Abstract
Hydrogen sulfide (H2S) is an important gaseous signaling molecule in plants that participates in stress responses and development. l-Cys desulfhydrase 1, one of the enzymatic sources of H2S in plants, participates in abscisic acid-induced stomatal closure. We combined pharmacological and genetic approaches to elucidate the involvement of H2S in stomatal closure and the interplay between H2S and other second messengers of the guard cell signaling network, such as hydrogen peroxide (H2O2) and phospholipase D (PLD)-derived phosphatidic acid in Arabidopsis (Arabidopsis thaliana). Both NADPH oxidase isoforms, respiratory burst oxidase homolog (RBOH)D and RBOHF, were required for H2S-induced stomatal closure. In vivo imaging using the cytosolic ratiometric fluorescent biosensor roGFP2-Orp1 revealed that H2S stimulates H2O2 production in Arabidopsis guard cells. Additionally, we observed an interplay between H2S and PLD activity in the regulation of reactive oxygen species production and stomatal movement. The PLDα1 and PLDδ isoforms were required for H2S-induced stomatal closure, and most of the H2S-dependent H2O2 production required the activity of PLDα1. Finally, we showed that H2S induced increases in the PLDδ-derived phosphatidic acid levels in guard cells. Our results revealed the involvement of H2S in the signaling network that controls stomatal closure, and suggest that H2S regulates NADPH oxidase and PLD activity in guard cells.
Stomata are formed by pairs of specialized guard cells that sense and integrate multiple stimuli (such as light, CO2, humidity, and hormones) to modulate stomatal pore size. Guard cells have become a model for studying signal transduction in plants. During stomatal closure, guard cells undergo depolarization of the plasma membrane through the inhibition of H+-ATPases, and an increase of cytosolic Ca2+ concentration. This response generates a net loss of osmotically active solutes (mainly K+) through regulation of inward and outward K+ rectifier channels, providing an intracellular environment that drives water out of the cells to reduce guard cell volume (Blatt, 2000; Schroeder et al., 2001). These processes are controlled by a complex signaling network, the architecture of which resembles that of a scale-free network (Hetherington and Woodward, 2003). Some of the pathways that constitute this network have been well studied, such as those triggered by abscisic acid (ABA) or CO2, while for others, our understanding is still lacking.
The production of reactive oxygen species (ROS) by the guard cells is a response common to most of the stimuli that induce stomatal closure (Song et al., 2014). Cytosolic ROS are generated primarily in the apoplast by NADPH oxidase (NADPHox) respiratory burst oxidase homolog (RBOH), which generates superoxide that dismutates to H2O2 (Kwak et al., 2003). In Arabidopsis (Arabidopsis thaliana), the RBOH family contains 10 members. Isoforms D (RBOHD) and F (RBOHF) are involved in the regulation of stomatal movement (Sierla et al., 2016). RBOH is composed of 6 transmembrane domains, a C-terminal tail containing the NADPH and FADH binding sites, and an N-terminal region, which contains two EF-hands and several phosphorylation sites, both of which are involved in activation (Suzuki et al., 2011; Sierla et al., 2016). Apart from the canonical regulation, other messengers can regulate the activity of RBOH. The Cys-890 amino acid residue of RBOHD is susceptible to modification by nitric oxide (NO; S-nitrosylation), which reduces the activity of NADPHox during the defense response to Pseudomonas syringae (Yun et al., 2011).
Another messenger that regulates NADPHox activity in guard cells is the signaling phospholipid, phosphatidic acid (PA; Zhang et al., 2009). PA, produced by phospholipase D (PLD), binds to RBOHD and RBOHF to induce ABA-dependent ROS production (Zhang et al., 2009). In plants, PA is generated via phospholipase C (PLC) activity in concert with diacylglycerol kinase, or directly through hydrolysis of structural lipids by PLD. PA has emerged as a key player in plant signaling (Testerink and Munnik, 2005; Wang et al., 2006). The Arabidopsis genome encodes 12 PLDs, which are divided into 6 subgroups [α(3), β(2), γ(3), δ, ε, and ζ(2)]. Only PLDα1 and PLDδ are associated with regulation of stomatal closure, and, in the ABA-dependent response, PLDα1 is required for ROS production (Zhang et al., 2009), while PLDδ is involved in NO-dependent regulation of stomatal closure (Distéfano et al., 2012).
Studies of signal transduction in plants and animals have revealed gasotransmitters as central messengers (Mustafa et al., 2009; Zhang, 2016), which are also evident in the guard cell signaling network (Scuffi et al., 2016). The first gasotransmitter described to participate in the modulation of stomatal movement was NO, which modulates the induction of stomatal closure and the inhibition of stomatal opening (García-Mata and Lamattina, 2001; Garcia-Mata and Lamattina, 2007). Recently, H2S has been recognized as a stomatal closure inductor and ascribed an active role in the signaling response to different stimuli (Scuffi et al., 2016). In plants, H2S is enzymatically produced by a Cys desulfhydrase in the catalyzed conversion of Cys to pyruvate, H2S, and NH3+. In Arabidopsis, the cytosolic protein DES1 was recently characterized as a novel l-Cys desulfhydrase (Alvarez et al., 2010). DES1 is involved in the response to heavy metal stress, plant immunity, autophagy, and stomatal closure (Alvarez et al., 2010, 2012a, 2012b; Scuffi et al., 2014). Null mutants of DES1 show an approximately 30% decrease in l-Cys desulfhydrase activity (Alvarez et al., 2010), suggesting that other sources also contribute to H2S production. Although other enzymes may produce endogenous H2S, we have shown that DES1 activity is required for ABA-dependent stomatal closure, and DES1 acts early in ABA-mediated signal transduction (Scuffi et al., 2014).
This raises the question of whether there is an interplay between H2S and other components of the guard cell signaling network, such as H2O2 production and PLD activity.
