Abstract
Early diagnosis of influenza infection maximizes the effectiveness of antiviral medicines. Here, we assess the ability for clinical characteristics and rapid influenza tests to predict PCR-confirmed influenza infection in a sentinel, cross-sectional study for influenza-like illness (ILI) in Thailand. Participants meeting criteria for acute ILI (fever > 38°C and cough or sore throat) were recruited from inpatient and outpatient departments in Bangkok, Thailand, from 2009–2014. The primary endpoint for the study was the occurrence of virologically-confirmed influenza infection (based upon detection of viral RNA by RT-PCR) among individuals presenting for care with ILI. Nasal and throat swabs were tested by rapid influenza test (QuickVue) and by RT-PCR. Vaccine effectiveness (VE) was calculated using the case test-negative method. Classification and Regression Tree (CART) analysis was used to predict influenza RT-PCR positivity based upon symptoms reported. We enrolled 4572 individuals with ILI; 32.7% had detectable influenza RNA by RT-PCR. Influenza cases were attributable to influenza B (38.6%), A(H1N1)pdm09 (35.1%), and A(H3N2) (26.3%) viruses. VE was highest against influenza A(H1N1)pdm09 virus and among adults. The most important symptoms for predicting influenza PCR-positivity among patients with ILI were cough, runny nose, chills, and body aches. The accuracy of the CART predictive model was 72.8%, with an NPV of 78.1% and a PPV of 59.7%. During epidemic periods, PPV improved to 68.5%. The PPV of the QuickVue assay relative to RT-PCR was 93.0% overall, with peak performance during epidemic periods and in the absence of oseltamivir treatment. Clinical criteria demonstrated poor predictive capability outside of epidemic periods while rapid tests were reasonably accurate and may provide an acceptable alternative to RT-PCR testing in resource-limited areas.
Introduction
Infection with influenza viruses poses a significant public health threat globally, with a disproportionate impact in the developing world [1]. Southeast Asia is a particularly important region of interest for influenza epidemiology and ecology, with a high burden of disease and complex transmission patterns. Many areas experience year-round transmission, promoting the emergence and seeding of viruses into other regions.
As influenza vaccine programs build throughout Asia, important questions remain regarding regional epidemiology and vaccine effectiveness to inform local and global vaccination programs. In Thailand, influenza vaccination is recommended for health care workers, pregnant women, individuals with underlying comorbidities, young children, and the elderly. While vaccine coverage has been increasing, awareness of influenza vaccine recommendations and acceptance of vaccine administration remain a challenge in some target populations [2]. Both live and inactivated vaccines, and northern and southern hemisphere formulations, are available in Thailand, however, the inactivated formulation of the southern hemisphere vaccine predominates (Sriluck Simasathien, personal communication).
Neuraminidase inhibitors have been shown to decrease the duration of influenza-associated illness [3] and the occurrence of influenza-related complications [4]. However, oseltamivir remains profoundly underutilized, even in high-risk populations [5]. In the developing world, the low use of oseltamivir may be related in part to the unavailability or delayed receipt of confirmation for influenza infection. There is a need for more accessible diagnostic tools for influenza infection to facilitate the prompt administration of oseltamivir and to decrease unnecessary additional testing [6]. Rapid influenza diagnostic tests for influenza have been readily deployed across a range of clinical settings, however, the sensitivity of these tests has been shown to vary by influenza subtype [7], lineage [8], timing of specimen collection relative to symptom onset[9], and influenza viral load [10, 11]. An improved understanding of the performance characteristics and limitations of rapid tests is essential for appropriate clinical application and interpretation.
Multiple studies have sought to predict influenza from among influenza-like illness (ILI) cases based upon clinical data such as symptoms and exposure histories. Some clinical algorithms have demonstrated promising results and relatively high accuracy [12, 13], however, most have reported problematically low positive predictive values [14–17]. These clinical algorithms are likely affected by overlap in the clinical presentation of influenza and other respiratory pathogens but may offer some promise in identifying likely influenza cases in resource-limited settings.
In this manuscript, we explore epidemiologic, demographic, and clinical factors associated with influenza infection, influenza vaccination, and vaccine effectiveness in a cohort of individuals presenting with ILI in Thailand over a five-year period. We present a predictive model to discern influenza from non-influenza ILI based upon clinical symptoms and explore factors related to performance characteristics of the QuickVue rapid influenza diagnostic test. We seek to improve the predictive capability for influenza infection in regions with limited laboratory capabilities and to therefore improve clinical management and clinical outcomes.
Materials and methods
Location
The study was conducted at Phramongkutklao (PMK) hospital in Bangkok, Thailand. PMK is a 1200 bed hospital serving active and retired Royal Thai Army military personnel, their families, and other civilians. The majority of the patient population seen is civilian (60–70%). The hospital has inpatient and outpatient departments and sees both children and adults.
Study population and study methods
Sentinel surveillance for ILI was established as part of the Armed Forces Research Institute of Medical Sciences (AFRIMS) influenza surveillance program at PMK hospital, initiated in 2009. The surveillance period for this manuscript was August 2009 through August 2014. The study is cross-sectional in design, with no specimen or data collection occurring after the initial visit. Participants were enrolled from inpatient and outpatient departments with the following inclusion criteria: fever > 38°C accompanied by cough or sore throat, age > = 6 months, presentation to PMK within 3 days of fever onset for outpatients and within 5 days of fever for inpatients, and provision of consent or assent for participation. Exclusion criteria were the presence of any immunocompromising condition or suspected tuberculosis.
Enrolled subjects provided demographic information and exposure information (recent travel, sick contacts, smoking history) as well as clinical information (presence / absence of specific symptoms, receipt of any medications for the illness prior to enrollment, presence of select comorbidities). Additionally, subjects were asked to report whether and when they had received an influenza vaccine within the last 12 months. A nasal swab was obtained for rapid influenza testing (QuickVue), which was performed onsite. An additional set of nasal and throat swabs were obtained, although two throat swabs were occasionally obtained from children per the parents’ or child’s wishes. Specimens were first tested for influenza A or B by reverse transcriptase polymerase chain reaction (RT-PCR); if positive for influenza A, they were further tested with primers specific for H1, H3 and H1 pandemic 2009 (pdm09). Primers and probes were designed by the US Centers for Disease Control and Prevention (CDC) [18, 19].
