Abstract
We have learned much about the short-term sequelae of congenital Zika virus (ZIKV) infection since the Centers for Disease Control and Prevention activated its ZIKV emergency response in January 2016. Nevertheless, gaps remain in our understanding of the full spectrum of adverse health outcomes related to congenital ZIKV infection and how to optimize health in those who are affected. To address the remaining knowledge gaps, support affected children so they can reach their full potential, and make the best use of available resources, a carefully planned public health approach in partnership with pediatric health care providers is needed. An essential step is to use population-based data captured through surveillance systems to describe congenital Zika syndrome. Another key step is using collected data to investigate why some children exhibit certain sequelae during infancy and beyond, whereas others do not, and to describe the clustering of anomalies and the timing of when these anomalies occur, among other research questions. The final critical step in the public health framework for congenital Zika syndrome is an intervention strategy with evidence-based best practices for longer-term monitoring and care. Adherence to recommended evaluation and management procedures for infants with possible congenital ZIKV infection, including for those with less obvious developmental and medical needs at birth, is essential. It will take many years to fully understand the effects of ZIKV on those who are congenitally infected; however, the lifetime medical and educational costs as well as the emotional impact on affected children and families are likely to be substantial.
The US Centers for Disease Control and Prevention (CDC) activated its Emergency Operations Center in January 2016 for the Zika virus (ZIKV) response. In many ways, the Zika epidemic and resulting response have been unprecedented, marking the first time that an arbovirus has been shown to cause birth defects and the first time in more than a half-century that a viral disease caused a birth defect epidemic.1 The response soon escalated to become the most complex outbreak response in the CDC’s history. The effort required contributions from across the agency as well as routine collaboration with state and global public health partners with both infectious and noninfectious disease expertise.
ZIKV primarily spreads through the bite of an infected Aedes mosquito (Aedes aegypti and Aedes albopictus); it can also be transmitted from mother to fetus during pregnancy or between sexual partners even if the infected partner is asymptomatic.2 Evidence of transmission is also found through blood transfusions as well as laboratory exposure.3 ZIKV has spread to >60 countries and territories, including Puerto Rico.4 In addition to travel-associated Zika cases in US residents, local mosquito-borne ZIKV transmission has also been reported in Florida and Texas.5
Although the presence of microcephaly first signaled the link between ZIKV infection during pregnancy and poor birth outcomes, other congenital anomalies may also be of concern; moreover, some affected infants do not have microcephaly. Congenital Zika syndrome (CZS) has been described as a recognizable pattern of congenital anomalies associated with ZIKV infection during pregnancy that is characterized by severe microcephaly with a partially collapsed skull; thin cerebral cortices with subcortical calcifications; eye anomalies, including macular scarring and focal pigmentary retinal mottling; congenital contractures or a limited range of joint motion; marked hypertonia; and symptoms of extrapyramidal involvement.6 Additionally, infants with a head circumference in the normal range at birth can have brain abnormalities that are consistent with CZS and the postnatal development of microcephaly.7
To date, estimates of health care and familial costs associated with the Zika epidemic have been limited to microcephaly as the primary phenotype. Li et al8 estimated the total lifetime cost for a surviving infant with Zika-associated microcephaly to be $3.8 million with the potential to reach $10 million for those who survive to adulthood. Therefore, the lifetime medical and educational costs as well as the emotional impact for children and families affected by the Zika epidemic (including the impact beyond microcephaly) are likely to be substantial.
In November 2016, the World Health Organization Emergency Committee recommended that the global Zika response transition to a longer-term approach with dedicated, sustained resources.9 By September 2017, the CDC transitioned its Zika emergency response activities to existing programs but noted that ZIKV continues to be a public health threat in the United States and internationally. Moving forward, broadening the public health approach beyond the immediate needs of pregnant women, fetuses, and infants to examine long-term child outcomes is needed. Many questions remain about the future of surviving children who are affected by CZS as they transition from infancy to childhood. The optimal way to address the remaining knowledge gaps, support affected children so they can reach their full potential, and make the best use of available resources is through a carefully planned and coordinated public health approach in partnership with pediatric health care providers.