RESULTS
H2S Requires RBOHF and RBOHD for the Induction of Stomatal Closure
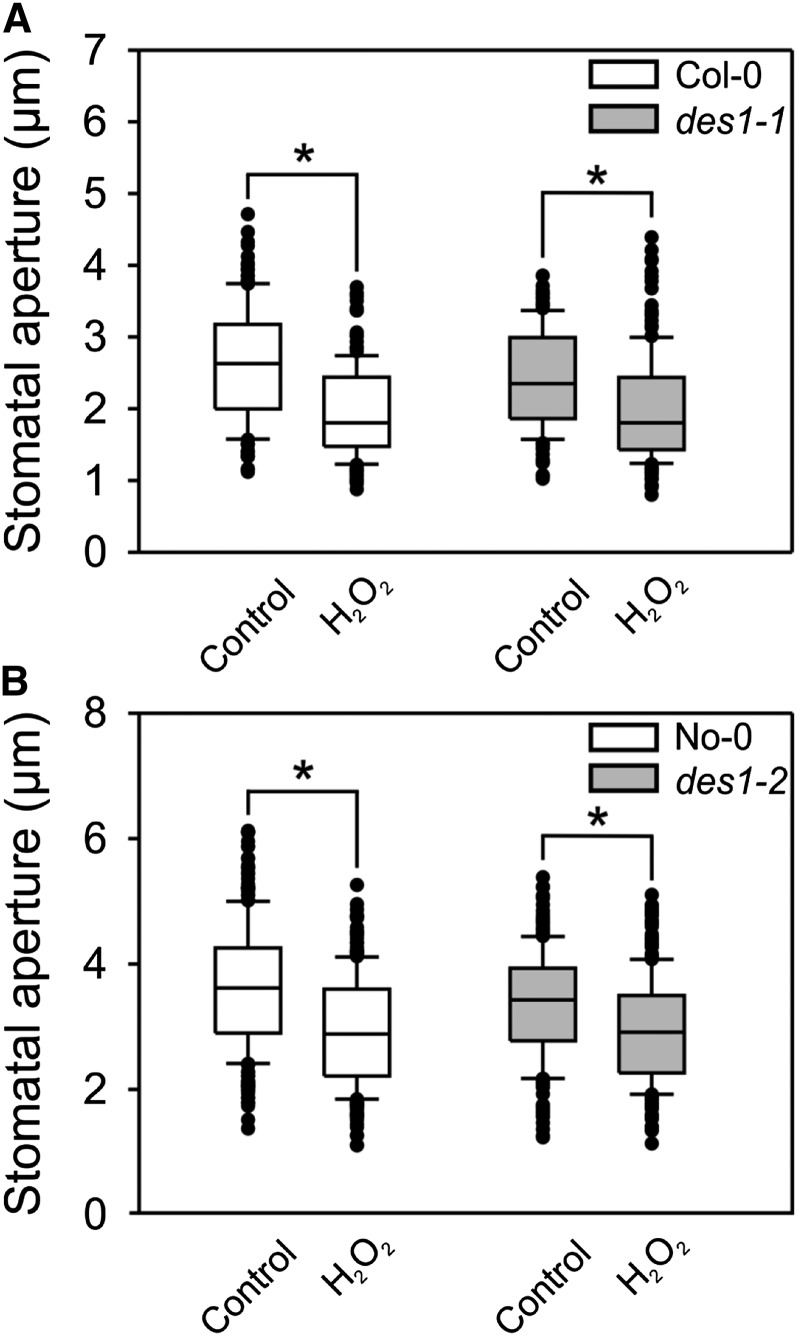
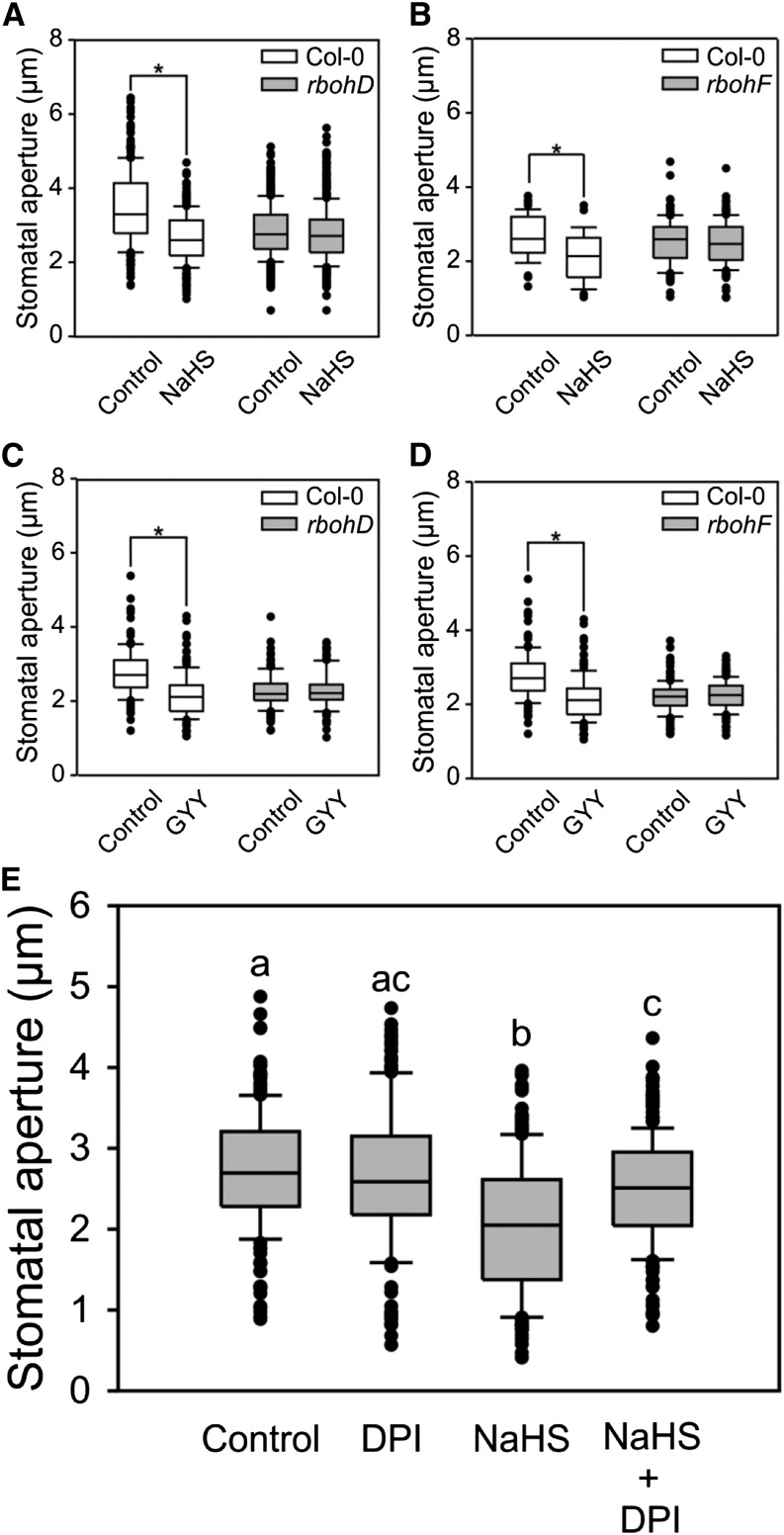
To analyze the putative interaction between H2S and H2O2, we assessed the ability of H2O2 to induce stomatal closure in H2S synthesis mutants, and the ability of H2S to induce stomatal closure in RBOHF and RBOHD mutants. First, we prepared isolated epidermal peels (epidermal strips) from wild-type Arabidopsis plants and from DES1 knockout mutants (des1-1 and des1-2). To induce stomatal opening, the epidermal strips were preincubated in opening buffer for 3 h in white light. Then, the strips were treated with 100 µm H2O2 for 90 min. H2O2 induced a 25% reduction of the stomatal pore width in both the wild type and des1 mutants, indicating that H2O2 acts either downstream or independently of H2S (Fig. 1, A and B). The NADPHox isoforms RBOHD and RBOHF are responsible for the majority of guard cell H2O2 production (Kwak et al., 2003; Nühse et al., 2007; Zhang et al., 2007). Accordingly, we analyzed H2S-induced stomatal closure in the rbohd and rbohf mutants. Epidermal strips from the wild type and rboh mutants were preincubated in opening buffer, and then treated with 100 µm of sodium hydrosulfide (NaHS) and morpholin-4-ium4 methoxyphenyl(morpholino) phosphinodithioate (GYY4137), which are H2S donors. Both H2S donors induced stomatal closure by about 20% in the wild type, confirming previous observations (Scuffi et al., 2014); however, no closure was evidenced in the rboh-mutant lines (Fig. 2, A–D). Moreover, stomatal closure was impaired in wild-type epidermal strips treated with 100 µm NaHS in the presence of the flavoenzyme inhibitor diphenyleneiodonium (DPI), used to inhibit NADPHox (Fig. 2E), validating our observations in the NADPHox mutants. These results indicated that there is interplay between H2S and H2O2 and suggested that NADPHox acts downstream of H2S.
Figure 1.
H2O2 induces stomatal closure in des1 mutants. Epidermal strips from two independent Arabidopsis mutants, des1-1 (A) and des1-2 (B), and their genetic backgrounds, Col-0 and No-0, respectively, were preincubated for 3 h in opening buffer (5 mm K-MES, pH 6.1, and 50 mm KCl) under light and subsequently treated for 90 min with 100 µm H2O2. The values of stomatal aperture are expressed in microns and represented in the box plot where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers. Data are from at least three independent experiments. Asterisks denote statistical differences with respect to the control treatment (Mann-Whitney rank sum test, P < 0.001).
Figure 2.
NADPH oxidase is involved in H2S-induced stomatal closure. Epidermal strips from Arabidopsis plants were incubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) under light for 3 h and subsequently subjected to different treatments for 90 min under light. Epidermal strips from the rbohD (A and C) and rbohF (B and D) mutants were treated for 90 min under light with 100 µm of H2S donors (NaHS [A and B] and GYY4137 [C and D]). Epidermal strips from the wild type (Col-0) were treated with 10 µm of the flavoenzyme inhibitor DPI, 100 µm of the H2S donor NaHS, or both (E). The values of stomatal aperture are expressed in microns and represented in the box plot where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers. Data are from at least three independent experiments. Asterisks denote statistical differences with respect to the control treatment (Mann-Whitney rank sum test, P < 0.001). Different letters indicate statistical differences among treatments (Dunn’s post hoc test, P < 0.05).
