Aneuploidy primes evolution of herbicide resistance in weeds.
Abstract
An increase in gene copy number is often associated with changes in the number and structure of chromosomes, as has been widely observed in yeast and eukaryotic tumors, yet little is known about stress-induced chromosomal changes in plants. Previously, we reported that the EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) gene, the molecular target of glyphosate, was amplified at the native locus and on an extra chromosome in glyphosate-resistant Amaranthus tuberculatus. Here, we report that the extra chromosome is a ring chromosome termed extra circular chromosome carrying amplified EPSPS (ECCAE). The ECCAE is heterochromatic, harbors four major EPSPS amplified foci, and is sexually transmitted to 35% of the progeny. Two highly glyphosate resistant (HGR) A. tuberculatus plants with a chromosome constitution of 2n = 32+1 ECCAE displayed soma cell heterogeneity. Some cells had secondary ECCAEs, which displayed size polymorphisms and produced novel chromosomal variants with multiple gene amplification foci. We hypothesize that the ECCAE in the soma cells of HGR A. tuberculatus plants underwent breakage-fusion-bridge cycles to generate the observed soma cell heterogeneity, including de novo EPSPS gene integration into chromosomes. Resistant soma cells with stable EPSPS amplification events as de novo insertions into chromosomes may survive glyphosate selection pressure during the sporophytic phase and are plausibly transmitted to germ cells leading to durable glyphosate resistance in A. tuberculatus. This is the first report of early events in aneuploidy-triggered de novo chromosome integration by an as yet unknown mechanism, which may drive rapid adaptive evolution of herbicide resistance in common waterhemp.
Herbicides are widely used in the management of weeds in agricultural production systems. Among the many classes of herbicides, glyphosate [N-(phosphonomethyl) Gly], popularly known as Roundup, is widely used in agriculture, especially on glyphosate-resistant (GR) transgenic crops. Vast adoption of GR crops across the globe and overreliance on glyphosate as a single means of weed control has spawned many GR weeds (Fernando et al., 2016). Glyphosate inhibits the 5-enopyruvlyshikimate-3-phosphate synthase (EPSPS) enzyme of the shikimate pathway, resulting in depletion of aromatic amino acid synthesis and, eventually, plant death (Amrhein et al., 1980; Steinrücken and Amrhein, 1980; Duke and Powles, 2008). Overexpression of the EPSPS gene as a result of gene amplification can lead to the evolution of GR weeds (Gaines et al., 2010; Salas et al., 2012; Jugulam et al., 2014; Malone et al., 2016; Molin et al., 2017). The EPSPS gene amplification may be local (Jugulam et al., 2014) or is postulated to be dispersed across the genome (Gaines et al., 2010; Molin et al., 2017).
Recently, we reported local amplification in low to moderately resistant plants and, surprisingly, an extra chromosome harboring amplified EPSPS copies in highly glyphosate-resistant (HGR) plants of Amaranthus tuberculatus (Dillon et al., 2017). Plants with the extra chromosome were correlated with increased expression of the EPSPS gene (Dillon et al., 2017). Molecular marker analysis showed that the extra chromosome originated from the autosomes; however, little else is known about the extra chromosome because it was only analyzed in mitotic preparations when chromosomes are highly condensed. Here, we report the cytogenetic structure of this chromosome using highly extended prometaphase chromosomes as well as sexual transmission, and the nature and mechanisms by which this extra chromosome is acquired and may disperse amplified EPSPS gene copies across the genome.
