The second B-box domain in BBX21 is essential for its direct binding of T/G-box cis-element present in the HY5 promoter, regulating HY5 and HY5-controlled gene expression, and promoting photomorphogenesis.
Abstract
B-box-containing (BBX) proteins play critical roles in a variety of cellular and developmental processes in plants. BBX21 (also known as SALT TOLERANCE HOMOLOG2), which contains two B-box domains in tandem at the N terminus, has been previously demonstrated as a key component involved in the COP1-HY5 signaling hub. However, the exact molecular and physiological roles of B-box domains in BBX21 are largely unclear. Here, we found that structurally disruption of the second B-box domain, but not the first one, in BBX21 completely abolishes its biological and physiological activity in conferring hyperphotomorphogenetic phenotype in Arabidopsis (Arabidopsis thaliana). Intact B-box domains in BBX21 are not required for interaction with COP1 and its degradation by COP1 via the 26S proteasome system. However, disruption of the second B-box of BBX21 nearly impairs its ability for binding of T/G-box within the HY5 promoter both in vitro and in vivo, as well as controlling HY5 and HY5-regulated gene expression in Arabidopsis seedlings. Taken together, this study provides a mechanistic framework in which BBX21 directly binds to the T/G-box present in the HY5 promoter possibly through its second B-box domain, which in turn controls HY5 and HY5-regulated gene expression to promote photomorphogenesis.
Sunlight is one of key environmental factors affecting the development and growth of plants throughout their life circle. In response to dynamically changing light conditions, plants have evolved complicated but delicate regulatory network ensuring the appropriate developmental processes (Jiao et al., 2007). Seedlings undergo two distinct developmental programs after seed germination depending on the absence or presence of light. Plants develop skotomorphogenesis showing elongated hypocotyls, apical hooks, and closed cotyledons in darkness. Upon light illumination, seedlings display photomorphogenic development with short hypocotyls and opened cotyledons (Sullivan and Deng, 2003). During this dark-to-light transition, approximately one-third of genes throughout the Arabidopsis (Arabidopsis thaliana) genome are altered, suggesting a large number of genes synergistically mediate in the deetiolation process (Ma et al., 2001).
Extensive genetic and biochemical studies have established that numerous positive and negative regulators coordinately participate in skotomorphogenic and photomorphogenic development in plants. A group of COP/DET/ FUS proteins consist of three distinct E3 ubiquitin ligase complexes, COP1-SPA complex, COP10-DET1-DDB1 complex, and COP9 signalosome, all of which directly target downstream photomorphogenic-promoting factors for ubiquitination and facilitate their degradation via the 26S proteasome system, thereby repressing photomorphogenesis in darkness (Lau and Deng, 2012; Huang et al., 2014). In the light, numerous factors, including a set of B-box-containing (BBX) proteins, work in concert to precisely regulate photomorphogenesis (Gangappa and Botto, 2014). The BBX family consists of 32 members, which are classified into five subgroups according to their domain structures in Arabidopsis (Khanna et al., 2009). Increasing studies show that multiple BBX proteins play critical and distinct roles in the light signaling. BBX4, BBX20, BBX21, BBX22, and BBX23 promote photomorphogenesis (Datta et al., 2006, 2007, 2008; Fan et al., 2012; Xu et al., 2016; Zhang et al., 2017), whereas BBX19, BBX24, BBX25, and BBX32 negatively participate in light signaling in Arabidopsis (Indorf et al., 2007; Holtan et al., 2011; Gangappa et al., 2013; Wang et al., 2015).
These BBX proteins contain one or two B-box domains in the N-terminal region (Khanna et al., 2009). B-box domains, whose specialized tertiary structures are stabilized by binding zinc ions, belong to a subgroup of zinc finger motif (Klug and Schwabe, 1995). Previous studies suggest that B-box domain plays a crucial role in protein-protein interactions. N-terminal B-box region of BBX32 interacts with soybean (Glycine max) GmBBX62 (Qi et al., 2012). Structurally intact B-box domains in BBX21, BBX22, and BBX25 are required for their interactions with ELONGATED HYPOCOTYL5 (HY5) to enhance or repress its transcriptional activity (Datta et al., 2007, 2008; Gangappa et al., 2013), suggesting a role for BBX as transcriptional regulators through directly associating with other transcription factors. In addition, an intact B-box domain plays a direct role in activating transcription in transient expression assays in Arabidopsis protoplasts (Datta et al., 2007, 2008; Gangappa et al., 2013), implying that B-box domain may be directly involved in regulating of transcription in plants. Recent progress has further established that BBX4 and BBX21 can directly bind to the promoters of their target genes and act as transcription factors in the regulation of plant development (Xu et al., 2016; Tripathi et al., 2017). Despite these advances, the exact biochemical roles of B-box domains in the DNA binding and its biological function remained largely unclear.
In this study, we demonstrated that the second B-box domain in BBX21 is indispensable for its biological function in plants. The structurally intact second B-box domain, but not the first one, is required and necessary for binding of HY5 promoter, activating HY5 expression and promoting photomorphogenesis. Taken together, our findings illuminate a molecular role for the B-box domain in the regulation of transcription and light signaling.