The study was approved by the Institutional Review Boards of the Royal Thai Army in Bangkok, Thailand, PMK hospital, and the Walter Reed Army Institute of Research (WRAIR). Written informed consent was obtained from all subjects.
Statistical analysis
Bivariate analyses were conducted using χ2 testing for categorical and ANOVA for continuous variables. Age was divided into groupings of 0–4 years, 5–14 years, 15–59 years, and greater than 60 years. Comorbidities studied were those recommended by the US CDC to receive influenza vaccination [20]. Epidemic periods were defined as months where confirmed influenza cases (by RT-PCR) represented > = 25% of all ILI cases. Vaccine effectiveness (VE) was calculated using the ‘test negative’ method for estimating vaccine effectiveness in ILI investigations, where VE = (1- the odds ratio for disease, comparing vaccinated and unvaccinated populations) * 100%[21]. VE for individual influenza subtypes was calculated excluding influenza positive cases due to other serotypes from the comparison group. Data on influenza lineages circulating in Thailand during the study period were derived from the Thai National Influenza Center and based upon hemaggulatination inhibition and sequencing results [22].
Classification and regression tree (CART) analysis was used to develop a predictive model of influenza PCR-positivity among ILI cases based upon symptoms reported. All symptom variables (S1 Table) and age were incorporated as binary variables. The initial tree was obtained by recursive partitioning using the rpart package in R, which identifies the initial split (the ‘root node’) that maximizes the separation of the variable of interest (in this case, RT-PCR positive or negative), then the next best split is identified (for ‘secondary nodes’), then the next, and so on. The tree was subsequently pruned using the ptree package, applying the complexity parameter that was found to minimize cross-validated error.
An initial training set was used to build the model, using data from the years 2009–2013. The model was subsequently applied to a test set of data from 2014. Measures of model performance were calculated by comparing the predicted outcome of RT-PCR (positive or negative for influenza) against the observed result. Performance measures for the QuickVue test were calculated using the RT-PCR result for influenza as the gold standard for comparison. All analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY) and R version 3.3.0 (R Core Team, Vienna, Austria).
Results
A total of 4572 individuals were enrolled between August 2009 and August 2014, of which 1493 (32.7%) had detectable influenza RNA by RT-PCR (hereafter described as influenza “cases”). The majority of influenza cases were attributable to influenza B (38.6%), then influenza A(H1N1)pdm09 (35.1%), followed by influenza A(H3N2) (26.4%). All influenza A infections were attributable to influenza A(H1N1)pdm09 or influenza A(H3N2) in the cohort. There was one case of dual infection with influenza B and influenza A(H1N1)pdm09 detected by RT-PCR.
Comparison of influenza-positive and influenza-negative cases (Table 1)
Table 1. Predictors of influenza positivity among patients presenting with influenza-like illness, predictors of reported vaccination within the last 12 months, and vaccine efficacy.
| Total | Flu + (%) | Pa | % Vaccinated | P | VE (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Overall | 4572 | (1493) 32.7% |
— | 21.0% | — | 49.5% (40.0–57.1%) | |
| Subtypeb | A(H3N2) | 392 | 26.4% | — | 17.0% | <0.01 | 36.2% (15.9–51.5%) |
| A(H1N1)pdm09 | 524 | 35.1% | — | 9.7% | 66.6% (54.9–75.3%) | ||
| Flu B | 257 | 38.6% | — | 16.0% | 41.1% (25.3–53.6%) | ||
| Age (years) | 0–4 | 2126 | 15.5% | <0.01 | 27.0% | <0.01 | 24.5% (0.01–43.2%) |
| 5–14 | 1433 | 46.5% | 19.1% | 40.1% (21.8–54.3%) | |||
| 15–59 | 950 | 49.0% | 9.8% | 56.7% (32.1–72.9%) | |||
| 60 + | 63 | 48.6% | 19.0% | 51.9% (-73.0–88.4%) | |||
| Gender | Female | 2489 | 31.5% | 0.169 | 22.5% | 0.028 | 50.1% (36.5–61.1%) |
| Male | 2083 | 33.5% | 19.8% | 48.1% (34.9–58.9%) | |||
| Comobiditiesc | |||||||
| Renal failure | 35 | 34.3% | 0.980 | 17.1% | 0.723 | ||
| Heart disease | 51 | 23.5% | 0.212 | 35.3% | 0.019 | ||
| COPD | 13 | 0.0% | 0.026 | 69.2% | <0.01 | ||
| Asthma | 157 | 29.3% | 0.401 | 42.7% | <0.01 | 37.2% (-26.7–69.7%) | |
| Obesity | 2 | 50.0% | 1.000 | 50.0% | 0.889 | ||
| Pregnancy | 0 | — | — | — | —- | ||
| Diabetes | 9 | 22.2% | 0.755 | 11.1% | 0.750 | ||
| Immunocompromised | 8 | 37.5% | 1.000 | 50.0% | 0.114 | ||
| Hematologic | 176 | 27.8% | 0.19 | 25.6% | 0.154 | 52.5% (-6.5%-80.9%) | |
| Liver disease | 2 | 0.00% | 1.000 | 0.00% | 1.000 | ||
| # Comorbid | None | 4121 | 33.2% | 19.6% | 49.3% (39.4–57.8%) | ||
| Conditions | 1 | 449 | 27.4% | 0.038 | 33.6% | <0.01 | 42.2% (0.09–64.1%) |
| 2 | 2 | 50.0% | 0.00% | — | |||
| Ward | Inpatient | 287 | 19.2% | <0.01 | 22.6% | 0.526 | 38.5% (-28.2–73.2%) |
| Outpatient | 4285 | 33.6% | 20.9% | 49.6% (40.2–57.6%) | |||
| Occupation | Healthcare | 183 | 38.2% | <0.01 | 22.4% | <0.01 | 79.1% (50.5–92.5%) |
| Homemaker | 52 | 63.5% | 11.5% | -17.2% (-810–79.4%) | |||
| Military | 194 | 53.6% | 9.3% | 85.1% (53.0–96.6%) | |||
| Office | 263 | 49.4% | 6.8% | 19.3% (-111–70.1%) | |||
| University | 248 | 49.2% | 5.6% | 37.2% (20.9–50.4%) | |||
| Year | 2009 | 237 | 19.4% | 16.0% | 41.6% (-47.1–80.9%) | ||
| 2010 | 1352 | 42.8% | <0.01 | 12.6% | <0.01 | 46.9% (25.3–62.8%) | |
| 2011 | 798 | 27.3% | 26.1% | 49.1% (25.3–66.0%) | |||
| 2012 | 802 | 35.5% | 20.1% | 37.6% (9.2%–57.7%) | |||
| 2013 | 593 | 18.4% | 21.9% | 17.9% (-36.1–52.3%) | |||
| 2014 | 790 | 32.4% | 32.0% | 59.1% (42.2–71.5%) | |||
a P-values were calculated using Mantel-Haenszel χ2 statistics, comparing numbers of individuals with and without influenza, or with and without a history of vaccination, across strata for each variable of interest. For comorbidities, χ2 statistics were based upon the presence or absence of each condition (e.g., numbers of individuals with influenza, among those with and without renal disease). Exact testing was performed for comparisons with cells with values < = 5. Comparisons that were statistically significantly with α = 0.05 are shown in bold.