RESULTS
HGR Plants Are Aneuploid, Involving a Ring Chromosome with Amplified EPSPS Gene Copies
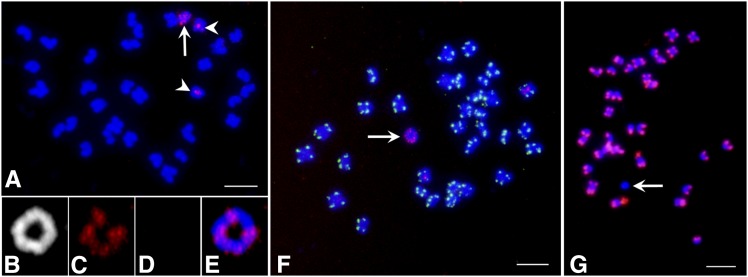
An HGR plant with 14 EPSPS copies had 2n = 33 chromosomes, and the extra chromosome was similar in size to the other chromosomes (Fig. 1A). Fluorescence in situ hybridization (FISH) mapping with the EPSPS gene probe showed one pair of chromosomes with signal in the centromeric region (Fig. 1A, arrowheads) and distinct signals on the extra chromosome (Fig. 1A, arrow). The 4′,6-diamidino-2-phenylindole (DAPI)-stained relatively long prometaphase chromosomes revealed that the extra chromosome is a ring chromosome (Fig. 1B) and showed four distinct clusters of EPSPS foci (Fig. 1, C and E) similar to the signals in the condensed mitotic metaphase stage (Fig. 1A). The linear chromosomes are protected at their ends by telomeric DNA repeats (TTTAGGGn), as shown by strong FISH signals at the telomeric ends using the Arabidopsis telomeric DNA probe (Fig. 1F). The ring chromosomes have no free ends and do not need telomeres. As expected, telomeric DNA signals were not detected in the extra chromosome (Fig. 1D and arrow in Fig. 1F), confirming the circular structure of this extra chromosome. We designated it as an extra circular chromosome carrying amplified EPSPS (ECCAE).
Figure 1.
Cytogenomic characterization of an ECCAE. An HGR A. tuberculatus plant showed chromosome constitution of 2n = 32+1 ECCAE (arrow); note EPSPS-FISH signals on the ECCAE and in the centromeric region of two other chromosomes (arrowheads in A). The ring structure of the ECCAE is clearly visible by DAPI staining at mitotic prometaphase stage (B). FISH signals of four EPSPS gene clusters in the ECCAE (C). The ECCAE lacks telomeric DNA FISH signal (D). Merged image of the ECCAE showing a circular structure and the EPSPS gene cluster (red; E). Two-color FISH using the telomeric DNA repeat probe did not detect any hybridization on the ECCAE (arrow), but strongly hybridized to the telomeres of all other chromosomes of the complement (green signals; F). Immunofluorescence of H4K5Ac on a HGR A. tuberculatus labeled the euchromatic regions of all the chromosomes except the ECCAE (arrow; G). Bars = 5 µm.
Next, we performed immunofluorescence using an antibody specific to histone H4 acetylation at Lys 5 (H4K5Ac), a hallmark of euchromatin. Strong immunofluorescence signals were detected on both ends of all metacentric chromosomes, or one end of all acrocentric chromosomes except the ECCAE (Fig. 1G). These results suggested that ECCAE is composed of heterochromatin and is derived from the pericentromeric region of a specific chromosome with amplified EPSPS copies. Because heterochromatin is relatively genetically inert, aneuploidy for such a heterochromatic chromosome has little effect on plant fitness (see next section). The ECCAE was observed in every metaphase cell. Thus, the ECCAE may have a functional centromere because it was transmitted stably during mitotic divisions.
ECCAE Is Sexually Transmitted to Progeny Plants
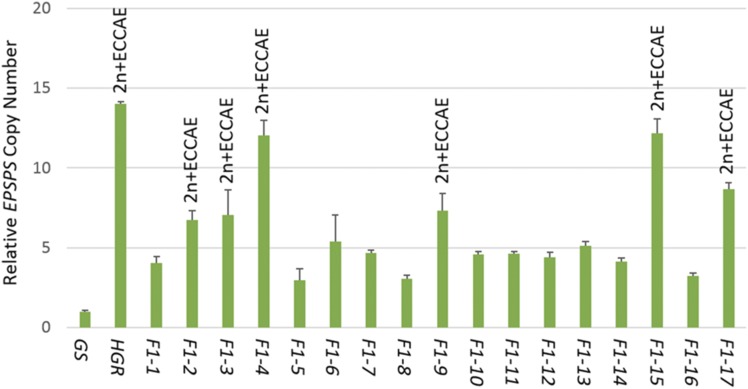
To study the sexual transmission of the ECCAE, we made crosses between an HGR plant with 14 EPSPS copies carrying the ECCAE (Supplemental Fig. S1A) and a glyphosate-susceptible (GS) plant with one EPSPS copy (Supplemental Fig. S1B). A total of 17 F1 plants were analyzed for EPSPS copy number variation and ECCAE transmission. The qPCR-based analysis revealed that 11 plants contained three to five copies of the EPSPS gene, four plants had seven to eight copies, and two plants had 12 copies (Fig. 2). Cytological analysis of these F1 plants showed that the 11 plants with three to five EPSPS copies did not have the ECCAE (Supplemental Fig. S1C) and the six F1 plants with 7 to 12 EPSPS copies had one copy of the ECCAE (Fig. 2; Supplemental Fig. S1, D and E). The transmission rate of ECCAE to the offspring was almost 35%, which is higher than the expected rate of 25% and similar to that of other such types of extra chromosomes of recent origin (Dhar et al., 2002). The lack of an adverse effect of the ECCAE on gamete function and, hence, plant fitness, further reinforces the heterochromatic nature of the ECCAE.