PUBLIC HEALTH APPROACH
In general, the public health approach is operationalized as a cycle that includes surveillance, research, and intervention (see Fig 1 for a public health approach to CZS). Since January 2016, the CDC, in collaboration with other public health partners, has conducted rapid surveillance on Zika-affected pregnant women and used findings to plan for services and update recommendations for care, including clinical guidance for health care providers who care for pregnant women and infants. Continued surveillance of affected children beyond birth will allow public health agencies to refine guidance and develop best practices for longer-term monitoring and care.
FIGURE 1.
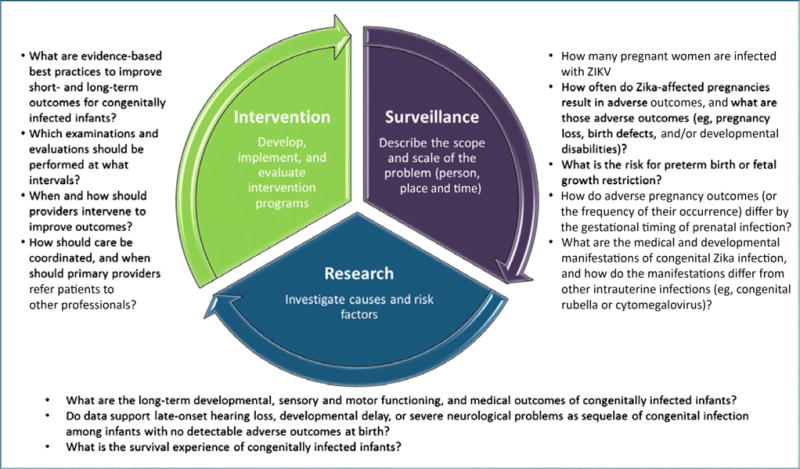
Framework for a public health approach to CZS.
Surveillance Objectives
An essential step in the public health approach is to use population-based data captured through surveillance systems to describe the problem. Surveillance of CZS is needed to answer several key questions. How many pregnant women are infected with ZIKV? How often do Zika-affected pregnancies result in adverse outcomes, and what are those adverse outcomes (eg, pregnancy loss, birth defects, and/or developmental disabilities)? What is the risk for preterm birth or fetal growth restriction? How do adverse pregnancy outcomes (or the frequency of occurrence) differ by the gestational timing of prenatal infection? What are the medical and developmental manifestations of congenital Zika infection,6 and how do the manifestations differ from other intrauterine infections (eg, congenital rubella virus or cytomegalovirus infection)?
Surveillance Efforts in the United States and US Territories
In February 2016, ZIKV and congenital ZIKV infections became nationally notifiable conditions in the United States, prompting health care providers to report laboratory-confirmed and symptomatic cases of ZIKV to their local, state, tribal, or territorial health departments and to collect clinical and follow-up information.10
US Zika Pregnancy and Infant Registry
In February 2016, in collaboration with local, state, tribal, and territorial health departments, the CDC established the US Zika Pregnancy and Infant Registry (USZPIR) to track women with laboratory evidence of ZIKV infection during pregnancy and collect data on pregnancy and infant outcomes in those pregnancies. All US states, the District of Columbia, and all US territories are collaborating in the USZPIR, which includes the US Zika Pregnancy Registry and the Puerto Rico Zika Active Pregnancy Surveillance System. The goal of the USZPIR is to better understand the effects of ZIKV infection during pregnancy, including the effects of timing and clinical presentation of infection, risk, and the spectrum of outcomes associated with maternal ZIKV infection. In addition, data collected from the USZPIR have provided initial estimates of the proportion of pregnancies affected by birth defects that are potentially associated with congenital Zika infection, which are 5% to 10% overall11,12 and 8% to 15% for those with first-trimester exposure.12,13
The USZPIR collects data during pregnancy and delivery, throughout infancy, and up to 24 months in the US states and most territories and 36 months in Puerto Rico. Women and children are monitored by using medical record abstraction of prenatal, emergency department, delivery and/or birth, and pediatric follow-up records. Pregnancy and delivery data are comprehensive and include (but are not limited to) ZIKV exposure, symptoms, and test results; pregnancy complications; and prenatal imaging findings. Neonatal assessment and infant and child follow-up data are derived from test results, physical examinations, growth and developmental assessments, neonatal neuroimaging, ophthalmologic examinations, hearing evaluations, and other consultations. Data about survival and the presence of birth defects also are collected. Knowledge gained from the USZPIR can lead to improved clinical guidance and facilitate planning for health services for affected children and their families. In Puerto Rico and some other US jurisdictions, all infants who are born to women with evidence of possible ZIKV infection during pregnancy are referred to the Children with Special Health Care Needs Program for close follow-up, monitoring, and needed services.14 Continued support from clinicians who provide health care to pregnant women and their infants is critical to the ability of these surveillance programs to provide quality data to address many unanswered questions.