b For influenza subtypes, data are displayed as column percents (i.e., the percent of influenza isolates that were influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B viruses, respectively). For the remaining categories, row percents are displayed (i.e., the percent of specimens for each stratum that was influenza).
c Comorbidities listed are those recommended by the US CDC to receive influenza vaccine. VE was not calculable for all strata due to low numbers.
Influenza cases were significantly older than non-influenza ILI cases, with mean ages of 14.8 and 8.7 years, respectively (p<0.01 by t-test, data not shown). 46.5% of ILI cases occurred in children less than 4 years of age, however, only 15.5% of ILI cases in young children were influenza positive by RT-PCR versus 49.0% of individuals aged 15–59 years. Individuals with ILI and no comorbidities were more likely to test positive for influenza infection by RT-PCR (33.2%) than those with one or more comorbidity (27.4%). Outpatients with ILI were more likely to test positive for influenza infection than inpatients (33.6% versus 19.2%). Healthcare workers presenting with ILI were significantly less likely to be influenza-positive (38.2%), compared with homemakers (63.5%) and members of the military (53.6%). The proportion of ILI cases that were influenza positive by RT-PCR varied significantly by year, with maximum positivity in 2010 (42.8%) and minimum positivity in 2013 (18.4%).
Vaccination history and vaccine effectiveness (Table 1)
21% (960 / 3612) of individuals with ILI reported a history of influenza vaccination in the last 12 months, with an associated overall vaccine effectiveness of 49.5% (95% confidence interval [CI] 40.0–57.1%). VE was highest for influenza A(H1N1)pdm09 (66.6%) and lowest for influenza A(H3N2) (36.2%). Young children were most likely to have been vaccinated within the last 12 months (27.0%) but VE was lowest in this age group at 24.5%. Individuals aged 15–59 years were least likely to report vaccination (9.8%) but VE was highest in this age group at 56.7%. 33.6% of those with any underlying comorbidity reported influenza vaccination, compared to 19.6% of those with none. Vaccination history varied by occupation, with healthcare workers the most likely to be vaccinated (22.4%) and office workers, university students, and members of the military the least. Notably, VE was very high in both healthcare workers (79.1%) and members of the military (85.1%). Likelihood of vaccination increased from 16.0% in 2009 to 32.0% in 2014. VE varied significantly by year, from a minimum of 17.9% in 2013 to a maximum of 59.1% in 2014.
Temporal trends in ILI cases
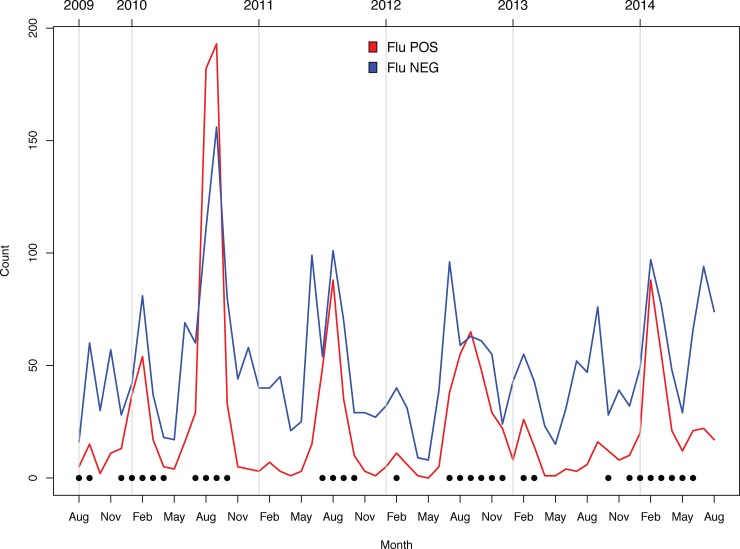
The seasonality of influenza cases roughly followed a biphasic pattern, with the largest peak often occurring between July and September and a smaller peak often occurring between January and March (Fig 1). An exception to this pattern was late 2012 –early 2014, where the pattern varied unpredictably and for reasons which remain unclear. In contrast to the biphasic peaks of influenza activity, the detection of influenza-negative ILI cases followed no discernable pattern and tended to occur year-round.
Fig 1. Temporal distribution of ILI cases testing positive for influenza infection (RED) and negative for influenza infection (BLUE).
Solid circles indicate months identified as experiencing influenza outbreaks (defined as months wherein > = 25% of ILI specimens tested positive for influenza infection by RT-PCR).