Figure 2.
qPCR analysis of EPSPS copy number in the parents and 17 F1 plants. Aneuploids detected by cytological analysis in the F1 progeny are also shown at the top of vertical bars. The vertical bars represent the relative β-tubulin:EPSPS gene copy number.
The ECCAE Chromosome Is the Source of Soma Cell Heterogeneity for ECCAE Size, Number, and Random EPSPS Chromosome Integration Events in Meristematic Tissues of the Sporophyte
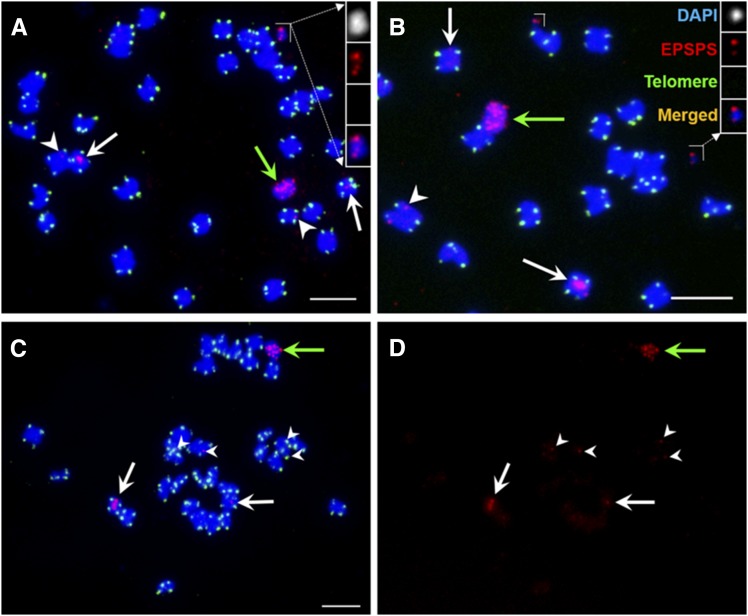
We assayed the chromosome constitution of six F1 plants with 2n+1 ECCAE using root meristematic tissues for ring chromosome structure and EPSPS gene amplification events. Cytological analysis of the four F1 plants with seven to eight EPSPS copies showed a chromosome constitution of 2n = 32+1 ECCAE in all cells of the meristematic tissues (Fig. 1F; Supplemental Fig. S1D). However, two F1 plants with 12 EPSPS copies revealed highly variable sizes and numbers of ECCAEs and EPSPS-FISH patterns among different metaphase cells in a single root tip meristem preparation (Fig. 3; Supplemental Fig. S1E). The chromosome constitution of a sample of 50 cells from one root meristem was analyzed for plant F1-15 (Fig. 3). The results revealed that all the cells contained one pair of EPSPS-carrying chromosomes (heteromorphic EPSPS hybridization signals; white arrows in Fig. 3) and an intact ECCAE derived from the parents (green arrows in Fig. 3). In 28% of the cells, in addition to the standard ECCAE, we observed one or two secondary ECCAEs (sECCAEs) of intermediate (inset in Fig. 3A) or very small size (insets in Fig. 3B). Dual-color FISH with a telomeric DNA probe gave negative results, indicating that the sECCAEs did not contain telomeres (insets in Fig. 3, A and B) and hence were ring chromosomes.
Figure 3.
Somatic cell chromosomes of plant F1-15 labeled with a telomeric DNA probe (green) and an EPSPS probe (red). Note the size variation in the sECCAEs and lack of telomeric signals (insets in A and B). In addition to the expected pericentromeric EPSPS signals on two chromosomes (white arrows), de novo formed EPSPS-FISH foci (arrowheads) were observed on an additional one to four chromosomes in 90% of the cells (A–D). Green arrows point to the ECCAE. Bars = 5 µm.