Zika Birth Defects Surveillance
The CDC guidance is for health care providers to screen all pregnant women for ZIKV exposure before and during pregnancy at every prenatal care visit. However, because ZIKV infections are almost always asymptomatic or mild,15 health care providers might miss the opportunity to ask patients about exposure and make decisions about testing for ZIKV infection per CDC recommendations. Consequently, numerous potential cases of prenatal exposure or infection might not be documented in the USZPIR. To identify cases of congenital ZIKV infection and birth defects that are potentially related to ZIKV infection and might be missed by the registry, the CDC established a population-based monitoring program: the Zika Birth Defects Surveillance system. Birth defect cases that are potentially related to ZIKV are systematically captured within defined catchment areas in the US states and territories with possible endemic transmission (ie, areas with an observed distribution of Aedes mosquitoes) or areas that are associated with a high volume of travel from endemic countries. Standard case definitions for possible Zika-related birth defects for the Zika Birth Defects Surveillance system and those that are monitored in the USZPIR ensure consistency across surveillance systems. The information collected will be used to understand the impact of these conditions on communities, develop population-level prevention strategies, and assist with medical and social services referrals for affected individuals and families; the data can also be used to assess the health and developmental outcomes of these children. This marks the first time that pregnancy surveillance and birth defect surveillance activities have combined their efforts to address a common goal.16 Moving forward, this combined infrastructure could serve as a model for addressing myriad public health problems that affect maternal and child health.
Research Objectives
Another key step in the public health approach to CZS is using the data to investigate why some children exhibit certain sequelae during infancy and beyond, whereas others do not. This knowledge can be used to inform clinical practice and interventions. An important future priority of the overall Zika response is to expand our understanding of the long-term outcomes of congenitally infected infants.17 Research gaps include a lack of knowledge about long-term developmental, sensory, and motor functioning and medical outcomes, as well as effective interventions; limited data on the potential for late-onset hearing loss, developmental delay, or severe neurologic problems as sequelae in congenitally infected infants with no detectable adverse outcomes at birth7; and survival. Extensive monitoring may be necessary for many years until a safe and effective vaccine is made available and widely used.
Ongoing and Planned Research Studies
The CDC is collaborating with partners to identify the full spectrum of CZS by monitoring affected infants during childhood. Several pregnancy cohort studies are being conducted around the world in Zika-affected countries. One cohort study is the Zika en Embarazadas y Niños en Colombia, a partnership between the CDC and Colombia’s national public health institute.18 This study will enroll up to 5000 pregnant women, their partners, and their infants (all regardless of Zika exposure) in several cities in Colombia with regular follow-up through pregnancy and until the infants are 6 months old. A subset of children, along with a parent or legal guardian, will be followed until the child is about four years of age. In addition, the US National Institutes of Health–funded Zika in Infants and Pregnancy study aims to enroll as many as 10000 pregnant women (with and without Zika infection) at up to 15 sites.19 These and other studies will help increase knowledge about the full spectrum of effects of prenatal ZIKV infection and other factors (eg, environment, previous infections, and nutrition) that might play a role in increasing the risk for adverse outcomes after exposure to ZIKV infection.
Studies to increase knowledge about the fetal and infant effects of ZIKV infection during pregnancy will require input from a broad range of experts, including obstetricians, maternal–fetal medicine specialists, pediatricians and pediatric subspecialists, and epidemiologists to lead data analyses and interpretation. These experts will need to work collaboratively to address the primary questions posed by the global Zika response.