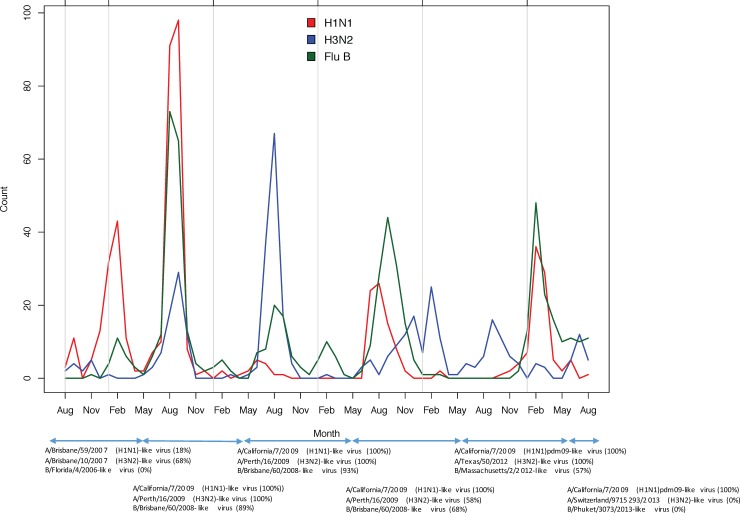
A large increase in influenza cases in 2010 was largely attributable to influenza A(H1N1)pdm09 (Fig 2) and 100% of H1N1 strains detected from 2010–2014 were A(H1N1)pdm09. Notably, there was a mismatch in the influenza B component of the vaccine in 2009 and a large influenza B outbreak in 2010. Subsequently, the influenza B vaccine strain represented 68%, 57%, and 0% of detected influenza B strains in 2012, 2013, and 2014, respectively. The vaccine strain of influenza A(H3N2) was changed several times during the study period and represented between 0 and 100% of detected influenza A(H3N2) strains during the study period 2009–2014. No distinct differences in seasonality were observed between subtypes.
Fig 2. Temporal distribution of subtypes, with southern hemisphere vaccine composition by year.
The percent of influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B isolates matching the vaccine strain for each year is shown in parentheses [23]. Note that the Southern hemisphere vaccine typically became available in May of each year.
Clinical predictors (Table 2)
Table 2. Predictors of clinical severity and receipt of antimicrobial medications among those with PCR-confirmed influenza infection.
| Total | % IPD | pa | % Oseltamivir | P | ||
|---|---|---|---|---|---|---|
| Overall | Inpatient | 55 | 3.7% | — | 25.4% | <0.001 |
| Outpatient | 1438 | — | 1.0% | |||
| Subtype | A(H3N2) | 392 | 4.1% | 3.6% | ||
| A(H1N1)pdm09 | 524 | 3.4% | 0.847 | 1.1% | 0.052 | |
| Flu B | 576 | 3.6% | 1.6% | |||
| Age (years) | 0–4 | 329 | 8.8% | 2.7% | ||
| 5–14 | 667 | 3.1% | <0.001 | 2.5% | 0.019 | |
| 15–60 | 467 | 0.9% | 0.4% | |||
| 60 + | 30 | 3.3% | 3.3% | |||
| Gender | Female | 658 | 4.4% | 0.238 | 1.8% | 0.917 |
| Male | 835 | 3.1% | 2.0% | |||
| # Comorbid | None | 1369 | 3.2% | 1.9% | ||
| conditions | 1 | 123 | 8.9% | 0.005 | 2.4% | 0.755 |
| 2 | 1 | 0.0% | 0.0% | |||
| Occupation | Healthcare | 70 | 0% | 0% | ||
| Homemaker | 33 | 0% | 0.052 | 0% | 0.171 | |
| Military | 104 | 0% | 0% | |||
| Office | 130 | 0% | 0% | |||
| University | 38 | 3.3% | 1.7% | |||
| Year | 2009 | 46 | 4.3% | 2.2% | ||
| 2010 | 579 | 5.5% | 0.010 | 1.2% | 0.092 | |
| 2011 | 218 | 1.8% | 2.3% | |||
| 2012 | 285 | 0.7% | 1.4% | |||
| 2013 | 109 | 3.7% | 5.5% | |||
| 2014 | 256 | 4.3% | 2.3% |
a P-values were calculated using Mantel-Haenszel χ2 statistics, comparing numbers of individuals recruited in inpatient or outpatient departments, or with and without a history of oseltamivir, across strata for each variable of interest. Exact testing was performed for comparisons with cells with values < = 5. Comparisons that were statistically significantly with α = 0.05 are shown in bold.
3.7% of influenza cases were hospitalized. Hospitalization was most common among young children with influenza infection (8.8%) and least common among those aged 15–59 years (0.9%). The rate of hospitalization among influenza cases varied significantly by year, from a minimum of 0.7% in 2012 to a maximum of 4.3% in 2014. 25.4% of inpatients with influenza infection received oseltamivir at some point prior to enrollment versus 1.0% of outpatients. Oseltamivir administration prior to enrollment was most common in the youngest and oldest age groups. Individuals with underlying comorbidities were more likely to be hospitalized (8.9% versus 3.2%) compared to those without comorbidities. 22.4% of influenza cases reported having received antibiotics at some point prior to enrollment; reported rates of antibiotic administration were higher in individuals aged 0–4 years and greater than 60 years and for inpatients.
Among ILI cases, individuals who were influenza-positive were more likely to have had fever at enrollment and more likely to report cough, sore throat, chills, malaise, and generalized body aches (Supplemental table). They were less likely to have difficulty breathing, diarrhea, and lung findings on exam than non-influenza ILI cases. Individuals with influenza A(H3N2) infection were more likely to report fever, runny, nose, or malaise at enrollment and less likely to report cough.