Most surprisingly, 90% of the cells revealed unambiguous EPSPS-FISH signals on a number of chromosomes; in addition, a wild-type signal was on one pair of chromosomes (arrowheads in Fig. 3). Most of these additional FISH signals were not located in the centromeric regions and thus were different from the wild-type EPSPS gene location in the centromeric region of one pair of chromosomes (arrowheads in Fig. 1, A and C). The number of these additional EPSPS-carrying chromosomes ranged from 1 to 4 in different cells (Fig. 3). These additional chromosomes, carrying EPSPS gene copies as well as the sECCAEs, were only detected in F1 plants that contained an ECCAE, suggesting that the ECCAE was the source of EPSPS genes for the observed chromosomal integration events.
DISCUSSION
Aneuploidy in Response to Glyphosate Selection Pressure
Aneuploidy, the gain of whole chromosomes, or segmental aneuploidy are the most prevalent events for genome adaptation to stressful conditions in yeast and mammalian cells, including cancer cells (Pavelka et al., 2010; Gordon et al., 2012; Yona et al., 2012). These extra chromosome copies usually contain genes needed to withstand a given stressful condition. Yona et al. (2012) reported that duplication of yeast chromosome III (trisomy) is acquired as a solution to heat stress. Two extra copies of chromosome 5L (iso5L) of Candida albicans were found to occur in response to antifungal drug selection (Selmecki et al., 2008). Cytogenetic abnormalities, including aneuploidy, have been found in cancer cells. In cancer cells, aneuploidy with the specific chromosomal duplication usually contains the chromosomal regions that harbor growth-promoting genes (Gordon et al., 2012). Thus, aneuploidy is likely a form of stress-inducible mutation in eukaryotes (Chen et al., 2012), but genome adaptation through aneuploidy may also occur spontaneously. To our knowledge, this and our earlier results (Dillon et al., 2017) are the first reports of aneuploidy-mediated gene amplification and adaptive response to herbicide glyphosate selection pressure in higher plants. But how are such aneuploids generated in the first place?
A primary aneuploid, trisomic for a chromosome carrying an amplified gene, may be acquired spontaneously through nondisjunction or chromosome missegregation as was shown in duplication of yeast chromosome III, which may have selective advantage under heat stress (Yona et al., 2012; see proposed model below). In cancer cells, defects in genes involving the spindle assembly checkpoint and chromatid cohesion are known to cause aneuploidy (Gordon et al., 2012). Chromosome loss after polyploidization also is reported to lead to high levels of aneuploidy in Drosophila melanogaster rectal cells (Schoenfelder et al., 2014).
Dhar et al. (2002) reported that the aneuploid triplo 2, trisomic for chromosome 2 of Plantago lagopus, resulted in rapid chromosomal changes in the extra copy of chromosome 2 leading to the formation of various types of ring or isochromosomes. Similar chromosomal structural changes were reported for extra copy of triplo 1L in barley (Hordeum vulgare; Singh, 2003). Alternatively, univalents at meiosis usually undergo anaphase lagging or centric misdivision giving rise to broken chromosomes at centromeric regions (Darlington, 1939). Aneuploid midget chromosomes were recovered from breakages in pericentromeric regions (Lukaszewski, 1997).