RESULTS
BBX21 Binding of the HY5 Promoter Is Independent of HY5
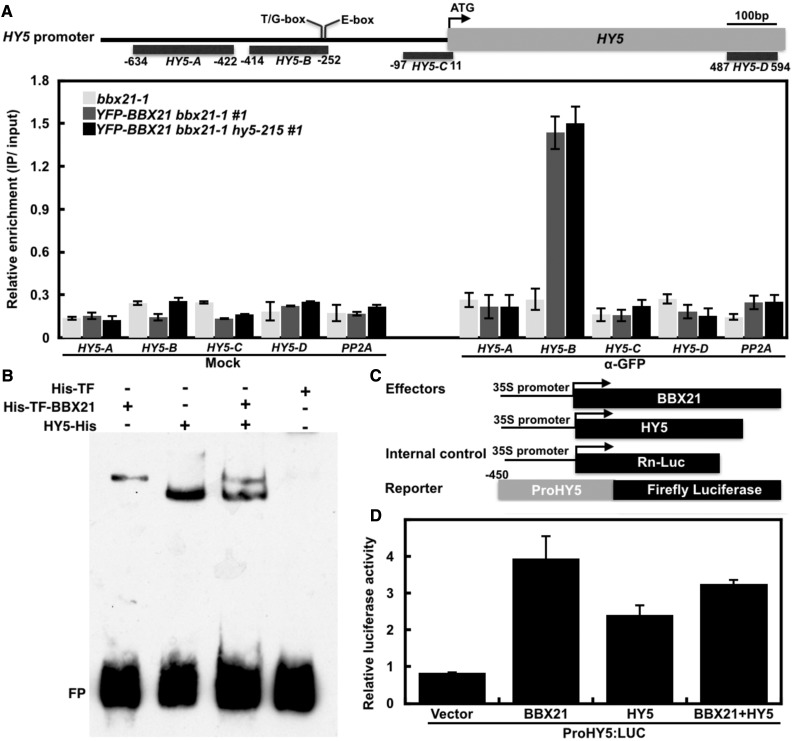
Upon light illumination, both accumulated BBX21 and HY5 itself directly binds to the same site (T/G-box) present in the HY5 promoter to activate its gene expression (Abbas et al., 2014; Binkert et al., 2014; Xu et al., 2016). We thus explored the possible interconnection between BBX21 and HY5 in binding of HY5 promoter. We found that the binding affinity for BBX21 on HY5 promoter (HY5-B: −414 to −252) was not detectably altered in the absence or presence of HY5 in our chromatin immunoprecipitation (ChIP) assays (Fig. 1A). Consistently, either BBX21 or HY5 alone can bind to the subfragment of HY5 promoter (−349 to −252) containing an intact T/G-box; however, BBX21 and HY5 together did not form a supershift in the electrophoretic mobility shift (EMSA) assays (Fig. 1B). In addition, either BBX21 or HY5 alone can activate the ProHY5:LUC, and the activation on ProHY5:LUC by BBX21 was not significantly affected in the presence of HY5 in the transient transfection assay in Arabidopsis protoplasts (Fig. 1, C and D). Together, these results indicate that BBX21 binding of HY5 promoter is in a HY5-independent manner.
Figure 1.
BBX21 binding of T/G-box in the HY5 promoter is independent on HY5. A, ChIP assays showing that BBX21 associated with the HY5 promoter in the presence and absence of HY5 in vivo. ChIP was performed with anti-myc monoclonal antibody, and the ChIP DNA was analyzed by real-time qPCR. The numbers represent the locations of the four HY5 promoter regions relative to the translation start site (referred to as position +1). Error bars represent sd of three technical replicates. B, EMSA assays showing BBX21 and HY5 independently bind to the subfragment of HY5 promoter (−349 to −252). TF, Trigger factor; FP, free probe. C, Schematic representation of various constructs used in the transient transfection assay in Arabidopsis protoplasts. Arrow after the 35S promoter indicates the transcriptional start site; −450 indicates the length of the HY5 promoter that was fused to the firefly luciferase to create the reporter construct. D, Bar graph showing activation by BBX21 and HY5 either alone or together on the ProHY5:LUC reporter. Error bars represent se (n = 3).
The Second B-Box Domain in BBX21 Promotes Photomorphogenesis
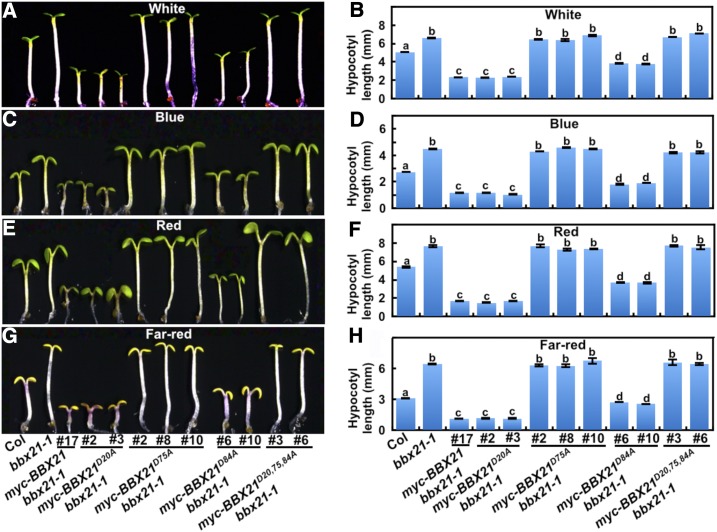
BBX21 possesses two B-box domains in tandem at the N terminus and promotes photomorphogenesis in the light (Datta et al., 2007; Xu et al., 2016). To test whether the B-box domains are involved in the regulation of seedling photomorphogenic development, we individually or together substituted three conserved Asp (D) residues in the B-box of BBX21 (D20A, D75A, and D84A or D20, D75, and D84A). D20 or D75 is the fourth zinc ion-coordinating residue in each of the respective B-box domains in BBX21, whereas D84 is a conserved residue in the second B-box domain, but is not required for zinc ion binding (Supplemental Fig. S1A; Datta et al., 2007). Substitution of D20A or D75A leads to the disruption of each of the B-box domains, and D84A might have slightly effect on the conformation of the second B-box structure. The substituted proteins were named BBX21D20A, BBX21D75A, BBX21D84A, and BBX21D20,75,84A (Supplemental Fig. S1B), and these transgenes tagged with myc or yellow fluorescent protein (YFP; myc-BBX21D75A, myc-BBX21 D75A, myc-BBX21D84A, myc-BBX21D20,75,84A, and YFP-BBX21D20,75,84A) were then introduced into bbx21-1 single mutant background. In these transgenic plants, the various mutated BBX21 genes were overexpressed, and the exogenous protein abundance were expressed at comparable levels (Supplemental Fig. S1, C–F). In the dark, these independent transgenic lines displayed similar etiolated phenotype with wild-type and bbx21-1 mutant seedlings (Supplemental Figs. S2 and S3, A and B). myc-BBX21 bbx21#17 transgenic seedlings exhibited dramatically shortened hypocotyls in various light conditions tested (white [W], blue [B], red [R], and far red [FR]), which was consistent with a previous study (Xu et al., 2016). The D20A amino acid substitution in BBX21 (BBX21D20A), which disrupts the first B-box domain structure of BBX21, was still able to confer light hypersensitivity in bbx21-1, as two independent myc-BBX21D20A bbx21-1 transgenic lines showed drastically short hypocotyls and the phenotypes resemble that of myc-BBX21 bbx21-1 #17 in various light conditions tested (W, B, R, and FR; Fig. 2). However, three individual myc-BBX21D75A bbx21-1 transgenic lines displayed similar phenotype with the bbx21-1 single mutant in hypocotyls grown in the various light conditions tested (W, B, R, and FR; Fig. 2), suggesting that disruption of the second B-box domain by D75A amino acid substitution in BBX21 results in the loss of its light responsiveness in bbx21-1 background. Overexpression of BBX21D84A in bbx21-1 displayed shorter hypocotyls than the wild type, but not as dramatic compared to the myc-BBX21 bbx21-1 #17 transgenic line (Fig. 2), implying D84A substitution (BBX21D84A) might only have partial effect on the function of the second B-box domain and only slightly reduce the ability in promoting photomorphogenesis in Arabidopsis. As expected, all the myc-BBX21D20,75,84A bbx21-1 and YFP-BBX21D20,75,84A bbx21-1 transgenic seedlings were indistinguishable from myc-BBX21D75A bbx21-1 and bbx21-1 grown in various light conditions tested (Fig. 2; Supplemental Fig. S3, C–J). Collectively, these data clearly suggest that BBX21D20A and BBX21D84A transgenes are biologically functional and could confer light hyperphotomorphogenic phenotype in bbx21-1; however, BBX21D75A and BBX21D20,75, 84A, both with an impaired second B-box domain, could not confer light responsiveness in bbx21-1 mutant background, demonstrating that the second B-box domain of BBX21 is required and essential for its biological and physiological function in plants.
