCC1 and CA2 in the pull-down assay. GST pull-down protein was detected by immunoblotting using anti-His antibody. D, Interaction between DCC1 and CA2 in coimmunoprecipitation assays. Immunoprecipitated protein with anti-GFP was detected by immunoblotting using an anti-MYC antibody.
Figure 3.
Corrected: DCC1 interacts with CA2. B, Interaction between DCC1 and CA2 in the pull-down assay. The 10% input and GST pull-down proteins were detected by immunoblotting using anti-His antibody (top row). CA2-GST and GST proteins were detected by immunoblotting using anti-GST antibody (bottom row). D, Interaction between DCC1 and CA2 in coimmunoprecipitation assays. The 10% input and immunoprecipitated proteins with anti-GFP (GFP-IP) were detected by immunoblotting using an anti-MYC antibody (top row). The 10% input and DCC1-GFP proteins were detected by immunoblotting using an anti-GFP antibody (bottom row).
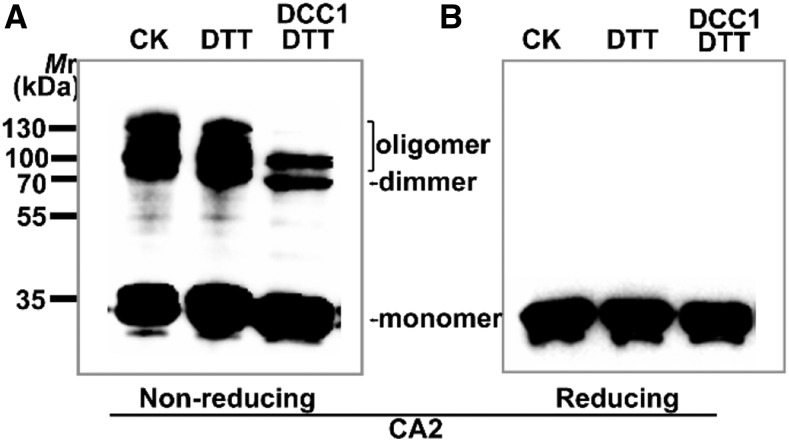
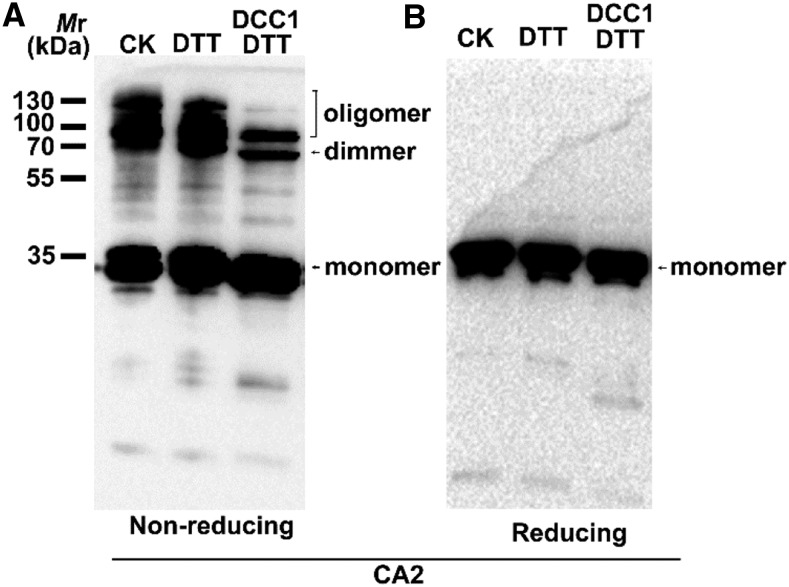
In the original Figure 5, A and B, the gel blot image was overly contrasted and tightly cropped. In the corrected Figure 5, A and B, the images are appropriately cropped and provided without any manipulations of contrast. The figure legend has not been changed.
Figure 5.
Original: DCC1 regulates Complex I activity via CA2 protein.
Figure 5.
Corrected: DCC1 regulates Complex I activity via CA2 protein.
In the original figures, the background of plantlet images in Figures 1, 4, 6, 7, 8, and 12a was cosmetically adjusted/cleaned. In the corrected figures, the plantlet images in Figures 1, 4, 6, 7, 8, and 12 are provided without any manipulations of adjusting/cleaning. The figure legends have not been changed.
Figure 1.
Original: Shoot regeneration is inhibited in the dcc1 mutant.
Figure 4.
Original: Loss of function of CA2 caused the decreased capacity of shoot regeneration.