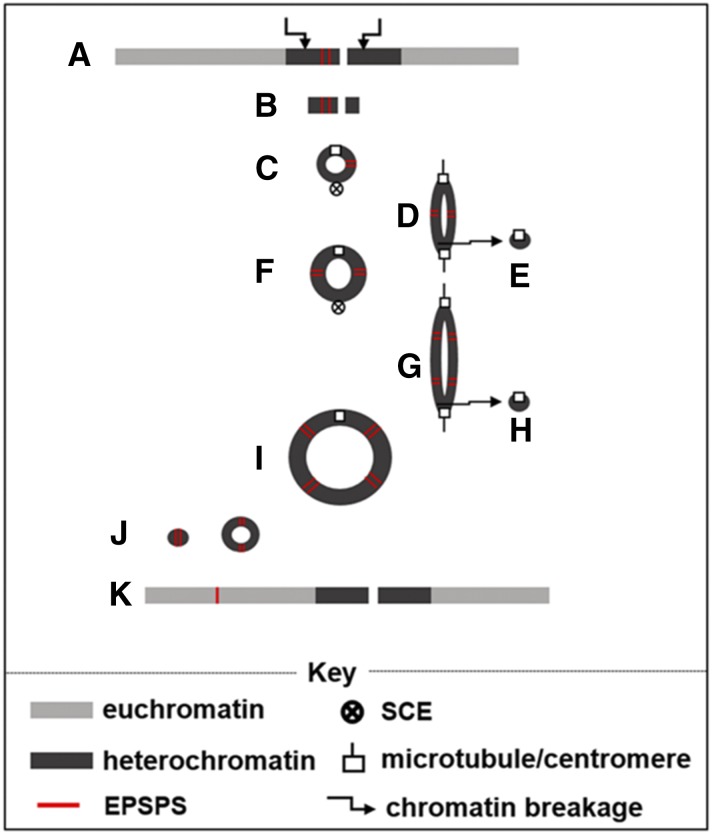
We propose the following steps for the origin of ECCAE. Chromosomal duplication (trisomy) involving chromosome-harboring amplified EPSPS may have occurred spontaneously (Fig. 4A). Aneuploidy tends to destabilize chromosomes (Darlington, 1939; Lukaszewski, 1997; Dhar et al., 2002; Singh, 2003; Passerini et al., 2016), and this may have triggered breakage of the chromosome around the centromere, including the amplified EPSPS locus (Fig. 4B). Broken chromosomes lacking telomeres are unstable but can be stabilized by the fusion of broken ends to form a ring chromosome (McClintock, 1938; Fig. 4C). We propose that ECCAE may have originated by this mechanism because no telomeric sequences were observed. Alternatively, the segmental duplications in the pericentromeric region may undergo ectopic recombination producing a ring chromosome (Appels et al., 1998), another plausible mechanism for the origin of a ring chromosome. We propose that such primordial ECCAEs with amplified EPSPS foci (Fig. 4C) had a selective advantage under glyphosate stress. Further selection pressure drove the evolution ECCAE; it acquired additional EPSPS foci and also grew in size as small chromosomes tend to be unstable and are lost during mitotic divisions (see next section).
Figure 4.
A proposed model for elucidating the BFB-mediated EPSPS gene amplification in GR A. tuberculatus. An extra chromosome carrying an amplified EPSPS may occur spontaneously and may be selected in response to glyphosate stress (A). The ring chromosome was formed by chromatin breakages in the proximal region of chromosome containing amplified EPSPS copies (B), followed by fusion of the proximal ends into a ring chromosome (C). Following chromosome replication, subsequent sister chromatid exchange (C) produce a double-sized dicentric ring chromosome (D). The dicentric ring chromosomes, following chromatid breakages at anaphase, produced different sized ring chromosomes, with (F) or without EPSPS genes (E). Additional rounds of BFB cycle (F–H) will produce ring chromosomes, such as the ECCAE observed (I). The ECCAE also produced sECCAEs with varying numbers of EPSPS gene copies (J; also shown in Fig. 3, A and B). The sECCAEs may undergo ectopic recombination with nonhomologous chromosomes and produce additional EPSPS foci in the genome (K).
A Model for the Evolution of ECCAE by Chromosome Breakage-Fusion-Bridge Cycles
Ring chromosomes can undergo breakage-fusion-bridge (BFB) cycles and produce different sized ring chromosomes and other chromosomal changes in somatic tissues during the sporophytic phase (McClintock, 1938; Gisselsson, 2001). We failed to detect BFB events due to lack of reliable anaphase cell spreads in our root tip preparation. Nonetheless, the ECCAE observed in this study very likely evolved through BFB cycles involving the primordial ECCAE (Fig. 4, C–I). Sister chromatid exchanges (Fig. 4C) in ring chromosomes frequently produce dicentric chromosomes (Fig. 4D), doubling the size of the original ring chromosome. The dicentric ring chromosomes suffer chromatid breaks at anaphase (Fig. 4D) and produce different sized ring chromosomes (Fig. 4, E and F). Additional rounds of the BFB cycle (Fig. 4, F–H) will produce ring chromosomes such as the ECCAE as observed in this study (Fig. 4I). Thus, ECCAE behavior provides an intrinsic mechanism of gene amplification and somatic cell heterogeneity for ECCAE polymorphisms. The glyphosate selection pressure led to the selection of observed ECCAE in HGR A. tuberculatus. However, as discussed above, ECCAEs are intrinsically unstable and also are transmitted only to a portion of the progeny. The GR A. tuberculatus plants in the absence of selection pressure will revert to sensitivity. How can ECCAE trigger more durable evolution of herbicide resistance?