Figure 2.
BBX21D75A bbx21-1 transgenic seedlings are hyposensitive to light. Hypocotyl length and phenotype of 4-d-old Col, bbx21-1, BBX21, and various mutated BBX21 transgenic seedlings grown in W (33.3 μmol/m2/s; A and B), B (0.62 μmol/m2/s; C and D), R (6.78 μmol/m2/s; E and F), and FR (1.46 μmol/m2/s; G and H) conditions. Data are means ± se; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis. The experiments were performed three times with similar results. The graphs depict one of these experiments.
BBX21 positively regulates anthocyanin accumulation in the light (Datta et al., 2007; Xu et al., 2016); we thus asked whether B-box in the BBX21 affects anthocyanin accumulation of seedlings. To this end, we measured the anthocyanin levels in various transgenic lines grown in W light for 4 d. Consistent with previous studies (Datta et al., 2007; Xu et al., 2016), bbx21-1 single mutant accumulated less anthocyanin, whereas myc-BBX21 bbx21-1 #17 transgenic seedlings accumulated more anthocyanin. Overexpression of myc-BBX21D20A in bbx21-1 background induced the accumulation of anthocyanin, similar to that of in myc-BBX21 bbx21-1 #17. myc-BBX21D84A bbx21-1 transgenic plants accumulated significantly more anthocyanin compared to Col and bbx21-1, but markedly less anthocyanin than myc-BBX21 bbx21-1 #17. As expected, all the myc-BBX21D75A bbx21-1 and myc-BBX21D20,75,84A bbx21-1 transgenic lines accumulated comparable anthocyanin content than bbx21-1, in which was significantly less compared with Col (Supplemental Fig. S4). These finding suggest that the second B-box domain in BBX21 is essential for positively regulating anthocyanin accumulation in the light.
B-Box Domains of BBX21 Are Not Required for Its Degradation by COP1
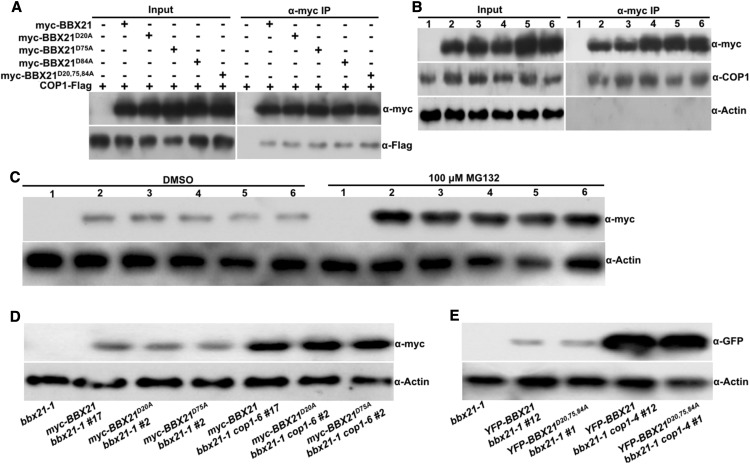
BBX21 interacts with COP1 and undergoes COP1-mediated degradation via the 26S proteasome pathway in darkness (Xu et al., 2016). To investigate whether the B-box domain is involved in this biological event, we performed coimmunoprecipitation (co-IP) assays by transiently expressing of 35:COP1-Flag together with 35S:myc-BBX21, 35S:myc-BBX21D75A, 35S:myc-BBX21D75A, 35S:myc-BBX21D84A, or 35S:myc-BBX21D20,75,84A in Arabidopsis protoplasts. Immunoprecipitation of myc-BBX21, myc-BBX21D75A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75,84A pulled down comparable levels of COP1-Flag (Fig. 3A). Consistently, myc-BBX21, myc-BBX21D75A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75,84A coimmunoprecipitated with a similar amount of COP1 in myc-BBX21, myc-BBX21D75A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75,84A Arabidopsis seedlings (Fig. 3B). These results indicate that mutations in the B-box domains of BBX21 do not affect its association with COP1.
Figure 3.
COP1 promotes the degradation of BBX21 with impaired B-box domains in darkness. A, Co-IP analysis showing that myc-BBX21D20A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75, 84A interacts with COP1. Total protein was extracted from Arabidopsis protoplasts expressing 35S:COP1-Flag, 35S:myc-BBX21, 35S:myc-BBX21D20A, 35S:myc-BBX21D75A, 35S:myc-BBX21D84A, and 35S:myc-BBX21D20,75,84A. The immunoprecipitates were detected using anti-Flag and anti-myc antibodies. B, Co-IP analysis showing that myc-BBX21D20A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75,84A interacts with COP1 in Arabidopsis seedlings. Four-day-old white-light-grown bbx21-1 (1), myc-BBX21 bbx21-1 #17 (2), myc-BBX21D20A bbx21-1 #2 (3), myc-BBX21D75A bbx21-1 #2 (4), myc-BBX21D84A bbx21-1 #6 (5), and myc- BBX21D20,75, 84A bbx21-1 #3 (6) seedlings were transferred to darkness for 16 h and then subjected to a co-IP assay using anti-myc antibodies, with the immunoprecipitates detected using anti-COP1 and anti-myc antibodies. Actin served as a negative control. C, Immunoblot analysis of myc-tagged wild-type or various mutated BBX21 protein levels in dark-grown bbx21-1 (1), myc-BBX21 bbx21-1 #17 (2), myc-BBX21D20A bbx21-1 #2 (3), myc-BBX21D75A bbx21-1 #2 (4), myc-BBX21D84A bbx21-1 #6 (5), and myc- BBX21D20,75, 84A bbx21-1 #3 (6) transgenic seedlings treated with DMSO or 100 μm MG132 for 3 h. E, Immunoblot analysis of myc-BBX21, myc-BBX21D20A, and myc-BBX21D75A protein levels in myc-BBX21 bbx21-1 #17, myc-BBX21D20A bbx21-1 #2, myc-BBX21D75A bbx21-1 #2, myc-BBX21 bbx21-1 cop1-6 #17, myc-BBX21D20A bbx21-1 cop1-6 #2, and myc-BBX21D75A bbx21-1 cop1-6 #2 transgenic seedlings grown in the dark for 4 d. F, Immunoblot analysis of YFP -BBX21 and YFP -BBX21D20,75,84A protein levels in YFP-BBX21 bbx21-1 #12, YFP-BBX21D20,75,84A bbx21-1 #2, YFP-BBX21 bbx21-1 cop1-4 #12, and YFP-BBX21D20,75,84A bbx21-1 cop1-4 #2 transgenic seedlings grown in the dark for 4 d. In D to F, bbx21-1 served as a negative control, and anti-actin served as a loading control.