H2S Increases H2O2 Production in Guard Cells
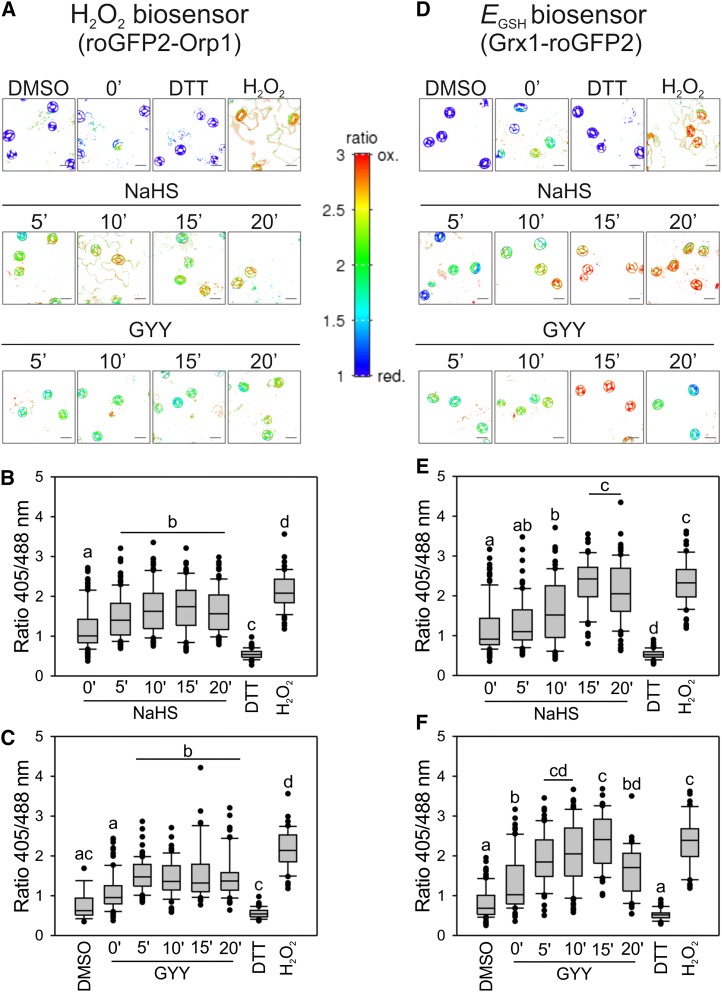
To analyze H2O2 production in response to H2S inside the guard cells, we used the fluorescent protein biosensor roGFP2-Orp1, which consists of the yeast peroxidase Orp1 fused to redox-sensitive roGFP2 to generate a proximity-based thiol relay that passes on the H2O2-induced oxidation of Orp1 to roGFP2 by thiol-disulfide exchange (Schwarzländer et al., 2016). This biosensor can dynamically monitor intracellular H2O2 fluctuations in vivo (Gutscher et al., 2009). We generated stable Arabidopsis lines expressing roGFP2-Orp1 in the cytosol. Since H2S is a reductant and H2O2 is an oxidant, and both are likely to affect the cellular thiol systems, we also used plants expressing the biosensor Grx1-roGFP2 to monitor the cytosolic glutathione redox potential (EGSH). This construct is a fusion of roGFP2 and human glutaredoxin 1 (Grx1) for rapid equilibration with the local glutathione pool to provide a readout for EGSH in real time (Gutscher et al., 2008). Epidermal strips from plants expressing the H2O2 biosensor roGFP2-Orp1 and from plants expressing the EGSH biosensor Grx1-roGFP2 were used to assess the effect of the H2S donors on H2O2 production and EGSH. We observed that immediately after peeling, both roGFP2-Orp1 and Grx1-roGFP2 biosensors showed pronounced oxidation, probably as a response to the mechanical stress associated with the procedure (Supplemental Fig. S1). Therefore, we extended the preincubation time in opening buffer to allow for the recovery of guard cell redox physiology (Supplemental Fig. S1). Oxidation started to decrease only after 4 h, reaching steady-state levels comparable to the intact leaf after 7 h of preincubation (Supplemental Fig. S1). Accordingly, a 7-h preincubation was used for the assays. We then treated epidermal strips with 100 µm NaHS. This H2S donor led to a rapid oxidation of roGFP2-Orp1 in guard cells (Fig. 3, A and B); however, EGSH also responded as indicated by an oxidative shift of the Grx1-roGFP2 sensor (Fig. 3, D and E). We then assayed GYY4137 as an alternative, tissue-permeating, slow release H2S donor (Li et al., 2008). GYY4137 also induced oxidation of roGFP2-Orp1 and Grx1-roGFP2 (Fig. 3, A, C, D, and F).
Figure 3.
H2S donors lead to oxidation of roGFP2-Orp1 and Grx1-roGFP2 in guard cells. Epidermal peels from Arabidopsis leaves expressing the H2O2 biosensor, roGFP2-Orp1 (A–C), and the EGSH biosensor, Grx1-roGFP2 (D–F), in the cytosol were incubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) for 7 h under light and then treated with: 0.1% (v/v) DMSO for 15 min, 100 µM of NaHS or GYY4137 for 0, 5, 10, 15, or 20 min, 20 mm DTT for 10 min, and 10 mm H2O2 for 10 min under the same conditions. Pseudo-color ratio images of representative peels (A and D). Scale bar = 10 µm. The values are expressed as the ratio of 405/488 nm and are represented in the box plots where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers (B, C, E, and F). Data are from at least three independent experiments. Different letters denote statistical differences between treatments (Dunn’s post hoc test, P < 0.05).
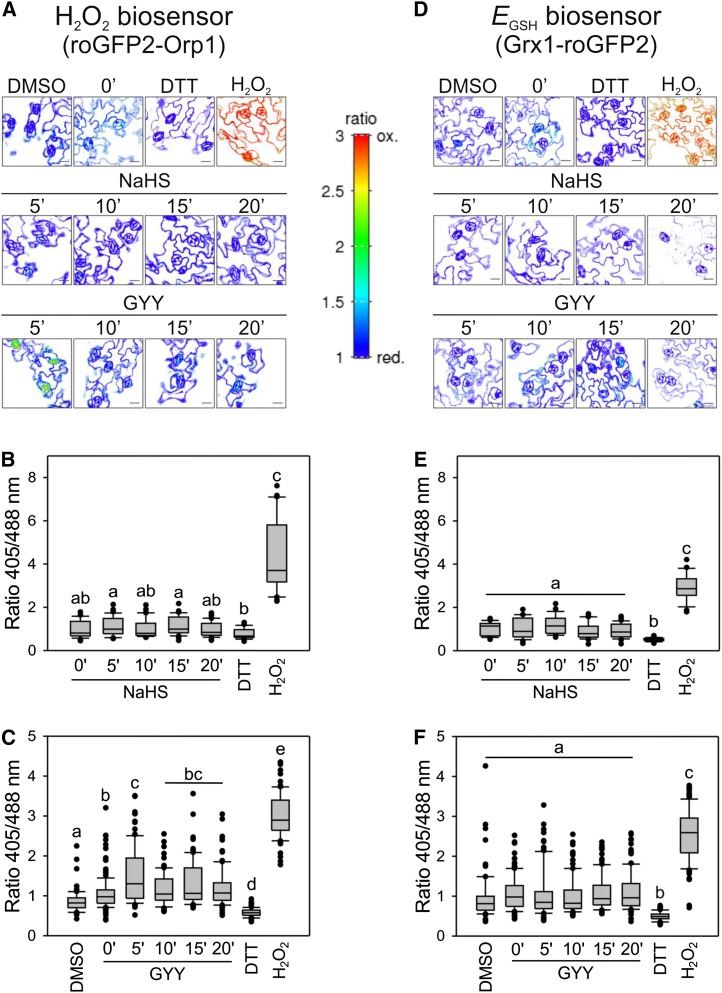
Epidermal strips have been a valuable and widely used model to study guard cell dynamics, but our observation of transient oxidation suggests that epidermal strips have a weakened redox buffering capacity, even after the extended recovery period. To test whether the observed oxidative shifts in guard cells of epidermal strips also occur in intact leaves, we assessed H2S treatments in intact leaves. Leaves from plants expressing roGFP2-Orp1 or Grx1-roGFP2 were incubated in opening buffer for 30 min and then treated with NaHS and GYY4137. Both sensors were reduced under the control conditions and NaHS showed no oxidative effect in the guard cells (Fig. 4, A, B, D, and E). By contrast, GYY4137 induced a rapid increase in roGFP2-Orp1 oxidation (Fig. 4, A and C), whereas the redox status of Grx1-roGFP2 was unchanged compared to the control (Fig. 4, D and F). Interestingly, when the roGFP2-Orp1 fluorescence ratio was analyzed in the pavement cells, there was no response to the H2S treatment (Supplemental Fig. S2), indicating that oxidation occurred with some specificity in guard cells. These observations indicated that oxidation of roGFP2-Orp1 in the guard cells is due to the production of H2O2 in H2S-treated leaves.