CART analysis of symptoms predicting influenza RT-PCR positive and negativity
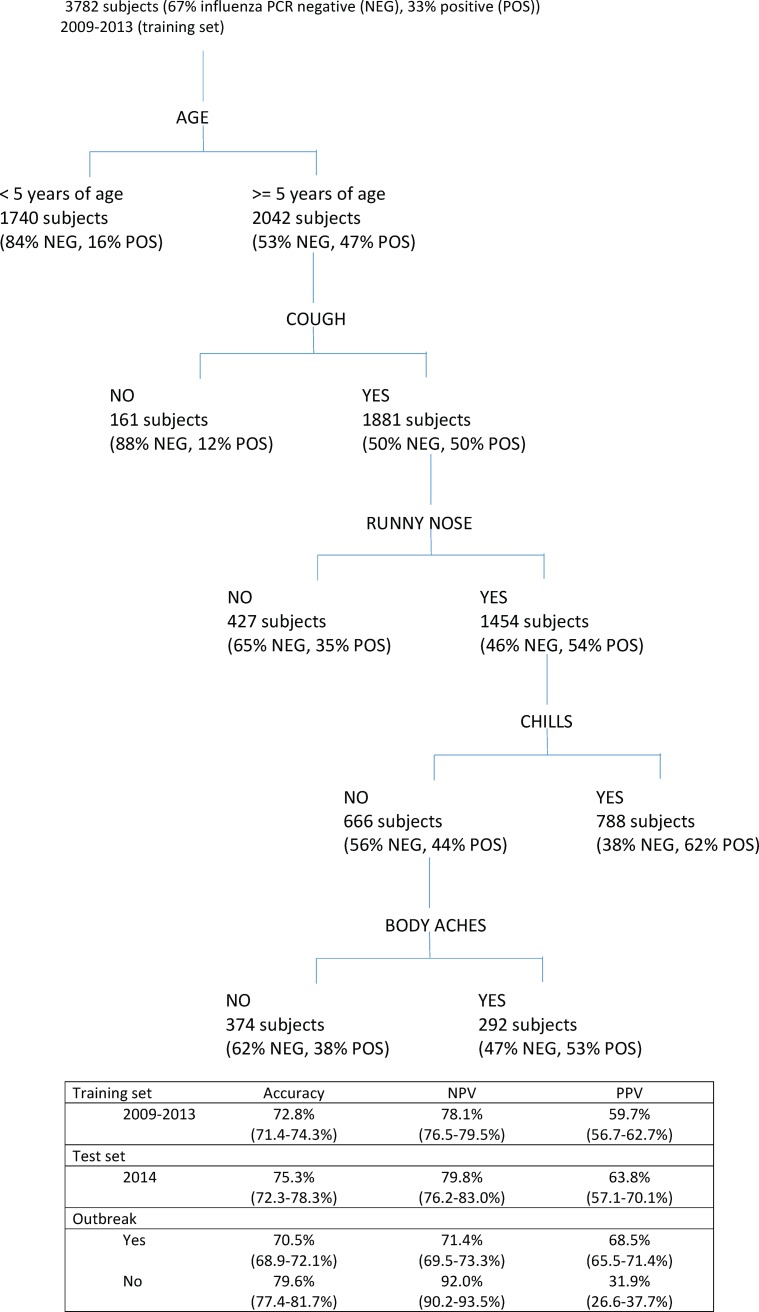
Age was the first cut-point identified by the recursive partitioning algorithm, with 84% of children less than 5 years of age with ILI testing negative by influenza RT-PCR (Fig 3). Cough was the next cut-point assigned to children older than 5 years, with 88% of those lacking cough testing negative by RT-PCR. Among those with cough, 65% of those without runny nose tested negative by RT-PCR. Among those with cough and runny nose, 62% of those with chills tested positive for influenza infection by RT-PCR, while those without chills were further divided by the presence or absence of body aches. 62% of those without chills or body aches tested negative by RT-PCR.
Fig 3. CART analysis to predict influenza RT-PCR positivity on the basis of clinical symptoms.
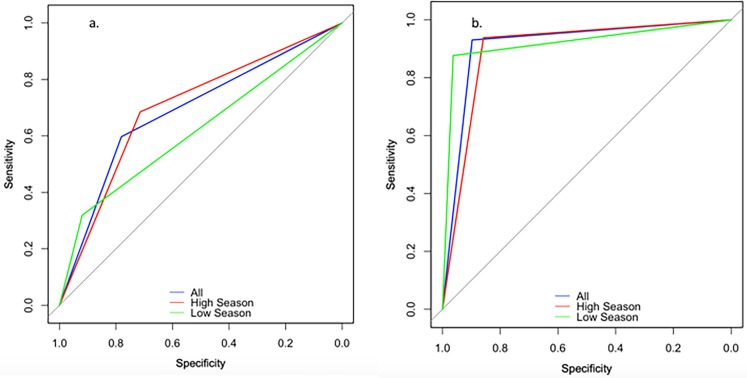
The overall accuracy of the model based upon the training set (data limited to years 2009–2013) was 72.8%. The specificity and negative predictive value (NPV) of the model were superior to the sensitivity and positive predictive value (PPV) for the model, at 82.9% and 78.4% versus 52.1% and 59.7%. When applied to the test set (data from 2014), the accuracy for correctly predicting influenza RT-PCR result based upon symptom data was similar (75.3%). Notably, PPV doubled when the algorithm was applied to epidemic periods (68.5% versus 31.9% for non-epidemic periods). Conversely, NPV was highest during non-epidemic periods (92.0% versus 71.4% for epidemic periods). The receiver operating characteristic (ROC) curves for the CART analysis overall, epidemic, and non-epidemic periods are shown in Fig 4A. Area under the curve (AUC) was 0.689 overall, 0.619 for non-epidemic periods, and 0.700 for epidemic periods.
Fig 4. Receiver operating characteristic (ROC) curves for (Fig 3A) CART analysis and (Fig 3B) QuickVue, compared to RT-PCR for identifying influenza infection among individuals with ILI.
Sensitivity and specificity of QuickVue (Table 3)
Table 3. QuickView test performance, as compared to RT-PCR (“gold standard”).