BBX21 is degraded via the 26S proteasome system in the dark and stabilized by the MG132 treatment (a proteasome inhibitor). myc-BBX21 significantly accumulated in etiolated myc-BBX21 bbx21-1 #17 transgenic seedlings by the treatment of 100 μm MG132 for 3 h (Fig. 3C) as reported previously (Xu et al., 2016). Similarly, myc-BBX21D75A, myc-BBX21D75A, myc-BBX21D84A, or myc-BBX21D20,75,84A protein levels were evidently increased in respective dark-grown transgenic lines with treatment of 100 μm MG132 for 3 h, suggesting that all these distinct mutated versions of BBX21 are subjected to 26S proteasome-mediated degradation in darkness. Next, a cop1 mutation was introduced into myc-BBX21D20A bbx21-1 #2, myc-BBX21D75A bbx21-1 #2, and YFP-BBX21 D20,75,84A bbx21-1 #1 transgenic lines by genetic crossing. Like myc-BBX21, markedly more myc-BBX21D20A, myc-BBX21D75A, and YFP-BBX21D20,75,84A accumulated in cop1 bbx21-1 than those in bbx21-1 background in darkness (Fig. 3, D and E). Together, these data suggest that disruption of B-box domains might have no effect on COP1-promoted degradation of BBX21 via the 26S proteasome pathway.
The Second B-Box Domain in BBX21 Is Essential for BBX21 Ability to Bind to the HY5 Promoter
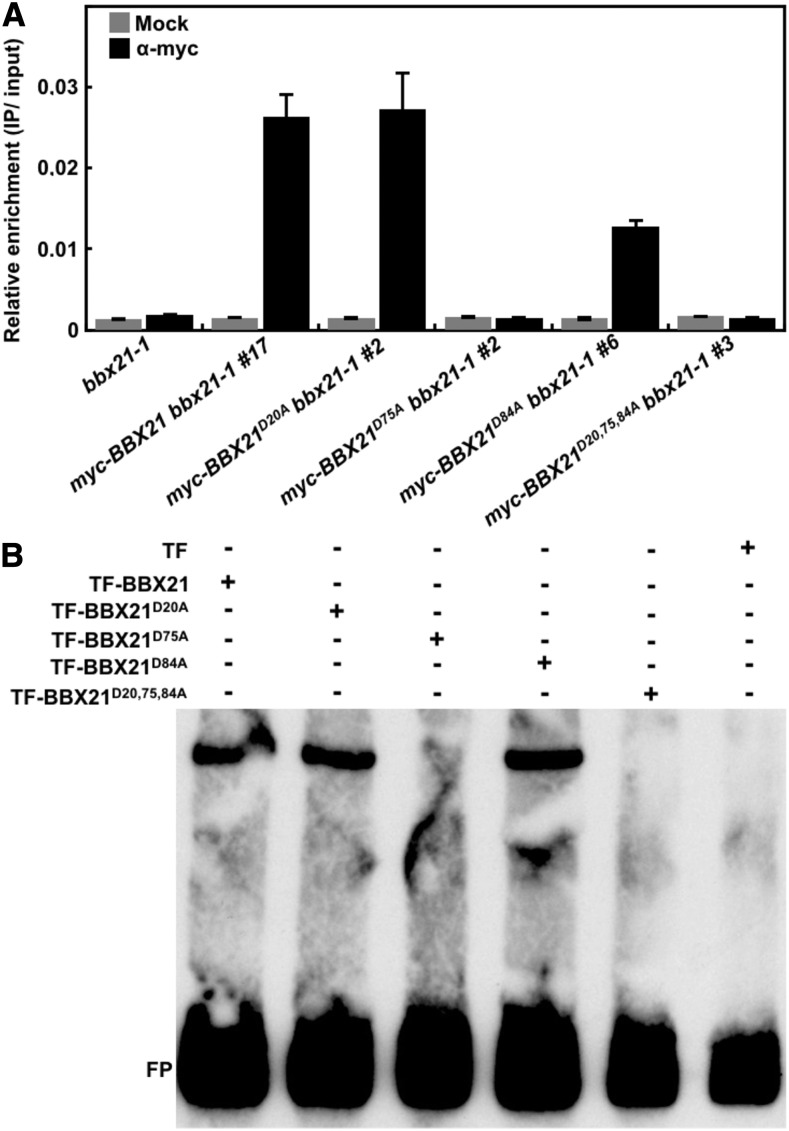
It was reported that BBX21 directly binds to the T/G-box present in the HY5 promoter and activates its gene expression (Xu et al., 2016). To test whether the B-box domain of BBX21 is responsible for the binding of HY5 promoter, we performed the ChIP-qPCR assays using anti-myc monoclonal antibodies and distinct transgenic lines carrying wild-type or various mutated BBX21 versions. As shown in Figure 4A, BBX21, BBX21D20A, and BBX21D84A associated with the HY5 promoter fragment (HY5-B: −414 to −252). Consistent with the observed phenotypes in hypocotyl length and anthocyanin levels, the binding affinity of BBX21D84A was lower than BBX21 and BBX21D20A (Fig. 4A). Neither BBX21D75A nor BBX21D20,75,84A could interact with the same region of HY5 promoter. These data indicate the disruption of the second B-box domain in BBX21 completely impaired its ability for binding HY5 promoter in vivo. Furthermore, we performed EMSA assays to verify the ChIP data. Like BBX21, either BBX21D20A or BBX21D84A was able to bind to the 98-bp HY5 promoter subfragment (−349 to −252) containing an intact T/G-box, whereas neither BBX21D75A nor BBX21D20,75,84A could bind to the same HY5 promoter fragment (Fig. 4B). Taken together, these data demonstrate that the second B-box, but not the first one, in BBX21 is indispensable for binding of HY5 promoter.
Figure 4.