ECCAE as a Trigger for de Novo Chromosome Integration Events and Potentially More Stable Herbicide Resistance
The dynamics of variation in EPSPS copy number and spread to additional chromosomes were revealed by pedigree analysis of two F1 plants with 12 EPSPS copies. Along with one large and one small EPSPS hybridization site in the pericentromeric region in one chromosome pair (arrows in Fig. 3), one to four unambiguous de novo EPSPS-FISH foci were detected in most of the cells (arrowheads in Fig. 3). This suggested that ECCAE during mitotic divisions in soma cells was the source of de novo EPSPS gene amplification. The size of the signal in the de novo chromosome insertions was much smaller than the size of the EPSPS loci in the ECCAE. Thus, we can rule out integration of wild-type ECCAE in different chromosome integration events. Our results suggest at least two mechanisms of de novo chromosome insertion of EPSPS foci. First, tiny sECCAEs with a few EPSPS loci may insert into chromosomes by ectopic recombination. Second, ECCAE and sECCAE may be the source of extra circular DNAs or episomes. For example, Molin et al. (2017) have documented an EPSPS cassette that may be extra circular DNAs. The episomes may undergo ectopic recombination with nonhomologous chromosomes and reintegrate into other chromosomes as postulated in the deletion-plus-episome model (Maurer et al., 1987; Carroll et al., 1988; Wahl, 1989) and produce additional foci in the genome. Taken together, our results demonstrate that the formation of ECCAE may trigger an endless chain of BFB events, thereby constantly generating the novel chromosomal integration events (see Fig. 4) that may eventually lead to durable glyphosate resistance in A. tuberculatus.
CONCLUSION
McClintock (1984) predicted the existence of innate systems in sporophytic genomes that may rapidly respond to challenge. We have shown that chromosome mechanisms, such as ECCAE, exist in the sporophytic genomes that can rapidly generate copy number variation for the target gene and soma cell heterogeneity. This soma cell heterogeneity, upon selection pressure in the presence of herbicide, leads to selection of acquired herbicide resistance during the sporophytic phase and is transmitted to germ cells. The evolution of stable herbicide resistance is a multistep process. The first step is a local tandem gene amplification event of the EPSPS gene, the molecular target of herbicide action (Dillon et al., 2017). We postulate that the EPSPS amplification event and the intrinsic property of the chromosome segment where it is located triggered an aneuploidy event. The aneuploidy in response to glyphosate selection pressure on the sporophyte triggered somatic cell heterogeneity, including de novo EPSPS gene integration events. We hypothesize that de novo chromosome integration events are the early steps toward the evolution of more durable herbicide resistance.
MATERIALS AND METHODS
Production of F1 Progeny by Crossing GS and GR Amaranthus tuberculatus and Estimation of EPSPS Copies in F1 Progeny
A male plant of GS (2n = 32, with no ECCAE) and female HGR (2n = 33, with one copy of ECCAE) of A. tuberculatus were grown individually in Miracle-Gro potting mix in 10-cm3 plastic pots and watered from the top in a greenhouse (25/20°C temperature; 15/9-h light day/night, supplemented with 120 mmol m−2 s−1 illumination using sodium vapor lamps). After flower initiation, the inflorescences of GS (male) and HGR (female) plants were covered together with a plastic bread bag (33 × 60 cm) containing microperforations. Upon plant maturity, seed were harvested from the HGR A. tuberculatus plant. Seed of this cross were planted and about 25 progeny were generated. The root tips from 17 F1 plants were harvested for FISH analysis. Additionally, leaf tissues from these 17 plants were harvested for DNA extraction and EPSPS copy determination.