Figure 4.
The GYY4137 treatment leads to oxidation of the roGFP2-Orp1 sensor specifically in guard cells. Whole Arabidopsis leaves expressing the H2O2 biosensor, roGFP2-Orp1 (A–C), and the EGSH biosensor, Grx1-roGFP2 (D–F), in the cytosol were incubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) for 30 min under light and then treated with: 0.1% (v/v) DMSO for 15 min, 100 µM of NaHS or GYY4137 for 0, 5, 10, 15, or 20 min, 20 mm DTT for 10 min, and 10 mm H2O2 for 20 min under the same conditions. Pseudo-color ratio images of representative leaves (A and D). Scale bar = 10 µm. The values are expressed as the ratio of 405/488 nm and are represented in the box plots where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers (B, C, E, and F). Data are from at least three independent experiments. Different letters denote statistical differences between treatments (Dunn’s post hoc test, P < 0.05).
In an independent set of experiments, we used the ROS-sensitive probe H2DCF-DA to examine ROS levels in H2S-treated plants. First, epidermal strips from wild-type plants were loaded with H2DCF-DA and then treated with 100 µm of NaHS. H2S induced a 2-fold increase in DCF fluorescence compared to the control (Supplemental Fig. S3, A and B). To determine if NADPHox activity is required for the increase of ROS production in guard cells after H2S treatment, the epidermal strips were treated with NaHS in the presence of DPI. DPI inhibited the H2S-induced ROS production, indicating that H2S induces ROS production through the activity of NADPHox (Supplemental Fig. S3, A and B). Taken together, our results suggest that H2S induces the production of H2O2 in guard cells.
H2S Requires PLDα1 and PLDδ for the Induction of Stomatal Closure
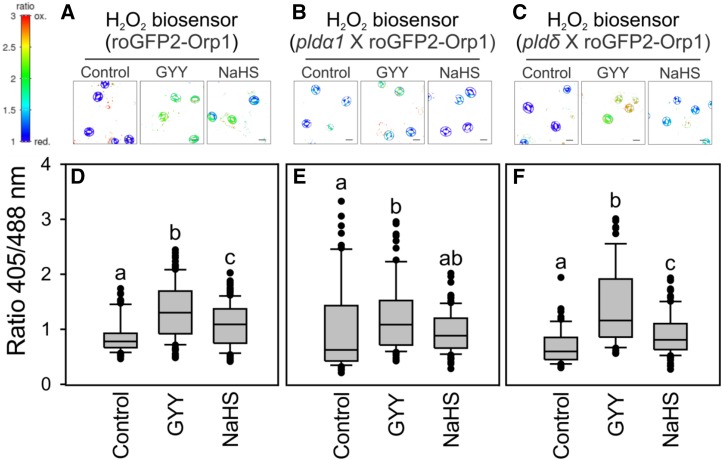
PLDα1 and PLDδ are the two members of the PLD multigene family expressed in guard cells. Both isoforms participate in ABA-dependent stomatal closure, although only one of them, PLDα1, is involved in the regulation of ROS production (Zhang et al., 2009; Distéfano et al., 2012). Therefore, we assessed the participation of the two PLDs in the regulation of H2O2 production in response to H2S. Epidermal peels from the pld mutant plants expressing roGFP2-Orp1 (pldα1 x roGFP2-Orp1 and pldδ X roGFP2-Orp1) were incubated in opening buffer for 7 h and subsequently treated with NaHS and GYY4137 for 15 min. The H2S donor GYY4137 shifted the redox state of roGFP2-Orp1 to around 50% sensor oxidation in the guard cells of wild-type plants and to 90% in the pldδ mutants. In addition, the NaHS treatment caused an increase in sensor oxidation in the guard cells of the wild type and the pldδ mutants, but not in the pldα1 mutants (Fig. 5). In contrast, the pldα1 background, despite showing some biological variability, displayed a 20% shift in the redox state of roGFP-Orp1 in response to H2S treatment (Fig. 5). In addition, when epidermal strips were loaded with H2DCF-DA and then treated with 100 µm NaHS, the wild type and the pldδ mutants, but not the pldα1 mutants, showed increased levels of DCF fluorescence (Supplemental Fig. S3, A and C). These results suggest that PLDα1, but not PLDδ, is required for H2S-induced H2O2 production.
Figure 5.
PLDα1 is required for H2S-dependent induction of H2O2 levels in guard cells. Epidermal peels from Arabidopsis Col-0 (roGFP2-Orp1) (D), pldα1 (pldα1 × roGFP2-Orp1) (E), and pldδ (pldδ × roGFP2-Orp1) (F) leaves expressing the H2O2 biosensor, roGFP2-Orp1, in the cytosol were incubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) for 7 h under light and then treated with opening buffer (control), or 100 µm of the H2S donors, GYY4137 (GYY) or NaHS, for 15 min. Pseudo-color ratio images of representative peels (A–C). Scale bar = 10 µm. The values are expressed as the ratio of 405/488 nm and are represented in the box plots where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers (D–F). Data are from at least three independent experiments. Different letters denote statistical differences between treatments (Dunn’s post hoc test, P < 0.05).