Disruption of the second B-box in BBX21 impairs its ability for DNA binding. A, ChIP assays showing that BBX21D75A did not associate with the HY5 promoter region B (−414 to −252) shown in Figure 1A in vivo. ChIP was performed with anti-myc monoclonal antibody, and the ChIP DNA was analyzed by real-time qPCR. Error bars represent sd of three technical replicates. B, EMSAs using His-TF-BBX21 and various mutated His-TF-BBX21 and the HY5 promoter subfragment (−349 to −252) as the probe. T, Trigger factor; FP, free probe.
BBX21 DNA-Binding Domain Is Necessary for Its Transcriptional Activity
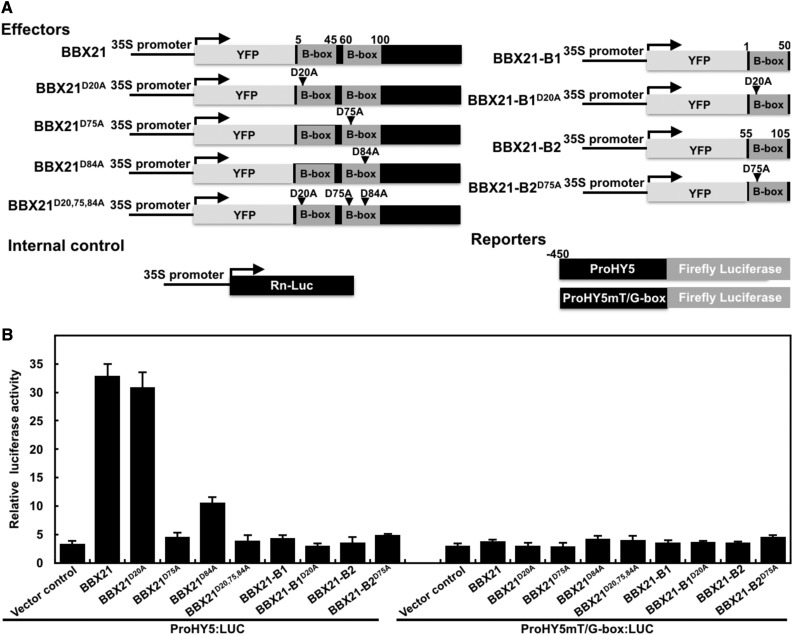
To explore the biological significance of the second B-box in BBX21 binding to the HY5 promoter, we performed transient transfection assays in Arabidopsis protoplasts using a reporter, in which the luciferase (LUC) reporter gene was under the control of a 450-bp promoter fragment of HY5. The ProHY5:LUC reporter was transfected into Arabidopsis mesophyll protoplasts along with 35S:RnLUC (an internal control) and the various effectors as indicated (Fig. 5A). Similar protein levels of various wild-type, mutated, and truncated BBX21 transiently expressed in Arabidopsis protoplasts after transfection (Supplemental Fig. S5). Either BBX21 or BBX21D20A alone robustly activated the ProHY5:LUC reporter in Arabidopsis protoplasts, whereas both BBX21D75A and BBX21D20,75,84A did not show any activation ability. BBX21D84A exhibited intermediated activation on ProHY5:LUC reporter. Neither the first B-box (BBX21-B1) nor the second B-box (BBX21-B2) of BBX21 alone could activate the ProHY5:LUC (Fig. 5B). These results indicate that the second B-box alone may not possess transcriptional activation ability, and DNA binding is necessary for the transcriptional function of BBX21. In addition, none of the wild-type and various mutated BBX21 or B-box domains was able to activate the ProHY5mT/G:LUC reporter carrying a mutated T/G-box (in which CACGTT was mutated to ACCGGG; Fig. 5B), indicating that BBX21 is acting through this T/G-box cis-element in activating HY5.
Figure 5.
Disruption of the second B-box in BBX21 impairs its transcriptional activity. A, Schematic representation of various constructs used in the transient transfection assay in protoplasts. Arrow after the 35S promoter indicates the transcriptional start site. D-n-A indicates the Ala substitution of the three Asp residues in the B-boxes at positions 20, 75, and 84 (n); −450 indicates the length of the HY5 promoter carrying a wild-type or a mutated T/G-box that was fused to the firefly luciferase to create the reporter constructs. B, Bar graph showing activation by wild-type, various mutated versions, and two respective B-box domains of BBX21 on the ProHY5:LUC reporter and the effect of mutating the T/G-box (CACGTT was mutated to ACCGGG) in the HY5 promoter. Error bars represent se (n = 3).
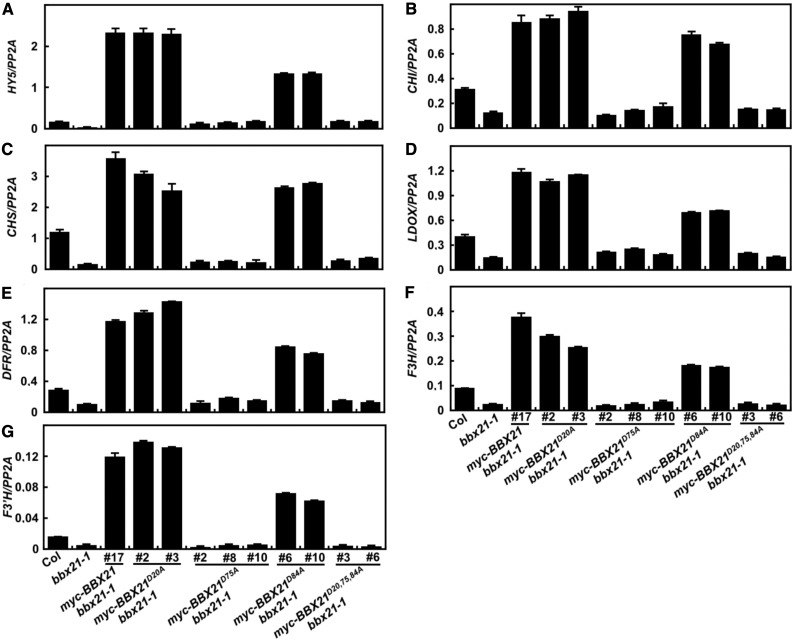
To further test whether the second B-box domain of BBX21 is required for activation of HY5 in plants, we examined the HY5 and six HY5-regulated gene (CHS, CHI, DFR, LDOX, F3H, and F3′H; Shin et al., 2007) expression in various transgenic lines. Similar to myc-BBX21 bbx21-1 #17, HY5 as well as six HY5-regulated genes tested were up-regulated in myc-BBX21D20A bbx21-1 and myc-BBX21D84A bbx21-1 transgenic seedlings. These gene expression levels in myc-BBX21D84A bbx21-1 were lower compared with myc-BBX21 bbx21-1 #17 and myc-BBX21D20A bbx21-1. However, these gene expression levels were significantly down-regulated in myc-BBX21D75A bbx21-1 and myc-BBX21D20,75,84A bbx21-1 transgenic plants (Fig. 6), which was similar with bbx21-1 single mutant. Taken together, these data strongly suggest that the second B-box domain in BBX21 is required for controlling HY5 and HY5-regulated gene expression in plants.