The qPCR reaction was performed using a StepOnePlus real-time detection system (Applied Biosystems) to determine the EPSPS gene copy number in the 17 F1 A. tuberculatus plants. The qPCR reaction mix consisted of 8 µL of SYBR Green mastermix (Thermo Fisher Scientific), 2 µL each of forward and reverse primers (5 µm), and 2 µL of genomic DNA (20 ng/µL) to make the total reaction volume up to 14 µL. EPSPS gene copy number was measured relative to the β-tubulin gene (reference gene). PCR conditions were 95°C for 15 min and 40 cycles of 95°C for 30 s and 60°C for 1 min. A melt curve profile was included following the thermal cycling protocol to determine the specificity of the qPCR reaction. The following primer sequences were used: EPSPS F 5′-ATGTTGGACGCTCTCAGAACTCTTGGT-3′ and EPSPS R 5′-TGAATTTCCTCCAGCAACGGCAA-3′; β-tubulin F 5′-ATGTGGGATGCCAAGAACATGATGTG-3′;and β-tubulin 5′-TCCACTCCACAAAGTAGGAAGAGTTCT-3′. The EPSPS gene copy number was measured with three technical replicates. Gene copy number was determined using the 2ΔCT method, where CT is the threshold cycle and ΔCT is CTTarget gene (EPSPS) – CTReference gene (β-tubulin).
FISH and Immunofluorescence
Molecular cytogenetic mapping of EPSPS gene and telomeric DNA followed published protocols (Koo and Jiang, 2009; Dillon et al., 2017). To prepare the EPSPS FISH probe, the sequence of A. tuberculatus EPSPS mRNA (GenBank accession no. FJ869881) was used to develop the PCR primers (forward, 5′-GCCAAGAAACAAAGCGAAAT-3′; reverse, 5′-TTTCAGCATCATAATTCATAACCC-3′; product length 1,804 bp). The PCR product was cloned in 2.1-TOPO TA vector (Invitrogen), and the clone was labeled with digoxigenin-11-deoxyuridine triphosphate (Roche Diagnostics) using a standard nick translation reaction. The clone, Arabidopsis (Arabidopsis thaliana) telomeric DNA (TTTAGGGn; Koo et al., 2016) was labeled with biotin-16-dUTP (Roche). Digoxigenin- and biotin-labeled probes were detected with rhodamine-conjugated antidigoxigenin (Roche) and Alexa Fluor 488 streptavidin (Invitrogen), respectively. The conditions for hybridization of antibody specific to H4K5Ac, detection of fluorescent signal, and image analysis were carried out as described (Jin et al., 2004).
Image Analysis
Chromosomes were counterstained with DAPI in Vectashield antifade solution (Vector Laboratories). The images were captured with a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy) using a cooled CCD camera CoolSNAP HQ2 (Photometrics) and AxioVision 4.8 software. The final contrast of the images was processed using Adobe Photoshop CS5 software.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number FJ869881.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Inheritance of ECCAE in crosses of a highly glyphosate resistant A. tuberculatus, with glyphosate resistant A. tuberculatus.
Supplemental Figure S2. Two-color FISH detection of the EPSPS gene and telomere-specific probes.
Acknowledgments
We thank John Raupp and Dr. Jiming Jiang for critical review of the manuscript and Duane Wilson and A.R. Vennapusa for technical assistance.
Footnotes
Articles can be viewed without a subscription.
References
- Amrhein N, Deus B, Gehrke P, Steinrücken HC (1980) The site of the inhibition of the shikimate pathway by glyphosate II. Interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiol 66: 830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appels R, Morris R, Gill BS, May CE (1998) Chromosome Biology. Kluwer Academic Publishers, Boston/Dordrecht/London [Google Scholar]
- Carroll SM, DeRose ML, Gaudray P, Moore CM, Needham-Vandevanter DR, Von Hoff DD, Wahl GM (1988) Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol 8: 1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bradford WD, Seidel CW, Li R (2012) Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 482: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CD. (1939) Misdivision and the genetics of the centromere. J Genet 37: 343–365 [Google Scholar]
- Dhar MK, Friebe B, Koul AK, Gill BS (2002) Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma 111: 332–340 [DOI] [PubMed] [Google Scholar]
- Dillon A, Varanasi VK, Danilova TV, Koo D-H, Nakka S, Peterson DE, Tranel PJ, Friebe B, Gill BS, Jugulam M (2017) Physical mapping of amplified 5-enolpyruvylshikimate-3-phosphate synthase gene copies in glyphosate-resistant Amaranthus tuberculatus. Plant Physiol 173: 1226–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64: 319–325 [DOI] [PubMed] [Google Scholar]