Insights into the longer-term consequences of CZS are starting to surface. A recent study showed that after congenital infection, ZIKV can continue to replicate in infants’ brain tissue after birth.20 In addition, researchers of a Brazilian case series described 13 infants with brain anomalies and laboratory evidence of congenital ZIKV infection and normal head size at birth.7 On follow-up, all had a decrease in the rate of head circumference growth, with postnatal microcephaly diagnosed in 11 of the 13 infants by the end of their first year. This case series illustrated that microcephaly at birth is not a necessary feature of the syndrome but can be seen later in infancy. A second study in 2017 reported eye findings in infants without microcephaly or other brain anomalies who were born to mothers with laboratory evidence of ZIKV infection.21 These findings have important implications for infants who are born without visible evidence of congenital Zika infection. Information is lacking to better characterize the complete phenotype of CZS, describe the clustering of anomalies, and define the timing of when these anomalies occur.
The long-term effects of this emerging infection are difficult to predict; however, parallels can be drawn to other congenital viral infections. For example, hearing loss because of congenital cytomegalovirus infection can sometimes develop during the first year of life even when it is absent at birth.22 As another example, rubella virus infection in utero is associated with autism (presenting in early childhood) and adult-onset diabetes.23,24 However, ZIKV infection has a unique natural history that continues to unfold in new and challenging ways. It will take many years to fully understand the effects of ZIKV on the fetus and congenitally infected infant. This is, in part, because some conditions might be absent or difficult to detect at birth; in addition, we do not have enough cases of congenital infection in infants compiled to sufficiently power epidemiologic studies on the long-term consequences of ZIKV infection during pregnancy.
Intervention Objectives
The final critical element in a public health approach to CZS is an intervention strategy with evidence-based best practices. An effective Zika strategy would determine which examinations and evaluations should be performed at what intervals; it would continue to assess practices such as when and how to intervene to improve outcomes and how to best coordinate care, including when to refer patients to other professionals.
Best Practices for Obstetric and Maternal-Fetal Care
Public health surveillance, research studies, and the development of evidence-based interventions for CZS will inform obstetric health care practices and improve counseling for Zika-exposed pregnant women and their families. Currently, counseling pregnant women with possible ZIKV infection is challenging because the long-term outcomes among children who are born to women with ZIKV infection during pregnancy are largely unknown. Obstetric health care providers should be prepared to counsel pregnant women with exposure to ZIKV regarding what to expect for child outcomes,25 including the spectrum of birth defects and other outcomes that are associated with prenatal ZIKV infection, levels of risk, and effects of the timing of infection and other cofactors. Counseling pregnant women in the setting of medical uncertainty regarding child outcomes is not unique to ZIKV infection.26 Other congenital infections have a wide range of presentations and varying long-term outcomes. To the extent possible, pregnant women with exposure to ZIKV should be informed of the potential fetal risks, availability and limitations of diagnostic testing and monitoring during pregnancy, and recommended services for infants who are born to mothers with ZIKV exposure during pregnancy. The CDC has developed interim guidance in the form of an obstetrics clinical tool kit to assist health care providers who serve pregnant women who are exposed to ZIKV.27,28 As new information becomes available, these guidelines will be updated accordingly. Additionally, Zika Care Connect (ZCC) is a resource for pregnant women who seek clinical services in accordance with the CDC’s interim guidance. ZCC establishes a network of specialty providers, including those in maternal–fetal medicine and obstetrics and/or gynecology, who are dedicated to the care of families affected by ZIKV.29
Best Practices for Pediatric and Interdisciplinary care
Partnerships between pediatric health care providers and public health professionals are essential to ensuring appropriate care for those who are affected by CZS. A recent CDC report highlighted gaps in evaluating and managing infants with possible congenital ZIKV infection.13 Initial USZPIR data showed that most infants with possible congenital ZIKV infection (ie, those who are born to women with [possible or confirmed] laboratory evidence of ZIKV infection) are not receiving CDC-recommended postnatal neuroimaging or ZIKV testing at birth.13,30 Adherence to recommended evaluation and management procedures for infants with possible congenital ZIKV infection is essential to characterize the full spectrum of CZS. The CDC has produced a pocket guide summarizing pediatric guidance on initial evaluation and outpatient management.31 Continued monitoring and follow-up with careful physical examinations and developmental evaluations beyond infancy will help elucidate the wide range of possible sequelae of congenital Zika infection.32
To inform the pediatric response to CZS, it will be critical to engage experts from countries that have greater experience with children affected by Zika. Communities in Brazil were affected earliest in the global spread of ZIKV and now have affected children entering their third year of life. Partnering with experts in Brazil to better characterize the medical and habilitative needs of children who were congenitally affected remains critical. The CDC is collaborating with the Brazilian Ministry of Health, the Secretariat of Health, and local clinicians on several investigations to evaluate children beyond infancy. One collaboration is a systematic assessment of the developmental and physical health of young children with CZS at ~12 to 24 months of age in several northeastern states of Brazil. Follow-up information from affected infants is key to understanding what services and support systems will be most beneficial to families that are caring for such children.