Lower limit of 95% confidence interval for each proportion is shown in parentheses.
| Sens | Spec | NPV | PPV | ||
|---|---|---|---|---|---|
| Overall | 77.0% (74.8%) | 97.2% (96.6%) | 89.7% (88.6%) | 93.0% (91.5%) | |
| Subtype | |||||
| Flu A | 73.4% (70.8%) | 98.4% (97.9%) | 93.7% (92.9%) | 91.9% (89.6%) | |
| Flu B | 80.6% (77.1%) | 98.7% (98.3%) | 97.2% (96.7%) | 90.3% (87.4%) | |
| Outbreak | |||||
| Yes | 77.4% (75.0%) | 96.4% (95.4%) | 85.8% (84.2%) | 93.8% (92.2%) | |
| No | 74.5% (97.6%) | 98.4% (97.5%) | 96.3% (95.1%) | 87.7% (81.4%) | |
| Disposition | |||||
| Inpatient | 74.5% (61.0%) | 98.7% (96.2%) | 93.2% (90.5%) | 94.2% (81.3%) | |
| Outpatient | 77.1% (74.9%) | 97.1% (96.4%) | 89.4% (88.2%) | 93.0% (91.4%) | |
| Days since onset | |||||
| 0 | 62.9% (49.7%) | 98.8% (95.7%) | 87.7% (82.1%) | 95.1% (83.5%) | |
| 1 | 78.5% (75.2%) | 97.1% (96.0%) | 88.8% (87.0%) | 93.9% (91.6%) | |
| 2 | 79.8% (76.0%) | 97.3% (96.1%) | 91.1% (89.3%) | 93.3% (90.5%) | |
| 3 | 72.0% (65.8%) | 96.5% (94.7%) | 89.3% (86.6%) | 89.6% (84.3%) | |
| 4+ | 64.3% (35.1%) | 100.0% (94.0%) | 92.3% (82.9%) | 100.0% (66.4%) | |
| Age | |||||
| 0–4 | 80.2% (75.5%) | 97.3% (96.5%) | 96.4% (95.5%) | 84.9% (80.4%) | |
| 5–14 | 78.0% (74.6%) | 96.1% (94.4%) | 83.3% (80.7%) | 94.5% (92.3%) | |
| 15–59 | 72.8% (68.5%) | 98.1% (96.4%) | 78.9% (75.3%) | 97.4% (95.2%) | |
| >60 | 86.7% (69.3%) | 100.0% (89.4%) | 89.2% (74.6%) | 100.0% (86.8%) | |
| Oseltamivir | |||||
| Yes | 65.5% (45.7%) | 95.7% (85.5%) | 81.8% (69.1%) | 90.5% (69.6%) | |
| No | 77.2% (75.0%) | 97.2% (96.6%) | 89.8% (88.7%) | 93.1% (91.5%) | |
The overall sensitivity of the QuickVue test was 77.0%, specificity was 97.2%, NPV 89.7%, and PPV 93.0%. The sensitivity of the QuickVue test was higher for influenza B than for influenza A viruses (80.6% versus 73.4%); specificity was high for both at >98%. 16 specimens were positive for both influenza A and influenza B viruses by QuickVue testing; 11 (68.8%) of these subsequently tested negative by RT-PCR (data not shown). The performance of the QuickVue test was not notably different by inpatient versus outpatient status. The sensitivity of QuickVue was 62.9% if performed on the day of illness onset and peaked at 2 days post-onset of symptoms (79.8%). Sensitivity was lowest in those aged 15–59 years of age (72.8%) and highest at the extremes of age. Sensitivity was lower in those with a history of oseltamivir administration (65.5%) compared to those without a history of oseltamivir (77.2%). PPV was highest during epidemic periods (93.7%) compared to non-epidemic periods (87.7%). The ROC curves for QuickVue overall, epidemic, and non-epidemic periods are shown in Fig 4B. Area under the curve (AUC) was 0.914 overall, 0.920 for non-epidemic periods, and 0.898 for epidemic periods.
Discussion
Our clinical prediction algorithm to discern influenza from non-influenza ILI had moderate accuracy (73%) but poor PPV (59.7%). This is consistent with prior studies which indicated that it is more feasible to identify what is not influenza than what is influenza among patients with ILI [14–17], given significant overlap of clinical symptoms between influenza and other respiratory pathogens. PPV improved to 69% during epidemic months, however, overall test performance (by AUC) was not dramatically improved. For this study, our efforts were likely further limited by a lack of longitudinal data regarding the time course of symptoms. Future efforts will include retrospective chart reviews of influenza cases and hospitalized patients to inform the development of more dynamic clinical prediction algorithms and to attempt to identify individuals at high risk of progression to severe disease.
Sensitivity of the QuickVue rapid test was >70% overall for both influenza A and B viruses, superior to prior studies reporting sensitivity in ambulatory populations of approximately 20–55% [24–26]. In our study, the timing of rapid test administration was found to be an important predictor of test performance, with peak sensitivity 1–2 days after the onset of symptoms, likely due to the dynamics of viral shedding in the nasopharynx after infection. As for the CART analysis, PPV improved during epidemic periods with a higher proportion of influenza-positive ILI cases. The lower sensitivity of the QuickVue test for adults, inpatients, and individuals who had previously received oseltamivir should serve as a caution to providers that a negative test result should not be interpreted to mean that influenza infection has been ruled out or to indicate that oseltamivir should not be given or continued.
Early treatment with neuraminidase inhibitors is recommended in individuals hospitalized with influenza and individuals at high risk of progression to severe disease. Antiviral coverage was low in the study population, though notably this was a cross-sectional study and individuals may have received oseltamivir later in their treatment course. There was a relatively high rate of reported antimicrobial administration (22%), which did not differ significantly among individuals with PCR-confirmed influenza infection by the presence or absence of an infiltrate on chest X-ray, by lung findings on physical exam, or by the presence or absence of comorbidities that may predispose to severe disease (data not shown). The high rate of antibiotic use and low rate of oseltamivir administration prior to study enrollment in this population underscore the need for early and enhanced diagnosis of influenza influenza to facilitate patient triage and the appropriate targeting of antimicrobials.
Influenza vaccine coverage has been increasing in recent years in the study population but remains relatively low at 20–30%. Vaccine effectiveness varied by year but was moderate overall at 49.5%, consistent with an earlier study in this cohort [27]. Notably, we report a VE in young children of 24%, which is much lower than the 55–60% estimated previously [28]. This may be explained by a disproportionate burden of influenza A(H3N2) infection in young children in our study (data not shown), as influenza A(H3N2) not well matched to the vaccine strain for some years. Vaccine coverage in those with relevant comorbidities was low at 33.0%. Further, vaccine coverage was low in members of the military and healthcare workers, at 22% and 9%, respectively, despite high VE (>75%) in both groups. These findings indicate that there are populations of adults within the study population that could greatly benefit from targeted vaccination campaigns, to include military and healthcare professionals and individuals with comorbidities. Studies of vaccine acceptance among adult patients and medical providers would provide insight into reasons for this low uptake and indicate areas for targeted intervention.