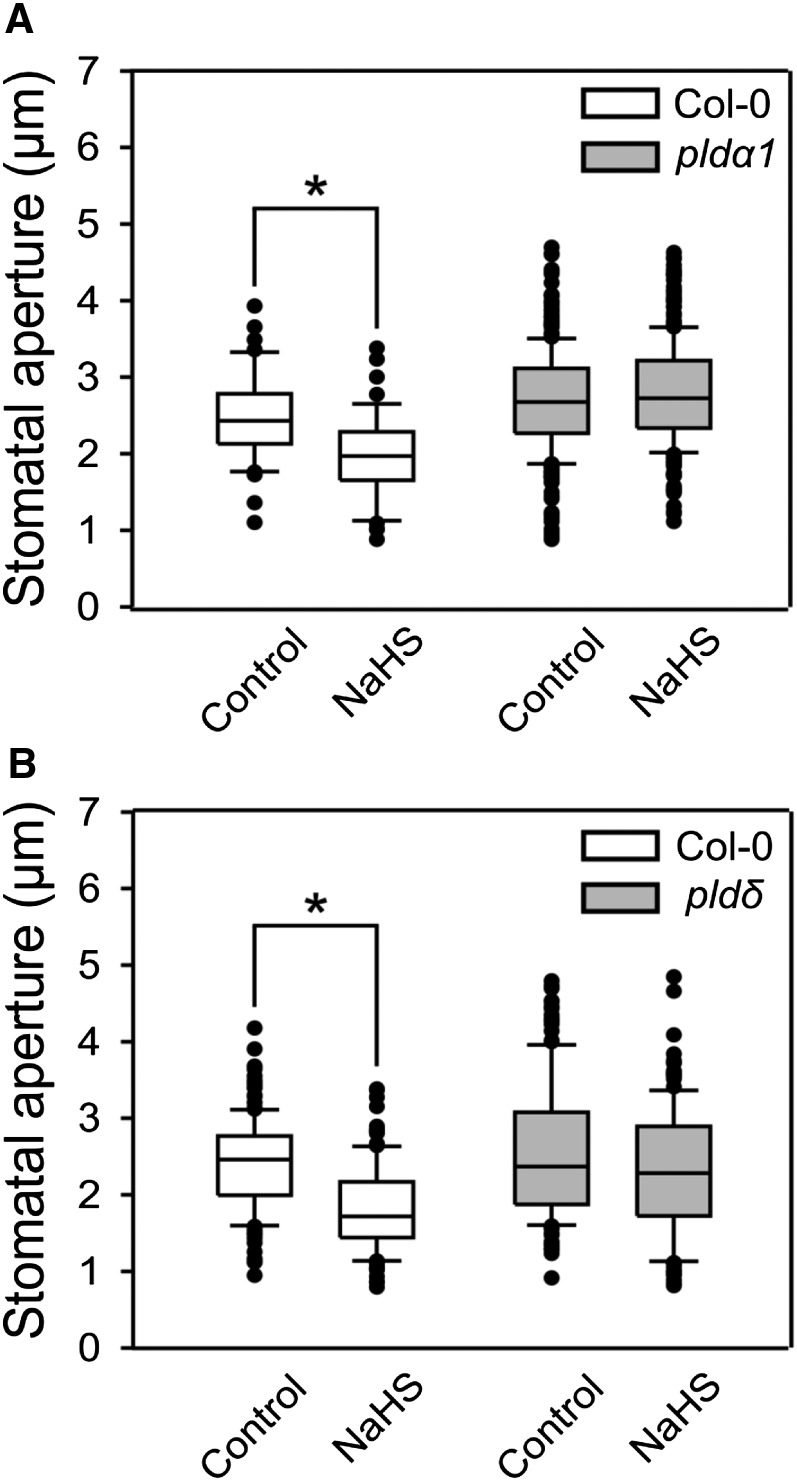
To further investigate the participation of PLDs in H2S-dependent signaling, we assessed the stomatal closure response to H2S in epidermal strips from the pldα1 and pldδ mutants. Despite our finding that only PLDα1 is involved in the regulation of ROS production, H2S-dependent stomatal closure was blocked in both pldα1 and pldδ lines (Fig. 6, A and B), suggesting that both PLD isoforms are required for H2S-mediated stomatal closure.
Figure 6.
PLDα1 and PLDδ are required for H2S-induced stomatal closure. Epidermal strips from Arabidopsis pldα1 (A) and pldδ (B) mutants and wild-type (Col-0) plants were preincubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) under light for 3 h and then treated for 90 min under light with 100 µm of the H2S donor, NaHS. The values of stomatal closure are expressed in microns and are represented in the box plots where the box is bound by the 25th to 75th percentile, whiskers span 10th to 90th percentile, and the line in the middle is the median. The individual points represent outliers. Data are from at least three independent experiments. Asterisks denote statistical differences with respect to the control treatment (Mann-Whitney rank sum test, P < 0.001).
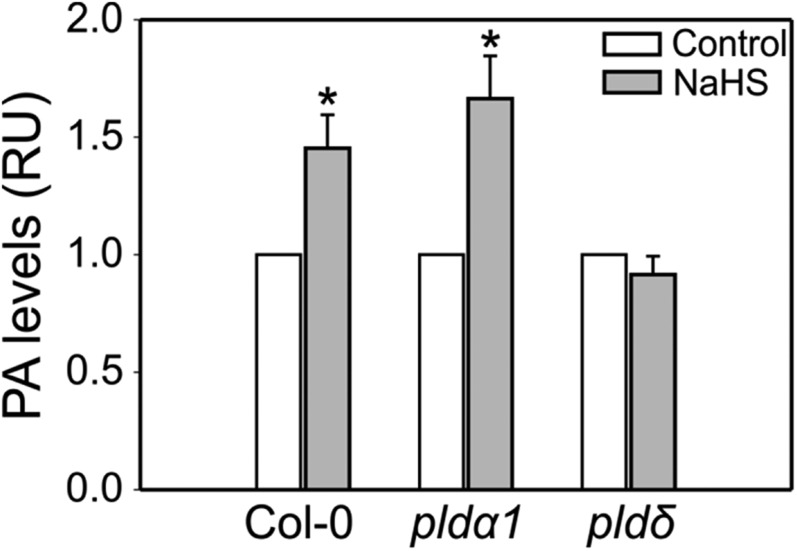
We also assessed the effect of H2S on the production of PA as the active messenger generated by the PLDs. Radioactive labeling and quantification of guard cell phospholipids in epidermal strips (where guard cells represent around 90% of the living cells) was previously performed in broad bean (Vicia faba), since the leaf size and straightforward epidermal extraction procedures facilitate obtaining the required epidermal tissue mass for the analysis (Distéfano et al., 2008). Yet, the repertoire of genetic tools for V. faba is limited and suitable PLD mutants have not been reported. Hence, we used Arabidopsis epidermal strips from wild-type and PLD mutant plants to quantify PA production. Epidermal strips were labeled with 32Pi for 3 h in opening buffer under white light and then treated for 1 h with 100 µM NaHS under the same conditions. Lipids were extracted, separated, and quantified using a phosphor imaging. H2S induced a 40% increase in PA levels in epidermal strips of wild-type and pldα1 plants (Fig. 7). However, NaHS treatment did not increase PA levels in epidermal strips of the pldδ mutants. These results indicated that H2S requires the activity of PLDδ for the induction of PA.
Figure 7.
H2S induces PA levels through PLDδ activity in Arabidopsis guard cells. Epidermal peels from the wild type (Col-0), and pldα1 and pldδ mutant Arabidopsis plants were incubated in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) in the presence of 32Pi for 3 h under light in a wet chamber and then treated with 100 µm of the H2S donor, NaHS, for 1 h. Lipids were extracted, separated by EtAc TLC, and PA levels were quantified and expressed as a fold increase with respect to the controls. The values are expressed as means ± se as relative units (RU) from at least three independent experiments. Asterisks denote statistical difference with respect to the control treatment (Mann-Whitney rank sum test; P = 0.003 for Col-0, P = 0.305 for pldδ and t test; P = 0.006 for pldα1). The percentage of PA in control treatments were: Col-0 = 6.6340 ± 0.8, pldα1 = 7.5380 ± 0.7570, and pldδ = 8.5170 ± 0.6320.
We next analyzed the production of PA in response to H2S at the whole-leaf level. Arabidopsis leaf discs were labeled overnight with 32Pi in opening buffer, and then treated with 100 µm NaHS for 1 h. After 1 h of H2S treatment, PA levels increased by about 40% in the wild type (Supplemental Fig. S4). To identify the source of the PA increase, we performed similar experiments using leaf discs from both pld mutants. Both the wild type and the pldα1 mutant showed a comparable increase of PA, while PA levels in the pldδ mutant remained unchanged (Supplemental Fig. S4). These results indicated that PLDδ is also required for the production of PA at the whole-leaf level, similar to our observations in the epidermal strips.