Figure 6.
Disruption of the second B-box in BBX21 impairs its ability for activating the HY5 expression in Arabidopsis. Quantitative RT-PCR analyses of HY5, CHS, CHI, DFR, LDOX, F3H, and F3′H transcript levels in Col, bbx21-1, BBX21, and various mutated BBX21 transgenic seedlings grown under W light for 4 d.
DISCUSSION
Numerous B-box-domain-containing proteins have been documented to be participating in various cellular and developmental processes ranging from plants to humans (Gangappa and Botto, 2014). It has been established that one mode of action of B-box domain is for protein-protein interactions. B-boxes in animal PML and MID are responsible for interacting with the GATA-2 and α4, respectively (Tsuzuki et al., 2000; Tao et al., 2008). In Arabidopsis, BBX21, BBX22, and BBX25 associate with HY5 through their B-box domains (Datta et al., 2007, 2008; Gangappa et al., 2013), and BBX19 interacts with COP1, ELF3, as well as CO through its first B-box domain (Wang et al., 2014, 2015). In this study, we demonstrate that the second B-box domain of BBX21 is essential and required for its DNA-binding ability, which is integral to its function as a transcription factor.
There are 32 proteins containing at least one or two B-box domains in tandem at the N terminus in Arabidopsis (Khanna et al., 2009). These BBX proteins act at least via two distinct mechanisms for regulating transcription. First, BBX21, BBX22, BBX24, and BBX25 all interact with HY5 through their B-box domains. As a result, BBX21 and BBX22 activate transcriptional activity of HY5, whereas BBX24 and BBX25 repress HY5 action (Datta et al., 2007, 2008; Gangappa et al., 2013), demonstrating that BBX proteins can act as transcriptional activators or repressors through forming heterodimers with other transcription factors. In addition, either BBX21 or BBX22 can activate the transcription through G-box cis-element in Arabidopsis protoplasts (Datta et al., 2007, 2008). BBX4 directly binds to the promoter of FT in the presence of BBX32 to repress flowering (Tripathi et al., 2017), and BBX21 directly binds to the T/G-box present in the HY5 promoter and activates HY5 expression (Xu et al., 2016). All these findings clearly demonstrate that BBX proteins possess DNA-binding ability and can act as transcription factors in plants. The second B-box domain, but not the first one, in BBX21 was indispensable for its binding of HY5 promoter both in vitro and in vivo, regulating HY5 and HY5-controlled gene expression, as well as its biological ability in conferring hyperphotomorphogenic phenotype in plants (Figs. 2 and 4–6). However, the second B-box domain alone was not able to activate transcription when transiently expressed in Arabidopsis protoplasts (Fig. 5). In agreement, a recent study showed that the C-terminal region of BBX21, but not the B-box domains, shows self-activation in yeast cells (Xu et al., 2017). It appears that the second B-box of BBX21 is required for binding to specific DNA cis-element, and its C terminus is essential for activating transcription. The crucial role of the second B-box in the functionality of BBX21 demonstrates that DNA-binding domain of BBX21 is necessary for BBX21 function. Both BBX21 and BBX22 activate transcription through G-box cis-element in transient expression assays (Datta et al., 2007, 2008), and the second B-box of BBX21 may directly bind to the T/G-box within the HY5 promoter (Figs. 4–6), suggesting that the G-box and T/G-box are likely at least two of the binding sites for B-box domain in plants.
Mutations of conserved residues in B-boxes of BBX1/CO cause late flowering (Robson et al., 2001). Disruption of the first B-box domain in BBX19, but not the second one, results in loss of its biological function in the regulation of photomorphogenesis and controlling flowering (Wang et al., 2014, 2015). Substitution of the fourth zinc ion-ligating residue in the second B-box domain of BBX21 completely impairs its physiological role in plants (Fig. 2). All these lines of evidence demonstrate an important biological and physiological function of the B-box domain in plant development. B-box domain appears to predominantly function as a protein-protein or protein-DNA interacting motif in the regulation of plant development and growth. Two B-box domains in BBX21 appear to play different roles, while the intact of each B-box domains is likely inessential for protein-protein interactions (Datta et al., 2007), and the second B-box is responsible for DNA-binding, controlling gene expression, and promoting photomorphogenesis (Figs. 2 and 4–6). Unlike BBX21, only the first B-box in BBX19, but not the second one, is indispensable for its biological activity in plants, as only this domain is required for interaction with COP1, ELF3, and CO (Wang et al., 2014, 2015). It appears that the functions of individual B-box domain in the BBX proteins might diverge at molecular level, though they are highly conserved. All these facts demonstrate that at least one of the B-box motifs in BBX proteins is the key functional domain and the structurally integrity of B-box(es) is essential and necessary for their biochemical activity in plants.
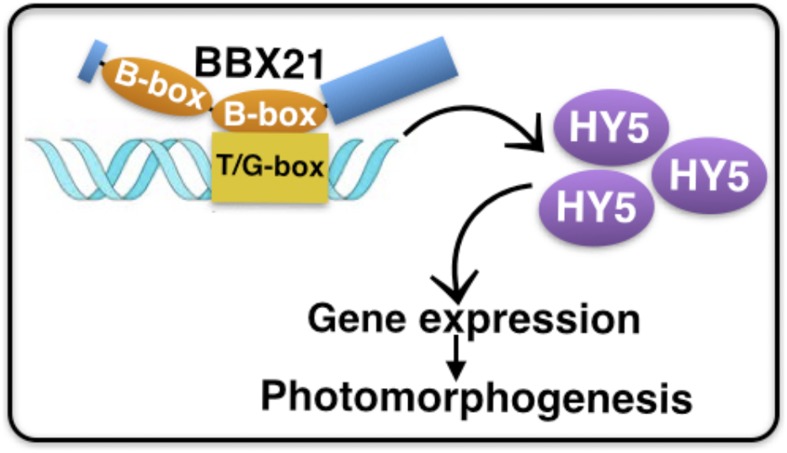
Collectively, this study provides direct primary genetic and molecular evidence demonstrating how the second B-box domain of BBX21 exerts an effect on plant growth at the seedling stage in the light. Upon seed germination and emerging from soil, BBX21 directly binds to T/G-box within the HY5 promoter through its second B-box domain. Hence, this B-box-mediated association controls HY5 and HY5-regulated gene expression and subsequently promotes photomorphogenic development (Fig. 7).