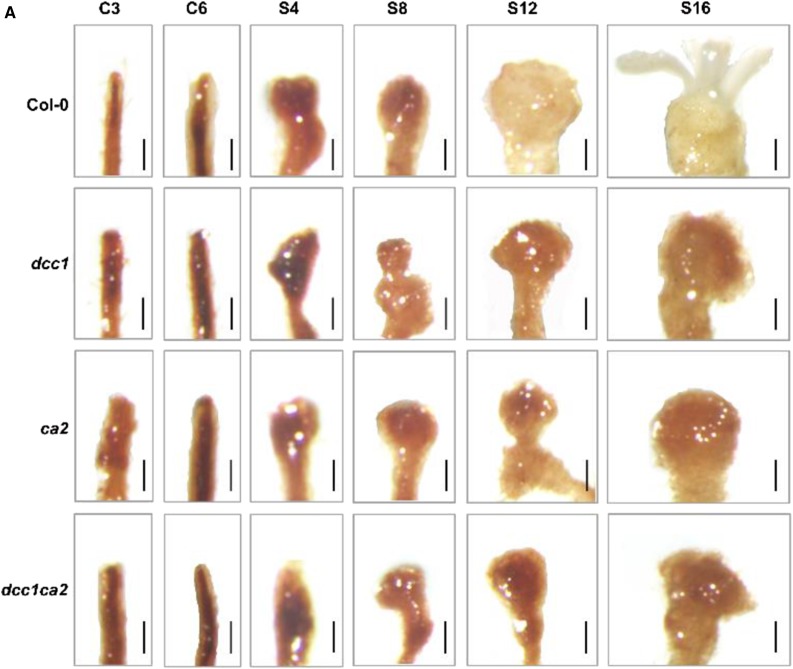
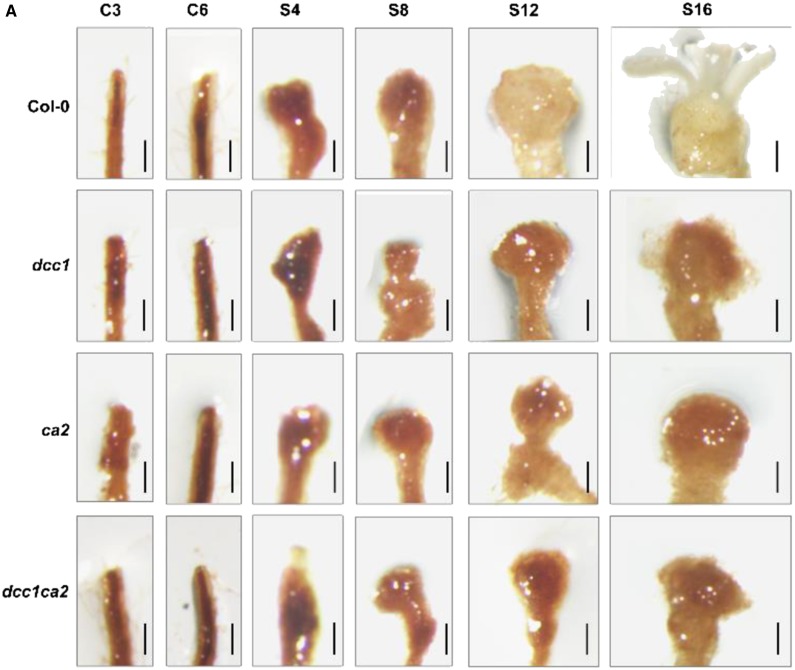
Figure 6.
Original: Mutation of DCC1 or CA2 results in increased ROS levels.
Figure 7.
Original: Treatment with exogenous ROS inhibits shoot regeneration.