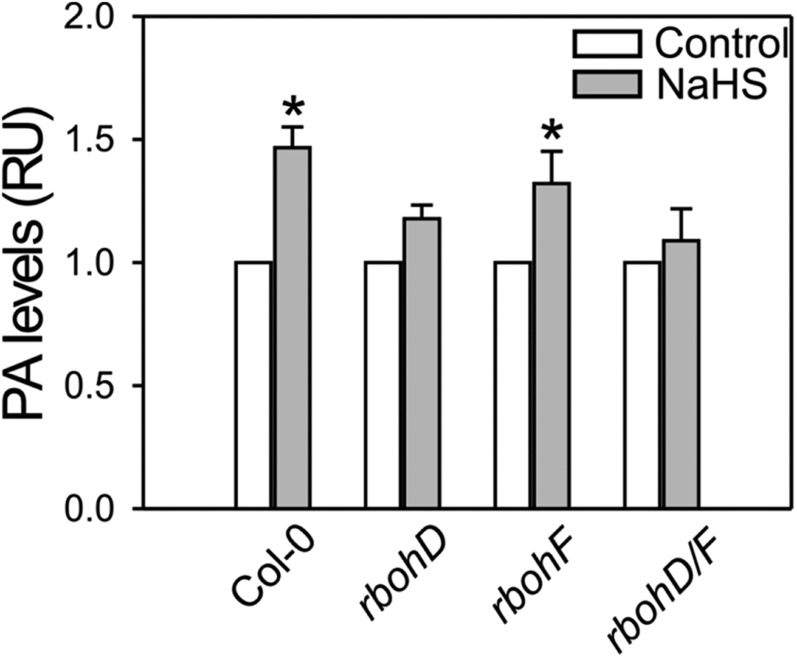
Previous reports postulated that PLDδ acts downstream of ROS production in guard cells (Distéfano et al., 2012; Guo et al., 2012). To investigate if H2S-induced PLDδ activity is also RBOH dependent, we analyzed PA production in rboh mutant leaf discs. Increased PA levels were found in leaf discs of the wild type and, to a lesser extent, in the rbohF mutants (about 30%; Fig. 8). This increase was not observed in the rbohD or rbohD/F double mutant plants, suggesting that H2S requires RBOHD to induce PLDδ-derived PA.
Figure 8.
H2S requires RBOHD to induce PA levels in Arabidopsis leaf discs. Leaf discs from the wild type (Col-0), and rbohD, rbohF, and rbohD/F double mutant Arabidopsis plants were labeled with 32Pi overnight in opening buffer (5 mm MES, pH 6.1, and 50 mm KCl) under light in a wet chamber and then treated with 100 µm of the H2S donor, NaHS, for 1 h. Lipids were extracted and separated by EtAc TLC. PA levels were quantified and expressed as a fold increase in relation to the controls. Error bars represent se of means (n ≥ 3). Asterisks denote statistical differences with respect to each control treatment (Student’s t test, P < 0.001 for Col-0, P = 0.111 for rbohD, P = 0.013 for rbohF, and P = 0.717 for rbohD/F). The percentage of PA in control treatments were: Col-0 = 0.4690 + 0.0358, rbohD = 0.5779 ± 0.0403, rbohF = 0.5602 ± 0.0402, and rbohD/F = 0.4827 ± 0.0553.
DISCUSSION
The activities of the different isoforms of NADPHox, which participate in the process of stomatal closure, can vary according to the stimulus that triggers the response. In pathogen-associated molecular pattern-induced stomatal closure, ROS production is dependent on RBOHD activity (Nühse et al., 2007; Zhang et al., 2007), whereas ROS production in the ABA-induced response depends mostly on RBOHF activity (Kwak et al., 2003). The stomatal responses to H2S treatments showed additional differences. Our data indicate that H2S requires the ROBHD and RBOHF isoforms to induce stomatal closure. The role of RBOHF acting downstream of H2S matches our previous finding that H2S participates in ABA-dependent stomatal closure (Scuffi et al., 2014). In addition, the fact that RBOHD is required for the induction of stomatal closure suggests that H2S could be a key component in those responses for which ROS production is largely dependent on RBOHD activity, such as plant-pathogen interactions. Preliminary data obtained in our laboratory indicate that the H2S/DES1 pathway participates in the response to the bacterial elicitor flagellin. The H2S scavenger hypotaurine partially blocked stomatal closure in response to the 22-amino acid flagellin fragment (flg22). In addition, des1 mutant plants do not close their stomata in response to treatment with flg22 (Supplemental Fig. S5), supporting our hypothesis that H2S can act in different signaling pathways that lead to stomatal closure.
H2DCF-DA is the most widely used probe for intracellular detection of ROS in planta. However, the probe has numerous limitations and is prone to measurement artifacts and inappropriate interpretation of the results (Bonini et al., 2006). The lack of alternative techniques to monitor intracellular ROS has encouraged the development of new tools for in vivo H2O2 monitoring. A particularly elegant concept has been the utilization of H2O2-sensitive proteins, such as peroxidases, to generate genetically encoded sensors (Winterbourn, 2014; Buettner, 2015). In this study, we used the fluorescent biosensor roGFP2-Orp1 to monitor H2O2 production in guard cells. roGFP2-Orp1 is a ratiometric (i.e. self-normalizing) biosensor, and its reactivity and selectivity for H2O2 has been demonstrated in vitro as well as in vivo in yeast and animal cells (Gutscher et al., 2009; Albrecht et al., 2011). The pH stability of the sensor is an important advantage over H2O2 sensors of the HyPer family, which can introduce pH artifacts to in vivo measurements even through minor intracellular pH changes. Yet, similar to the HyPer sensors, the dynamic response of roGFP2-Orp1 also depends on the endogenous thiol redox systems (discussed in detail by Schwarzländer et al., 2016). We observed that the preparation of the epidermal strips gives rise to sensor oxidation, similar to the response to tissue wounding (Meyer et al., 2007). The occurrence of the oxidation event revealed by our study should be considered when guard cell physiology, and the involvement of redox-related events in particular, are investigated using epidermal peels. However, the oxidation event observed in our study was transient, allowing measurements to be performed after recovery. Although NADPHox-dependent ROS are produced in the apoplast, we monitored intracellular ROS and redox dynamics, since we expressed roGFP2-based biosensors in the cytosol. The influence of apoplastic ROS production on rapid guard cell cytosolic signaling was supported by different reports showing that ROS can rapidly permeate into the cytosol through aquaporins. Two members of the plasma membrane intrinsic proteins (AtPIP2;1 and AtPIP1;4) were shown to permeate H2O2 in response to ABA and pathogen-associated molecular patterns (Grondin et al., 2015; Tian et al., 2016). Moreover, the permeation of H2O2 into guard cells through PIP2;1 in response to ABA and flg22 was recently confirmed using the HyPer biosensor (Rodrigues et al., 2017). To validate the results obtained using roGFP-Orp1, we measured the production of ROS using H2DCF-DA. Although we only measured fluorescence in the cytosol, we observed a high signal from guard cell chloroplasts under H2S treatment. This phenomenon has been reported in ozone-treated guard cells (Vahisalu et al., 2010). Targeting of roGFP-Orp1 to different organelles in future studies may help to better understand H2O2 dynamics in different subcellular compartments.
Since roGFP2-based biosensors contain redox-sensitive Cys residues, this biosensor qualifies as a potential target for H2S, and we cannot rule out the possibility of direct sensor modification by H2S. H2S acts as a reductant inducing protein S-sulfhydration, while oxidation (i.e. formation of an intramolecular Cys disulfide) of the biosensor was observed. This makes it unlikely that the observed response was the result of direct sensor modification and supports H2S-triggered H2O2 production as a logical and sufficient interpretation. The responses of the biosensors in the epidermal strips may be explained by H2O2 oxidizing the glutathione pool, which serves as a source of reductant for several peroxidase systems. The absence of glutathione oxidation, as observed in whole-leaf guard cells, may be due to a higher redox buffering capacity. Differences in the maximal in vivo response of a sensor to saturating conditions have been consistently observed and can vary between tissues and compartments (Schwarzländer et al., 2008, 2016).
The existing models of guard cell signaling suggest that in addition to phosphorylation and to calcium binding, other mechanisms are involved in the regulation of NADPHox in guard cells (for a recent review, see Sierla et al., 2016). For example, the activation of PLDs and their product, PA, were suggested as regulators of ROS production in guard cells (Zhang et al., 2009; Distéfano et al., 2012; Uraji et al., 2012; Kalachova et al., 2013). PA binds to the stomatal closure-related proteins ABI1 and NADPHox (Zhang et al., 2004, 2009), to glyceraldehyde-3-phosphate dehydrogenase (Guo et al., 2012), MAPK6 (Yu et al., 2010), and microtubules (Zhang et al., 2012). In response to ABA, PA produced by PLDα1 generates ROS via NADPHox, and downstream ROS production acts as a signal to produce PA via PLDδ activity in an NO-dependent manner (Distéfano et al., 2012; Uraji et al., 2012). In this study, we showed that: (1) H2S induced the production of PA via the activity of PLDδ in a RBOHD-dependent pathway, (2) under our experimental conditions, PLDα1-derived PA in guard cells did not contribute to the PA pool measured after H2S treatments, and (3) the responses generated by H2S in the guard cells were not always identical to those generated by ABA, as was previously reported (Papanatsiou et al., 2015).