Figure 7.
A working model showing how B-box domains in BBX21 in promoting photomorphogenesis. BBX21 directly binds to the T/G-box present in the HY5 promoter through the second B-box domain to activate the HY5 gene expression, which in turn serves to increase the accumulation of HY5 abundance and regulate HY5-controlled gene expression to promote photomorphogenesis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All lines used in this study are of the Columbia (Col) ecotype. Seeds were surface-sterilized for 10 min with 30% commercial Clorox bleach and washed three times with sterile water, then sown on solid Murashige and Skoog medium supplemented with 0.4% Bacto-agar (Difco) and 1% Suc for phenotypic analysis and biochemical assays.
Gene Constructs and Transgenic Plants
pCombia1300-35S:COP1-Flag (Xu et al., 2015), pCold-His-TF-BBX21 (Xu et al., 2016), pET28a-HY5-His (Xu et al., 2016), pRTL2-BBX21 (Datta et al., 2007), and pRTL2-HY5 (Gangappa et al., 2013) constructs were described previously. To generate pCold-His-TF-BBX21D20A, pCold-His-TF-BBX21D75A, pCold-His-TF-BBX2D84A, and pCold-His-TF-BBX21D20,75,84A, the full-length BBX21D20A, BBX21D75A, BBX2D84A, and BBX21D20,75,84A were amplified by primer-based site-directed mutagenesis and then cloned into NdeI/XbaI sites of pCold-His-TF vector (Takara). The full-length BBX21D20A, BBX21D75A, BBX2D84A, and BBX21D20,75,84A open reading frames were cloned into the pDONR-221 vector (Invitrogen) by primer-based site-directed mutagenesis method and introduced into the plant binary vector pEarley Gateway 203 or pEarley Gateway 104 (Earley et al., 2006) using Gateway LR Clonase enzyme mix (Invitrogen). These constructs were used to transform Agrobacterium tumefaciens strain GV3101 by the freeze-thaw method, which were then introduced into Arabidopsis (Arabidopsis thaliana) bbx21-1 mutant plants via the floral dip method (Clough and Bent, 1998). Transformants were selected on Murashige and Skoog medium supplemented with 20 μg/mL glufosinate-ammonium (Sigma-Aldrich).
Co-IP Assays and Immunoblot Analysis
For co-IP experiments using Arabidopsis protoplasts, the constructs 35S:COP1-Flag and myc-tagged wild type or various mutated BBX21 driven by the cauliflower mosaic virus 35S promoter were transformed into Arabidopsis mesophyll protoplasts. After overnight incubation in light, the protoplasts were incubated in darkness for 3 h and then lysed. COP1-Flag proteins were immunoprecipitated by anti-myc antibodies (Sigma-Aldrich) coupled with 25 μL of Protein-A Sepharose (GE Healthcare) for 3 h at 4°C; 100 mm Gly (pH 2.5) and 100 mm NaCl were immediately neutralized by 2 m Tris-HCl, pH 9.0, and 100 mm NaCl and finally concentrated using StrataClean Resin (Stratagene) before immunoblot analyses.
For co-IP assays using Arabidopsis seedlings, 1 mg of total proteins was extracted from 4-d-old Arabidopsis seedlings in protein extraction buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 10% (v/v) glycerol, 0.1% Tween 20, 1 mm PMSF, and 1× cOmplete Protease Inhibitor Mixture (Roche). The extracts were incubated with 4 μL of anti-myc antibodies (Sigma-Aldrich) coupled with 25 μL of Protein-A Sepharose (GE Healthcare) for 3 h at 4°C. The Sepharose was then washed three times with protein extraction buffer. The precipitates were eluted into 100 mm Gly (pH 2.5) and 100 mm NaCl, immediately neutralized by 2 m Tris-HCl pH 9.0 and 100 mm NaCl, and finally concentrated using StrataClean Resin (Stratagene) before immunoblot analyses.
For immunoblots, Arabidopsis wild-type or mutant seedlings were homogenized in a protein extraction buffer containing 50 mm Tris, pH 7.5, 200 mm NaCl, 1 mm EDTA, 10% glycerol, 1 mm DTT, 1 mm PMSF, and 1× complete protease inhibitor cocktail (Roche). Primary antibodies used in this study were anti-COP1 (McNellis et al., 1994), anti-cMyc (Sigma-Aldrich), anti-GFP (Abmart), and anti-actin (Sigma-Aldrich).
Measurement of Hypocotyl Length
To measure the hypocotyl length of seedlings, seeds were cold-treated at 4°C for 3 d and then transferred to continuous white light for 8 h to induce uniform germination. Then, the seeds were transferred to white or monochromatic light (W, B, R, or FR) conditions and incubated at 22°C for 4 d. The hypocotyl lengths of seedlings were measured using ImageJ software.
Quantitative Real-Time PCR
Total RNA was extracted from 4-d-old Arabidopsis seedlings grown under white light using the RNeasy plant mini kit (Qiagen). cDNAs were synthesized from 2 mg of total RNA using the SuperScript II first-strand cDNA synthesis system (Fermentas) according to the manufacturer’s instructions. Then, cDNAs were subjected to real-time quantitative PCR using the CFX96 real-time PCR detection system (Applied Biosystems) and SYBR Green PCR Master Mix (Takara). PCR was performed in triplicate for each sample, and the transcript levels were normalized to that of a PP2A gene. All primers used for this assay are listed in Supplemental Table S1.
ChIP
The ChIP assays were performed as described by Xu et al. (2016). Chromatin isolation was performed using bbx21-1 and various mutated BBX21 transgenic seedlings grown under constant white light for 4 d. The resuspended chromatin was sonicated at 4°C to ?250- to 500-bp fragments. The sheared chromatin was immunoprecipitated, washed, reverse cross-linked, and finally amplified. About 10% of sonicated but nonimmunoprecipitated chromatin was reverse cross-linked and used as an input DNA control. Both immunoprecipitated DNA and input DNA were analyzed by real-time PCR (Applied Biosystems). Monoclonal anti-cMyc antibody (Sigma-Aldrich) was used for the assays. All primers used for this assay are listed in Supplemental Table S1.