Developmentally oriented, interdisciplinary care33 will be essential for the success and well-being of children who are affected by Zika and their families. Consultation with pediatric specialists, such as neurologists, developmental pediatricians, and gastroenterologists, is important to optimize health during infancy and childhood. As a child grows, developmental care and educational needs will grow, and services from occupational, speech and/or language, and other specialists may be needed. Issues that might affect a child’s health and development also include vision impairment or hearing loss. Although traditional interventions, such as glasses or hearing aids, might be used to optimize vision and hearing, vision and speech therapy will still be necessary for development. In addition, early intervention educators and physical therapists will be needed to optimize impaired motor and cognitive functioning. Families will likely need to access birth–to–3-years-old early-intervention programs34 for such integrated care; as the children grow, they will need access to early-childhood special education programs.35 The coordination of early-intervention services with medical services will be best accomplished through a child’s medical home.36 Therefore, having an informed primary health care provider will be essential for optimal, coordinated, and ongoing care. ZCC can also help families that are affected by ZIKV and their primary health care providers identify local specialized pediatric care and ensure long-term follow-up.29 Families will likely need much support, and family support organizations, such as Family Voices and Family-to-Family Health Information Centers,37 can help. These and other resources for health care providers who serve children who are affected by ZIKV are compiled on the CDC’s Web site.38
Some children with congenital Zika infection may have less obvious developmental and medical needs at birth, but it remains crucial to monitor their health and development. Monitoring growth and developmental milestones at each well-child visit is consistent with current well-child care guidelines.39 In addition, parents should receive information on monitoring development so that concerns are recognized and appropriate interventions are initiated in a timely manner. The CDC’s “Learn the Signs. Act Early” program teaches parents about typical and atypical development.40
CONCLUSIONS
We have learned much about the short-term sequelae of congenital ZIKV infection since the CDC activated its ZIKV emergency response in January 2016. Nevertheless, gaps remain in our understanding of the full spectrum of adverse health outcomes related to congenital ZIKV infection and how to optimize health in those who are affected. A carefully planned public health approach must include input from and partnerships among experts in multiple clinical and public health disciplines to improve the ability to anticipate needs, provide appropriate care for those who are affected, and ensure that patients reach their full potential. The emergent nature of congenital Zika infection has required an all-hands-on-deck approach to maximize the effectiveness of our public health actions. To advance health care and promote well-being in families and children who are impacted by Zika, this work must continue intensively, and collaboration across sectors must be ensured.
Acknowledgments
Dr Broussard conceptualized and designed the manuscript, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Shapiro-Mendoza, Peacock, Rasmussen, Mai, Petersen, Galang, Gilboa, Boyle, and Moore, Ms Newsome, and Ms Reynolds conceptualized and designed the manuscript and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING: No external funding.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- CZS
congenital Zika syndrome
- USZPIR
US Zika Pregnancy and Infant Registry
- ZCC
Zika Care Connect
- ZIKV
Zika virus
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 2.Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure - United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077–1081. doi: 10.15585/mmwr.mm6539e1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Zika virus: transmission. Available at: https://www.cdc.gov/zika/transmission/index.html. Accessed January 2, 2018.
- 4.Centers for Disease Control and Prevention. Travelers’ health: World map of areas with risk of Zika. Available at: https://wwwnc.cdc.gov/travel/page/world-map-areas-with-zika. Accessed January 2, 2018.
- 5.Centers for Disease Control and Prevention. Zika virus: 2017 case counts in the US. Available at: https://www.cdc.gov/zika/reporting/2017-case-counts.html. Accessed January 2, 2018.