Several plausible scenarios may explain the differences with the model proposed for ABA-dependent signaling. One may be due to the differences in the subcellular localization of PLD proteins and NADPHox in response to H2S. This “spatial mismatch” has been observed before. The Arabidopsis PLDδ isoform is found in the subcellular fraction corresponding to the plasma membrane, and PLDα1 was also detected in the plasma membrane, but predominantly in the soluble fraction (Wang and Wang, 2001). Another study reported that tomato PLDβ1 relocalized from the cytosol to punctuate structures close to the plasma membrane, while PLDα1 remained in the cytosol in suspension-cultured tomato cells treated with xylanase (Bargmann et al., 2006). Thus, PA derived from different PLDs will be differentially localized, giving specificity to the signal that triggers the response. The fact that we could not detect PLDα1-derived PA may be due to the techniques and experimental conditions used to measure PA, related with localization, quantity, and/or timing.
Another explanation is related to the organization of the guard cell cytoskeleton, a key structure for the modulation of stomatal movement (Higaki et al., 2010). Given that PLDδ modulates the cytoskeleton by forming physical bridges between the microtubules and the plasma membrane (Andreeva et al., 2009; Ho et al., 2009), and that H2S regulates microtubule organization in root hairs (Jia et al., 2015), it can be postulated that PLDδ-derived PA might affect stomatal closure in response to H2S via modulation of microtubule organization.
We previously found that PLD and PLC activity are required for NO to induce stomatal closure (Distéfano et al., 2008, 2012) and that H2S induces NO production in ABA-mediated responses (Scuffi et al., 2014). These data are not enough to establish if the PLC/diacylglycerol kinase pathway is linked to H2S-induced stomatal closure, but NO appears as a probable link between both pathways.
In summary, H2S appears to induce stomatal closure via the participation of RBOHD, RBOHF, PLDα1, and PLDδ through a bifurcated pathway. It can be concluded that H2S induces ROS production via the activation of PLDα1 and the production of PA occurs via deactivation of PLDδ and that the absence of either of these two pathways prevents H2S from inducing stomatal closure.
MATERIALS AND METHODS
Plant Materials, Chemicals, and Stomatal Assays
Arabidopsis (Arabidopsis thaliana) Columbia-0 (Col-0) and Nössen (No-0) wild types, and the des1-1 (SALK_103855) and des1-2 (RIKEN RATM13-27151_G) mutants were kindly provided by Dr. Cecilia Gotor; The pldδ (SALK_023247) and pldα1 (SALK_067533) mutants were kindly provided by Dr. Munnik, and the rbohD and rbohF mutants were kindly provided by Prof. Jonathan Jones at The Sainsbury Laboratory, Norwich. Arabidopsis sensor lines with stable, cyto-nuclear expression of the Grx1-roGFP2 biosensor were used as described by (Marty et al., 2009). To generate the roGFP2-Orp1 Arabidopsis lines, the roGFP2-Orp1 sequence (Cys-36 and Cys-82; Gutscher et al., 2009) was amplified, and Gateway cloning sites were attached by PCR with the primers 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCATGGTGAGCAAGGGCGAGGAG-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCTATTCCACCTCTTTCAAAAGTTC-3′. The PCR product was cloned into the pDONR207 vector (Thermo Fisher Scientific). After sequencing, the roGFP2-Orp1 insert of the Gateway entry clone was transferred to the pH2GW7 vector (Karimi et al., 2002) under the control of a CaMV35S promotor. The destination vector carrying the roGFP2-Orp1 insert was electroporated into Agrobacterium tumefaciens strain C58C1 (Deblaere et al., 1985). Arabidopsis plants were transformed by the floral dip method (Clough and Bent, 1998). Seeds were screened for fluorescence and propagated to the T3 generation to select homozygous plants. Plants were grown in soil:perlite:vermiculite (1:1:1, v/v/v) at 25°C under a 16-h-light/8-h-dark photoperiod. Mutant pld plants (pldα1 and pldδ-1) were crossed with the roGFP2-Orp1 biosensor lines. The F4 generation (pldα1 X roGFP2-Orp1 and pldδ X roGFP2-Orp1) plants used were previously characterized by fluorescence for roGFP2-Orp1 and by PCR for T-DNA insertions according to Distéfano et al. (2012).
NaHS, DPI, and hypotaurine were purchased from Sigma. Chemicals for lipid extraction and silica-60 thin-layer chromatography (TLC) plates were purchased form Merck. 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCF-DA) was purchased from Thermo Fisher. flg22 (QRLSTGSRINSAKDDAAGLQIA) was synthesized by Genbiotech.
The stomatal aperture treatments were performed on epidermal strips excised from the abaxial side of fully expanded Arabidopsis leaves. Immediately after stripping, the epidermal peels were floated in opening buffer (5 mm K-MES, pH 6.1, and 50 mm KCl) for 3 h in the light. The strips were subsequently maintained in the same opening buffer and exposed to different treatments. After 90 min, stomata were digitized using a Nikon DS-Fi 1 camera coupled to a Nikon Eclipse Ti microscope. The stomatal aperture width was measured using ImageJ analysis software (National Institutes of Health).
Epifluorescence Microscopy
H2O2 was visualized using the ROS-sensitive probe H2DCF-DA. Arabidopsis epidermal strips were preincubated in opening buffer for 3 h under light and then loaded in the dark with the dye in 10 mm Tris buffer, pH 7.2, for 20 min. The strips were washed three times with fresh 10 mm Tris buffer, pH 7.2, and exposed to different treatments for 10 min in opening buffer. Fluorescence images were obtained using a Nikon DS-Fi 1 digital camera coupled to a Nikon Eclipse E200 epifluorescence microscope with excitation at 488 nm and emission at 505 to 530 nm. The green fluorescence was quantified using ImageJ analysis software (National Institutes of Health) as the pixel intensity of guard cells, except for the chloroplasts, and subtracting the pixel intensity of the background. The fluorescence values are presented as relative units with respect to the control treatments and are expressed as the means + se.
Confocal Laser Scanning Microscopy
Epidermal peels from Arabidopsis leaves expressing the H2O2 biosensor, roGFP2-Orp1, or the EGSH biosensor, Grx1-roGFP2, in the cytosol were incubated in opening buffer for 30 min, 2 h, 4 h, or 7 h for recovery, or for 7 h and subsequently treated with 100 µm of the H2S-donors under the same conditions. Whole Arabidopsis leaves were incubated in opening buffer for 30 min under light before treatment with 100 µm of the H2S-donors. To determine the dynamic range of the spectral response of the biosensors in situ, we used treatments with 10 mm H2O2 and 20 mm DTT for full oxidation and reduction, respectively, of the sensors. The epidermal peels and whole leaves were mounted under a Zeiss confocal microscope LSM 780 (Carl Zeiss MicroImaging) as described previously (Wagner et al., 2015). Images were collected with a 63× lens (Plan-Apochromat, 1.40 numerical aperture, oil immersion) and the biosensors were excited sequentially at 405 and 488 nm (line-switching mode) and emission was detected at 508 to 535 nm.
Analysis of Ratiometric Images
Ratiometric images were analyzed using a custom MatLab program package (Fricker, 2016). A region of interest in the cytosol was defined for each guard or pavement cell.
Lipid Labeling, Extraction, and Quantification
Guard Cell Phospholipids Labeling
Guard cell phospholipids were labeled by floating epidermal peels in 600 µL of opening buffer containing 0.06 µCi 32Pi µL−1 (carrier free) in a 12-well plate in a wet chamber under light for 3 h. Subsequently, epidermal peels were subjected to different treatments for 1 h under light, as indicated. The treatments were stopped by transferring the epidermal peels to a 2-mL Eppendorf tube containing a mixture of 170 µL of opening buffer plus 20 µL of 50% perchloric acid (v/v).