It is generally thought that the influenza vaccine becomes significantly protective after two weeks, with efficacy waning considerably in the months following administration [29, 30]. The timing and number of peaks of influenza activity varied unpredictably in our study population, which complicates timing of vaccine delivery to optimize VE and, more broadly, selection of the northern versus southern hemisphere vaccine strains. Future studies should consider the potential for climatological data and prior influenza epidemic data to possibly predict these peaks in activity and inform vaccination programs.
These analyses were subject to multiple limitations. Foremost is that patient assessment was limited to a single point in time, precluding analysis of how symptoms, severity, and clinical management may have changed over time. Patient treatment and vaccination histories were self-reported and possibly subject to recall bias. This study was conducted at a single hospital in Thailand and may have limited generalizability beyond the Asia-Pacific region. A five-year period may be insufficient to assess patterns of seasonality and vaccine effectiveness, particularly given that 2009–2010 were unique years due to the dramatic emergence of influenza A(H1N1)pdm09 and the introduction of the swine flu vaccine. This study is intended to continue for ten years, which will allow assessment of patterns of influenza transmission and vaccine performance over a longer interval.
Conclusions
Despite the widespread availability of influenza vaccines and antivirals, the global burden of influenza-related disease continues to be high. In this manuscript, we report on predictors of vaccination, influenza infection, and vaccine effectiveness in a cohort of individuals presenting with ILI to an urban Thai hospital. Based upon the significant overlap in the clinical presentation between influenza and other influenza-like illnesses, we do not recommend the routine use of clinical algorithms for the identification of seasonal influenza. We suggest that the expanded use of rapid influenza diagnostic tests such as QuickVue would allow early detection of influenza infection in resource-limited settings and facilitate the appropriate allocation of antimicrobial medications. Finally, we suggest that there are multiple groups who would benefit from targeted vaccination campaigns such as members of the military, healthcare workers, and adults with medical comorbidities, given their relatively high rates of influenza infection but low rates of reported vaccination.
Supporting information
a P-values were calculated using Mantel-Haenszel χ2 statistics, with exact testing performed for comparisons with cells with values < = 5. Comparisons that were statistically significantly with α = 0.05 are shown in bold.
(DOCX)
Acknowledgments
We are grateful to the participants of this study as well as the research nurses at Phramongkutklao hospital and the clinical, laboratory and administrative personnel at AFRIMS. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Data Availability
Deidentified data associated with this study are available from Dryad (https://datadryad.org, doi:10.5061/dryad.t7n48).
Funding Statement
This work was supported by Global Emerging Infections Surveillance and Response System (https://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Global-Emerging-Infections-Surveillance-and-Response), Grant numbers: various. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–90. Epub 2013/02/02. doi: 10.1016/S0140-6736(12)61901-1 ; PubMed Central PMCID: PMCPMC3986472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ditsungnoen D, Greenbaum A, Praphasiri P, Dawood FS, Thompson MG, Yoocharoen P, et al. Knowledge, attitudes and beliefs related to seasonal influenza vaccine among pregnant women in Thailand. Vaccine. 2016;34(18):2141–6. Epub 2016/02/09. doi: 10.1016/j.vaccine.2016.01.056 ; PubMed Central PMCID: PMCPMC4811693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–37. Epub 2015/02/03. doi: 10.1016/S0140-6736(14)62449-1 . [DOI] [PubMed] [Google Scholar]
- 4.Vardakas KZ, Theocharis G, Tansarli GS, Rafailidis P, Falagas ME. Impact of oseltamivir use on the reduction of complications in patients with influenza: a prospective study. Arch Virol. 2016;161(9):2511–8. Epub 2016/07/03. doi: 10.1007/s00705-016-2941-5 . [DOI] [PubMed] [Google Scholar]
- 5.Loubet P, Samih-Lenzi N, Galtier F, Vanhems P, Loulergue P, Duval X, et al. Factors associated with poor outcomes among adults hospitalized for influenza in France: A three-year prospective multicenter study. J Clin Virol. 2016;79:68–73. Epub 2016/04/23. doi: 10.1016/j.jcv.2016.04.005 . [DOI] [PubMed] [Google Scholar]
- 6.Lacroix S, Vrignaud B, Avril E, Moreau-Klein A, Coste M, Launay E, et al. Impact of rapid influenza diagnostic test on physician estimation of viral infection probability in paediatric emergency department during epidemic period. J Clin Virol. 2015;72:141–5. Epub 2015/10/30. doi: 10.1016/j.jcv.2015.08.002 . [DOI] [PubMed] [Google Scholar]
- 7.Louie JK, Guevara H, Boston E, Dahlke M, Nevarez M, Kong T, et al. Rapid influenza antigen test for diagnosis of pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16(5):824–6. Epub 2010/04/23. doi: 10.3201/eid1605.091797 ; PubMed Central PMCID: PMCPMC2954007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurt AC, Baas C, Deng YM, Roberts S, Kelso A, Barr IG. Performance of influenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza Other Respir Viruses. 2009;3(4):171–6. doi: 10.1111/j.1750-2659.2009.00086.x ; PubMed Central PMCID: PMCPMC4634687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon A, Videa E, Saborio S, Lopez R, Kuan G, Reingold A, et al. Performance of an influenza rapid test in children in a primary healthcare setting in Nicaragua. PLoS One. 2009;4(11):e7907 Epub 2009/11/26. doi: 10.1371/journal.pone.0007907 ; PubMed Central PMCID: PMCPMC2774508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CK, Cowling BJ, Chan KH, Fang VJ, Seto WH, Yung R, et al. Factors affecting QuickVue Influenza A + B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65(1):35–41. Epub 2009/08/15. doi: 10.1016/j.diagmicrobio.2009.05.003 . [DOI] [PubMed] [Google Scholar]