- Fernando N, Manalil S, Florentine SK, Chauhan BS, Seneweera S (2016) Glyphosate resistance of C3 and C4 weeds under rising atmospheric CO2. Front Plant Sci 7: 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines TA, Zhang W, Wang D, Bukun B, Chisholm ST, Shaner DL, Nissen SJ, Patzoldt WL, Tranel PJ, Culpepper AS, et al. (2010) Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc Natl Acad Sci USA 107: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselsson D. (2001) Ring chromosomes: vicious circles at the end and beginning of life. Atlas Genet Cytogenet Oncol Haematol 6: 59–66 [Google Scholar]
- Gordon DJ, Resio B, Pellman D (2012) Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13: 189–203 [DOI] [PubMed] [Google Scholar]
- Jin W, Melo JR, Nagaki K, Talbert PB, Henikoff S, Dawe RK, Jiang J (2004) Maize centromeres: organization and functional adaptation in the genetic background of oat. Plant Cell 16: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugulam M, Niehues K, Godar AS, Koo D-H, Danilova T, Friebe B, Sehgal S, Varanasi VK, Wiersma A, Westra P, Stahlman PW, Gill BS (2014) Tandem amplification of a chromosomal segment harboring 5-enolpyruvylshikimate-3-phosphate synthase locus confers glyphosate resistance in Kochia scoparia. Plant Physiol 166: 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo D-H, Jiang J (2009) Super-stretched pachytene chromosomes for fluorescence in situ hybridization mapping and immunodetection of DNA methylation. Plant J 59: 509–516 [DOI] [PubMed] [Google Scholar]
- Koo D-H, Zhao H, Jiang J (2016) Chromatin-associated transcripts of tandemly repetitive DNA sequences revealed by RNA-FISH. Chromosome Res 24: 467–480 [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ. (1997) Construction of midget chromosomes in wheat. Genome 40: 566–569 [DOI] [PubMed] [Google Scholar]
- Malone JM, Morran S, Shirley N, Boutsalis P, Preston C (2016) EPSPS gene amplification in glyphosate-resistant Bromus diandrus. Pest Manag Sci 72: 81–88 [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Lai E, Hamkalo BA, Hood L, Attardi G (1987) Novel submicroscopic extrachromosomal elements containing amplified genes in human cells. Nature 327: 434–437 [DOI] [PubMed] [Google Scholar]
- McClintock B. (1938) The production of homozygous deficient tissues with mutant characteristics by means of the aberrant mitotic behavior of ring-shaped chromosomes. Genetics 23: 315–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. (1984) The significance of responses of the genome to challenge. Science 226: 792–801 [DOI] [PubMed] [Google Scholar]
- Molin WT, Wright AA, Lawton-Rauh A, Saski CA (2017) The unique genomic landscape surrounding the EPSPS gene in glyphosate resistant Amaranthus palmeri: a repetitive path to resistance. BMC Genomics 18: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini V, Ozeri-Galai E, de Pagter MS, Donnelly N, Schmalbrock S, Kloosterman WP, Kerem B, Storchová Z (2016) The presence of extra chromosomes leads to genomic instability. Nat Commun 7: 10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka N, Rancati G, Li R (2010) Dr Jekyll and Mr Hyde: role of aneuploidy in cellular adaptation and cancer. Curr Opin Cell Biol 22: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas RA, Dayan FE, Pan Z, Watson SB, Dickson JW, Scott RC, Burgos NR (2012) EPSPS gene amplification in glyphosate-resistant Italian ryegrass (Lolium perenne ssp. multiflorum) from Arkansas. Pest Manag Sci 68: 1223–1230 [DOI] [PubMed] [Google Scholar]
- Schoenfelder KP, Montague RA, Paramore SV, Lennox AL, Mahowald AP, Fox DT (2014) Indispensable pre-mitotic endocycles promote aneuploidy in the Drosophila rectum. Development 141: 3551–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J (2008) An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol 68: 624–641 [DOI] [PubMed] [Google Scholar]
- Singh RJ. (2003) Plant Cytogenetics, Ed 2 CRC Press, Boca Raton, FL [Google Scholar]
- Steinrücken HC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun 94: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Wahl GM. (1989) The importance of circular DNA in mammalian gene amplification. Cancer Res 49: 1333–1340 [PubMed] [Google Scholar]
- Yona AH, Manor YS, Herbst RH, Romano GH, Mitchell A, Kupiec M, Pilpel Y, Dahan O (2012) Chromosomal duplication is a transient evolutionary solution to stress. Proc Natl Acad Sci USA 109: 21010–21015 [DOI] [PMC free article] [PubMed] [Google Scholar]