EMSAs
EMSAs were performed using biotin-labeled probes and the Light Shift Chemiluminescent EMSA kit (Thermo Scientific) as described previously (Xu et al., 2016). The 98-bp promoter subfragment of HY5 upstream of ATG (−349 to −252) were PCR amplified and then mixed with biotin and kept in UV light for 30 min for biotin labeling. Next, 500 ng of purified His-TF-BBX21 or His-TF proteins was incubated together with 40 fmol biotin-labeled probes in a 20-μL reaction mixture containing 10 mm Tris-HCl, pH 7.5, 0.05% Nonidet P-40, 10 mm MgCl2, 5% (v/v) glycerol, and 0.1 μg/mL poly(dI∙dC). Reactions were incubated at 25°C for 20 min and separated on 6% native polyacrylamide gels in 0.5× TBE buffer. Then, gels were electroblotted to Hybond N+ (Millipore) nylon membranes in 0.5× TBE for 40 min, and the labeled probes were detected according to the manufacturer’s protocols provided with the EMSA kit. The sequence of HY5 promoter subfragment is list in Supplemental Table S1.
Protoplast Assay
Arabidopsis mesophyll cell protoplasts were prepared and transfected as described previously (Yoo et al., 2007). The promoter-reporter used was the 450-bp HY5 promoter driving firefly luciferase (pPCV814-ProHY5-LUC). The pRTL2-35S:BBX21, pRTL2-35:HY5, pEarley Gateway 104-35S:YFP-BBX21, pEarley Gateway 104-35S:YFP-BBX21D20A, pEarley Gateway 104-35S:YFP-BBX21D75A, pEarley Gateway 104-35S:YFP-BBX21D84A, pEarley Gateway 104-35S:YFP-BBX21D20,75,84A, pEarley Gateway 104-35S:YFP-BBX21-B1, pEarley Gateway 104-35S:YFP-BBX21-B1D20A, pEarley Gateway 104-35S:YFP-BBX21-B2, and pEarley Gateway 104-35S:YFP-BBX21-B2D75A were used as the effectors. The dual luciferase kit (Promega) was used for detection of reporter activity. Renilla luciferase driven by a full-length cauliflower mosaic virus 35S promoter was used as an internal control.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL data libraries under the following accession numbers: BBX21 (At1g75540) and HY5 (At5g11260).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Various mutated BBX21 gene and protein expression levels in various transgenic lines.
Supplemental Figure S2. Phenotypes of various mutated versions of BBX21 transgenic seedlings grown in darkness.
Supplemental Figure S3. YFP-BBX21D20,57,85A bbx21-1 transgenic seedlings are hyposensitive to light.
Supplemental Figure S4. BBX21D75A bbx21-1 transgenic seedlings accumulate less anthocyanin.
Supplemental Figure S5. Immunoblot assays showing the similar expression levels of BBX21 and substituted and truncated BBX21 proteins in Arabidopsis protoplasts.
Supplemental Table S1. List of primers used in this study.
Acknowledgments
We thank all our lab members at SUSTech for their discussion on the project.
References
- Abbas N, Maurya JP, Senapati D, Gangappa SN, Chattopadhyay S (2014) Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 26: 1036–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkert M, Kozma-Bognár L, Terecskei K, De Veylder L, Nagy F, Ulm R (2014) UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26: 4200–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi GH, Deng XW, Holm M (2006) Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18: 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi C, Johansson H, Holm M (2007) SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 19: 3242–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Johansson H, Hettiarachchi C, Irigoyen ML, Desai M, Rubio V, Holm M (2008) LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 20: 2324–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Fan XY, Sun Y, Cao DM, Bai MY, Luo XM, Yang HJ, Wei CQ, Zhu SW, Sun Y, Chong K, Wang ZY (2012) BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol Plant 5: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2014) The BBX family of plant transcription factors. Trends Plant Sci 19: 460–470 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Crocco CD, Johansson H, Datta S, Hettiarachchi C, Holm M, Botto JF (2013) The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 25: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, Shen Y, Maloof JN, Maszle DR, Ohto MA, Preuss S, et al. (2011) BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 156: 2109–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Deng XW (2014) Beyond repression of photomorphogenesis: role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol 21: 96–103 [DOI] [PubMed] [Google Scholar]
- Indorf M, Cordero J, Neuhaus G, Rodríguez-Franco M (2007) Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J 51: 563–574 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH (2009) The Arabidopsis B-box zinc finger family. Plant Cell 21: 3416–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Schwabe JWR (1995) Protein motifs 5. Zinc fingers. FASEB J 9: 597–604 [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis TW, von Arnim AG, Araki T, Komeda Y, Miséra S, Deng XW (1994) Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Gibson A, Fu X, Zheng M, Kuehn R, Wang Y, Wang Y, Navarro S, Morrell JA, Jiang D, et al. (2012) Involvement of the N-terminal B-box domain of Arabidopsis BBX32 protein in interaction with soybean BBX62 protein. J Biol Chem 287: 31482–31493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F, Costa MM, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, Putterill J, Coupland G (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619–631 [DOI] [PubMed] [Google Scholar]
- Shin J, Park E, Choi G (2007) PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Deng XW (2003) From seed to seed: the role of photoreceptors in Arabidopsis development. Dev Biol 260: 289–297 [DOI] [PubMed] [Google Scholar]
- Tao H, Simmons BN, Singireddy S, Jakkidi M, Short KM, Cox TC, Massiah MA (2008) Structure of the MID1 tandem B-boxes reveals an interaction reminiscent of intermolecular ring heterodimers. Biochemistry 47: 2450–2457 [DOI] [PubMed] [Google Scholar]
- Tripathi P, Carvallo M, Hamilton EE, Preuss S, Kay SA (2017) Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proc Natl Acad Sci USA 114: 172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S, Towatari M, Saito H, Enver T (2000) Potentiation of GATA-2 activity through interactions with the promyelocytic leukemia protein (PML) and the t(15;17)-generated PML-retinoic acid receptor alpha oncoprotein. Mol Cell Biol 20: 6276–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Guthrie C, Sarmast MK, Dehesh K (2014) BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 26: 3589–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Sarmast MK, Jiang J, Dehesh K (2015) The transcriptional regulator BBX19 promotes hypocotyl growth by facilitating COP1-mediated EARLY FLOWERING3 degradation in Arabidbopsis. Plant Cell 27: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Lin F, Jiang Y, Ling J, Hettiarachchi C, Tellgren-Roth C, Holm M, Wei N, Deng XW (2015) Arabidopsis COP1 SUPPRESSOR 2 represses COP1 E3 ubiquitin ligase activity through their coiled-coil domains association. PLoS Genet 11: e1005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang Y, Li J, Lin F, Holm M, Deng XW (2016) BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc Natl Acad Sci USA 113: 7655–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DB, Gao SQ, Ma YN, Wang XT, Feng L, Li LC, Xu ZS, Chen YF, Chen M, Ma YZ (2017) The G-protein β subunit AGB1 promotes hypocotyl elongation through inhibiting transcription activation function of BBX21 in Arabidopsis. Mol Plant 10: 1206–1223 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang X, Huai J, Shang F, Xu G, Tang W, Jing Y, Lin R (2017) A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiol 174: 2487–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]