- 11.Velasco JM, Montesa-Develos ML, Jarman RG, Lopez MN, Gibbons RV, Valderama MT, et al. Evaluation of QuickVue influenza A+B rapid test for detection of pandemic influenza A/H1N1 2009. J Clin Virol. 2010;48(2):120–2. Epub 2010/04/15. doi: 10.1016/j.jcv.2010.03.010 . [DOI] [PubMed] [Google Scholar]
- 12.Liao Q, Ip DK, Tsang TK, Cao B, Jiang H, Liu F, et al. A clinical prediction rule for diagnosing human infections with avian influenza A(H7N9) in a hospital emergency department setting. BMC Med. 2014;12:127 doi: 10.1186/s12916-014-0127-0 ; PubMed Central PMCID: PMCPMC4243192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160(21):3243–7. Epub 2000/11/23. . [DOI] [PubMed] [Google Scholar]
- 14.Michiels B, Thomas I, Van Royen P, Coenen S. Clinical prediction rules combining signs, symptoms and epidemiological context to distinguish influenza from influenza-like illnesses in primary care: a cross sectional study. BMC Fam Pract. 2011;12:4 doi: 10.1186/1471-2296-12-4 ; PubMed Central PMCID: PMCPMC3045895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campe H, Heinzinger S, Hartberger C, Sing A. Clinical symptoms cannot predict influenza infection during the 2013 influenza season in Bavaria, Germany. Epidemiol Infect. 2016;144(5):1045–51. Epub 2015/09/22. doi: 10.1017/S0950268815002228 . [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman RK, Balasubramani GK, Nowalk MP, Eng H, Urbanski L, Jackson ML, et al. Classification and Regression Tree (CART) analysis to predict influenza in primary care patients. BMC Infect Dis. 2016;16(1):503 Epub 2016/09/24. doi: 10.1186/s12879-016-1839-x ; PubMed Central PMCID: PMCPMC5034457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein J, Louie J, Flanders S, Maselli J, Hacker JK, Drew WL, et al. Performance characteristics of clinical diagnosis, a clinical decision rule, and a rapid influenza test in the detection of influenza infection in a community sample of adults. Ann Emerg Med. 2005;46(5):412–9. Epub 2005/11/08. doi: 10.1016/j.annemergmed.2005.05.020 . [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. CDC realtime RT-PCR (rRTPCR) protocol for detection and characterization of influenza (version 2007), CDC REF # I-007-05. 2007.
- 19.World Health Organization. CDC protocol of realtime RTPCR for swine influenza A(H1N1). Available at http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf (Accessed 28 April 2009). 2009.
- 20.Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016;65(5):1–54. Epub 2016/08/26. doi: 10.15585/mmwr.rr6505a1 . [DOI] [PubMed] [Google Scholar]
- 21.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31(30):3104–9. Epub 2013/04/30. doi: 10.1016/j.vaccine.2013.04.026 . [DOI] [PubMed] [Google Scholar]
- 22.Thai National Influenza Center. http://www.thainihnic.org/influenza/main.php?option=newsletter. Accessed December 15, 2017.
- 23.Bashyam HS, Toyosaki-Maeda T, Stephens HA, Mammen MP, Endy TP, Vaughn DW, et al. Frequency and phenotypic analysis of dengue epitope-specific T cells in infected Thai subjects during acute illness and convalescence. American Journal of Tropical Medicine and Hygiene. 2005;73(6):240–. PubMed PMID: WOS:000202990001149. [Google Scholar]
- 24.Stebbins S, Stark JH, Prasad R, Thompson WW, Mitruka K, Rinaldo C, et al. Sensitivity and specificity of rapid influenza testing of children in a community setting. Influenza Other Respir Viruses. 2011;5(2):104–9. Epub 2011/02/11. doi: 10.1111/j.1750-2659.2010.00171.x ; PubMed Central PMCID: PMCPMC4942005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouleau I, Charest H, Douville-Fradet M, Skowronski DM, De Serres G. Field performance of a rapid diagnostic test for influenza in an ambulatory setting. J Clin Microbiol. 2009;47(9):2699–703. Epub 2009/07/10. doi: 10.1128/JCM.00762-09 ; PubMed Central PMCID: PMCPMC2738100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen SJ, Kittikraisak W, Fernandez S, Suntarattiwong P, Chotpitayasunondh T. Challenges with new rapid influenza diagnostic tests. Pediatr Infect Dis J. 2014;33(1):117–8. doi: 10.1097/INF.0000000000000089 ; PubMed Central PMCID: PMCPMC4606927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy JW, Simasathien S, Watanaveeradej V, Bhoomiboonchoo P, Fernandez S, Jarman RG, et al. Influenza vaccine effectiveness in the tropics: moderate protection in a case test-negative analysis of a hospital-based surveillance population in Bangkok between August 2009 and January 2013. PLoS One. 2015;10(8):e0134318 doi: 10.1371/journal.pone.0134318 ; PubMed Central PMCID: PMCPMC4534293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kittikraisak W, Suntarattiwong P, Ditsungnoen D, Klungthong C, Fernandez S, Yoon IK, et al. Effectiveness of the 2013 and 2014 Southern Hemisphere Influenza Vaccines Against Laboratory-confirmed Influenza in Young Children Using a Test-negative Design, Bangkok, Thailand. Pediatr Infect Dis J. 2016;35(10):e318–25. doi: 10.1097/INF.0000000000001280 ; PubMed Central PMCID: PMCPMC5021558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrie JG, Ohmit SE, Truscon R, Johnson E, Braun TM, Levine MZ, et al. Modest Waning of Influenza Vaccine Efficacy and Antibody Titers During the 2007–2008 Influenza Season. J Infect Dis. 2016;214(8):1142–9. Epub 2016/04/21. doi: 10.1093/infdis/jiw105 . [DOI] [PubMed] [Google Scholar]
- 30.Ferdinands JM, Fry AM, Reynolds S, Petrie J, Flannery B, Jackson ML, et al. Intraseason waning of influenza vaccine protection: Evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis. 2016. Epub 2017/01/01. doi: 10.1093/cid/ciw816 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a P-values were calculated using Mantel-Haenszel χ2 statistics, with exact testing performed for comparisons with cells with values < = 5. Comparisons that were statistically significantly with α = 0.05 are shown in bold.
(DOCX)
Data Availability Statement
Deidentified data associated with this study are available from Dryad (https://datadryad.org, doi:10.5061/dryad.t7n48).