Whole-Leaf Phospholipids Labeling
Whole-leaf phospholipids were labeled by floating leaf discs in opening buffer containing 0.06 µCi 32Pi µL−1 (carrier free) in a 2-mL Eppendorf tube under light in a wet chamber overnight. Treatments were performed for 1 h in the same tube under light, as indicated.
Lipid Extraction and Quantification
Lipids were extracted by adding 750 µL of CHCl3/MeOH/HCl (50:100:1, v/v/v) and vortexing for 5 min. The samples were processed as described previously (Munnik and Laxalt, 2013). Lipids were chromatographed using an ethyl acetate solvent system as the mobile phase [the organic upper phase consisted of ethyl acetate/isooctane/formic acid/water (13:2:3:10, v/v/v/v)]. Radiolabeled lipids were visualized by autoradiography (BioMax XAR; Kodak) and quantified by phosphor imaging (Storm; Molecular Dynamics). For each treatment, radioactivity levels of 32PA were normalized to the amount of radioactivity in the phosphatidylethanolamine and phosphatidylglycerol spots. Finally, 32PA levels were expressed as a fold increase relative to the control treatment for at least three independent experiments.
Statistical Analyses
Data analyses were performed using Sigmaplot11 for Windows (Systat Software). The statistically significant differences were analyzed using one-way ANOVA, or Student’s t test, as indicated in the figure legends.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: DES1 (At5g28030), RBOHD (At5g47910), RBOHF (At1g64060), PLDα1 (At3g15730), and PLDδ (At4g35790).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Redox recovery of epidermal peels in opening buffer.
Supplemental Figure S2. H2S does not affect cytosolic redox balance in pavement cells.
Supplemental Figure S3. NADPH oxidase activity and PLDα1 are both required for H2S-dependent induction of ROS production in guard cells.
Supplemental Figure S4. H2S induces PA production through PLDδ activity in Arabidopsis leaf discs.
Supplemental Figure S5. H2S is involved in flagellin-induced stomatal closure.
References
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP (2011) In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 14: 819–829 [DOI] [PubMed] [Google Scholar]
- Álvarez C, Bermúdez MÁ, Romero LC, Gotor C, García I (2012a) Cysteine homeostasis plays an essential role in plant immunity. New Phytol 193: 165–177 [DOI] [PubMed] [Google Scholar]
- Alvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152: 656–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez C, García I, Moreno I, Pérez-Pérez ME, Crespo JL, Romero LC, Gotor C (2012b) Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 24: 4621–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva Z, Ho AYY, Barthet MM, Potocký M, Bezvoda R, Žárský V, Marc J (2009) Phospholipase D family interactions with the cytoskeleton: isoform delta promotes plasma membrane anchoring of cortical microtubules. Funct Plant Biol 36: 600. [DOI] [PubMed] [Google Scholar]
- Bargmann BOR, Laxalt AM, Riet BT, Schouten E, van Leeuwen W, Dekker HL, de Koster CG, Haring MA, Munnik T (2006) LePLDbeta1 activation and relocalization in suspension-cultured tomato cells treated with xylanase. Plant J 45: 358–368 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Bonini MG, Rota C, Tomasi A, Mason RP (2006) The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med 40: 968–975 [DOI] [PubMed] [Google Scholar]
- Buettner GR. (2015) Moving free radical and redox biology ahead in the next decade(s). Free Radic Biol Med 78: 236–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13: 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distéfano AM, García-Mata C, Lamattina L, Laxalt AM (2008) Nitric oxide-induced phosphatidic acid accumulation: a role for phospholipases C and D in stomatal closure. Plant Cell Environ 31: 187–194 [DOI] [PubMed] [Google Scholar]
- Distéfano AM, Scuffi D, García-Mata C, Lamattina L, Laxalt AM (2012) Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta 236: 1899–1907 [DOI] [PubMed] [Google Scholar]
- Fricker MD. (2016) Quantitative redox imaging software. Antioxid Redox Signal 24: 752–762 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L (2007) Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide 17: 143–151 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Devaiah SP, Narasimhan R, Pan X, Zhang Y, Zhang W, Wang X (2012) Cytosolic glyceraldehyde-3-phosphate dehydrogenases interact with phospholipase Dδ to transduce hydrogen peroxide signals in the Arabidopsis response to stress. Plant Cell 24: 2200–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutscher M, Pauleau A-L, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP (2008) Real-time imaging of the intracellular glutathione redox potential. Nat Methods 5: 553–559 [DOI] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP (2009) Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem 284: 31532–31540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Higaki T, Kutsuna N, Sano T, Kondo N, Hasezawa S (2010) Quantification and cluster analysis of actin cytoskeletal structures in plant cells: role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J 61: 156–165 [DOI] [PubMed] [Google Scholar]
- Ho AYY, Day DA, Brown MH, Marc J (2009) Arabidopsis phospholipase Dδ as an initiator of cytoskeleton-mediated signalling to fundamental cellular processes. Funct Plant Biol 36: 190. [DOI] [PubMed] [Google Scholar]
- Jia H, Hu Y, Fan T, Li J (2015) Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci Rep 5: 8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalachova T, Iakovenko O, Kretinin S, Kravets V (2013) Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem 66: 127–133 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan C-H, Moore PK (2008) Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360 [DOI] [PubMed] [Google Scholar]
- Marty L, Siala W, Schwarzländer M, Fricker MD, Wirtz M, Sweetlove LJ, Meyer Y, Meyer AJ, Reichheld J-P, Hell R (2009) The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc Natl Acad Sci USA 106: 9109–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot J-P, Hell R (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Munnik T, Laxalt AM (2013) Measuring PLD activity in vivo. In T Munnik, I Heilmann, eds, Plant Lipid Signaling Protocols. Methods in Molecular Biology (Methods and Protocols), Vol 1009. Humana Press, Totowa, NJ [DOI] [PubMed]
- Mustafa AK, Gadalla MM, Snyder SH (2009) Signaling by gasotransmitters. Sci Signal 2: re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanatsiou M, Scuffi D, Blatt MR, García-Mata C (2015) Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol 168: 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci USA 114: 9200–9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Dick TP, Meyer AJ, Morgan B (2016) Dissecting redox biology using fluorescent protein sensors. Antioxid Redox Signal 24: 680–712 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Scuffi D, Álvarez C, Laspina N, Gotor C, Lamattina L, García-Mata C (2014) Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166: 2065–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D, Lamattina L, García-Mata C (2016) Gasotransmitters and stomatal closure: is there redundancy, concerted action, or both? Front Plant Sci 7: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J (2016) Reactive oxygen species in the regulation of stomatal movements. Plant Physiol 171: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Miao Y, Song C-P (2014) Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol 201: 1121–1140 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14: 691–699 [DOI] [PubMed] [Google Scholar]
- Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10: 368–375 [DOI] [PubMed] [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H (2016) Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol 171: 1635–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraji M, Katagiri T, Okuma E, Ye W, Hossain MA, Masuda C, Miura A, Nakamura Y, Mori IC, Shinozaki K, et al. (2012) Cooperative function of PLDδ and PLDα1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 159: 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojärvi J, et al. (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62: 442–453 [DOI] [PubMed] [Google Scholar]
- Wagner S, Nietzel T, Aller I, Costa A, Fricker MD, Meyer AJ, Schwarzländer M (2015) Analysis of plant mitochondrial function using fluorescent protein sensors. Methods Mol Biol 1305: 241–252 [DOI] [PubMed] [Google Scholar]
- Wang C, Wang X (2001) A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol 127: 1102–1112 [PMC free article] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45: 250–278 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. (2014) The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 1840: 730–738 [DOI] [PubMed] [Google Scholar]
- Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188: 762–773 [DOI] [PubMed] [Google Scholar]
- Yun B-W, Feechan A, Yin M, Saidi NBB, Le Bihan T, Yu M, Moore JW, Kang J-G, Kwon E, Spoel SH, et al. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268 [DOI] [PubMed] [Google Scholar]
- Zhang H. (2016) Hydrogen sulfide in plant biology. In L Lamattina, C García-Mata, eds, Gasotransmitters in Plants. Signaling and Communication in Plants. Springer, Cham, Switzerland [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W (2012) Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24: 4555–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase D alpha 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101: 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009) Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21: 2357–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]