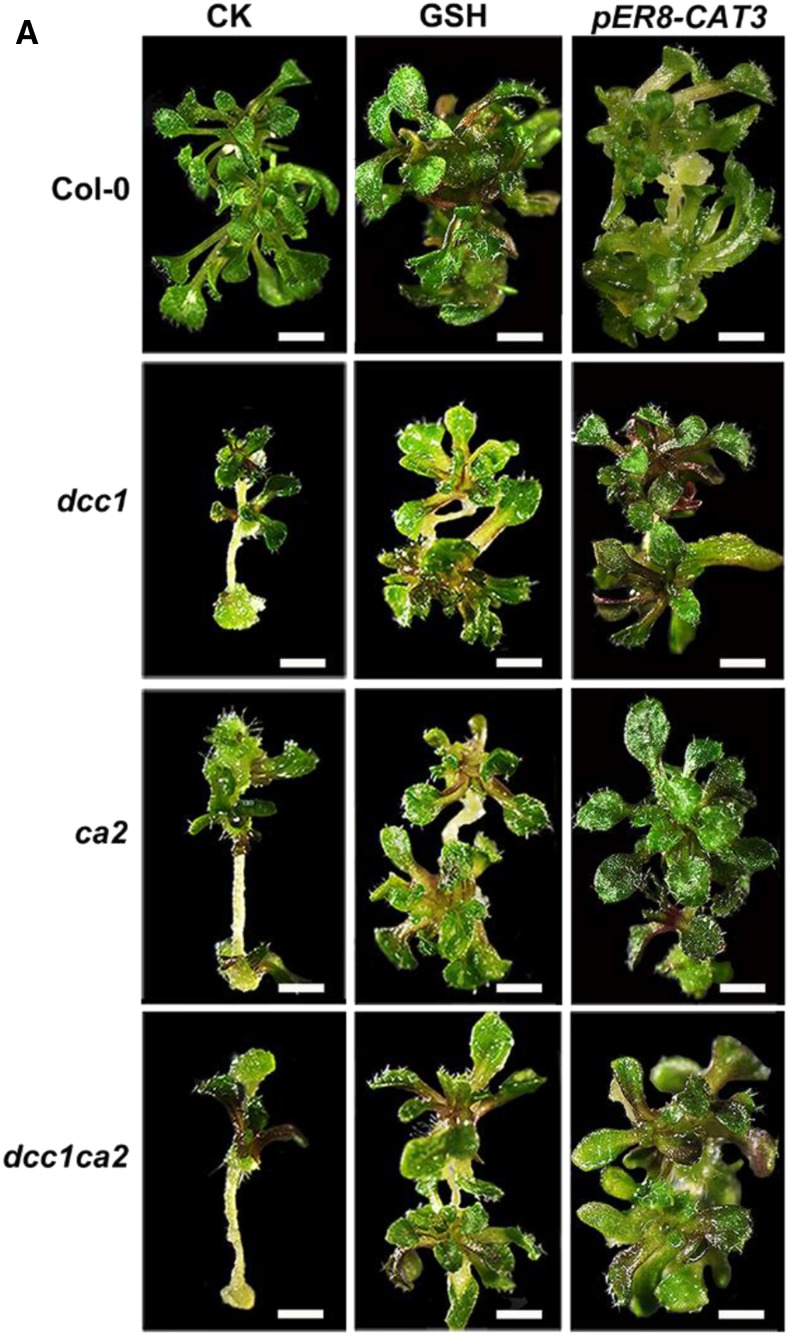
Figure 8.
Original: Decreased ROS level rescues phenotypes of dcc1ca2 double mutant.
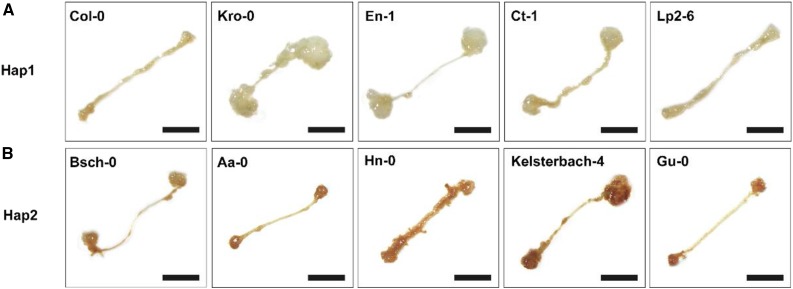
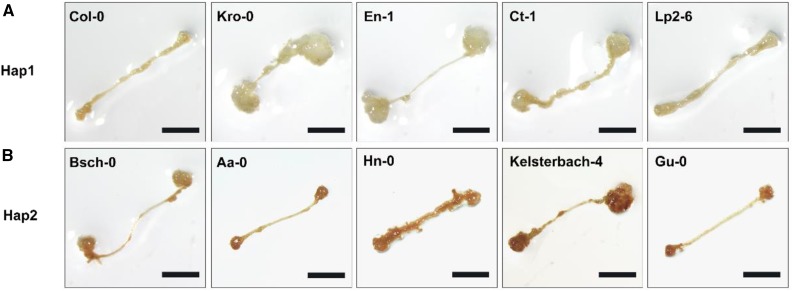
Figure 12.
Original: ROS levels are associated with the shoot regeneration capacities of different ecotypes in Arabidopsis.
Figure 1.
Corrected: Shoot regeneration is inhibited in the dcc1 mutant.
Figure 4.
Corrected: Loss of function of CA2 caused the decreased capacity of shoot regeneration.
Figure 6.
Corrected: Mutation of DCC1 or CA2 results in increased ROS levels.
Figure 7.
Corrected: Treatment with exogenous ROS inhibits shoot regeneration.
Figure 8.
Corrected: Decreased ROS level rescues phenotypes of dcc1ca2 double mutant.
Figure 12.
Corrected: ROS levels are associated with the shoot regeneration capacities of different ecotypes in Arabidopsis.