- 6.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171(3):288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015—January 2016 with congenital Zika virus infection without microcephaly at birth - Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Simmons KB, Bertolli J, et al. Cost-effectiveness of increasing access to contraception during the Zika virus outbreak, Puerto Rico, 2016. Emerg Infect Dis. 2017;23(1):74–82. doi: 10.3201/eid2301.161322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Statement on the fifth meeting of the Emergency Committee under the International Health Regulations 2005 regarding microcephaly, other neurological disorders, and Zika virus. 2016 Available at: www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/. Accessed March 17, 2017.
- 10.Council of State and Territorial Epidemiologists. (CSTE position statement 16-IC-01).Zika virus disease and Zika virus infection 2016 case definition. 2016 Available at: https://wwwn.cdc.gov/nndss/conditions/zika/case-definition/2016/06/. Accessed March 17, 2017.
- 11.Honein MA, Dawson AL, Petersen EE, et al. US Zika Pregnancy Registry Collaboration Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro-Mendoza CK, Rice ME, Galang RR, et al. Zika Pregnancy and Infant Registries Working Group Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621. doi: 10.15585/mmwr.mm6623e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds MR, Jones AM, Petersen EE, et al. US Zika Pregnancy Registry Collaboration Vital signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure - US Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(13):366–373. doi: 10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams L, Bello-Pagan M, Lozier M, et al. Update: ongoing Zika virus transmission - Puerto Rico, November 1, 2015-July 7, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(30):774–779. doi: 10.15585/mmwr.mm6530e1. [DOI] [PubMed] [Google Scholar]
- 15.Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 16.Gilboa SM, Mai CT, Shapiro-Mendoza CK, et al. Population-based pregnancy and birth defects surveillance in the era of Zika virus. Birth Defects Res. 2017;109(5):372–378. doi: 10.1002/bdr2.1007. [DOI] [PubMed] [Google Scholar]
- 17.Oussayef NL, Pillai SK, Honein MA, et al. Zika virus - 10 public health achievements in 2016 and future priorities. MMWR Morb Mortal Wkly Rep. 2017;65(52):1482–1488. doi: 10.15585/mmwr.mm6552e1. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. CDC newsroom: CDC and the Instituto Nacional de Salud of Colombia collaborate to understand long-term effects of Zika virus infection during pregnancy. Available at: https://www.cdc.gov/media/releases/2016/p0902-cdc-colombia-collaboration.html. Accessed January 2, 2018.
- 19.National Institutes of Health. NIH launches large study of pregnant women in areas affected by Zika virus. Available at: https://www.nih.gov/news-events/news-releases/nih-launches-large-study-pregnant-women-areas-affected-zika-virus. Accessed January 2, 2018.
- 20.Bhatnagar J, Rabeneck DB, Martines RB, et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis. 2017;23(3):405–414. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zin AA, Tsui I, Rossetto J, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr. 2017;171(9):847–854. doi: 10.1001/jamapediatrics.2017.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 23.Sever JL, South MA, Shaver KA. Delayed manifestations of congenital rubella. Rev Infect Dis. 1985;7(suppl 1):S164–S169. doi: 10.1093/clinids/7.supplement_1.s164. [DOI] [PubMed] [Google Scholar]
- 24.Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977;7(1):69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- 25.Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648. doi: 10.1097/AOG.0000000000001378. [DOI] [PubMed] [Google Scholar]
- 26.Gelber SE, Grunebaum A, McCullough LB, Chervenak FA. Fulfilling professional responsibilities when counselling patients about Zika infection. BJOG. 2017;124(4):550. doi: 10.1111/1471-0528.14388. [DOI] [PubMed] [Google Scholar]
- 27.Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127. doi: 10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. CDC US Zika Pregnancy Registry: Healthcare provider toolkit for health departments – for obstetricians. Available at: https://www.cdc.gov/pregnancy/zika/research/documents/uSZPR_Toolkit_OB.pdf. Accessed January 2, 2018.
- 29.Zika Care Connect. Available at: https://www.zikacareconnect.org/. Accessed January 2, 2018.
- 30.Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - united States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089–1099. doi: 10.15585/mmwr.mm6641a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Evaluation for infants with possible congenital Zika virus infection. Available at: https://www.cdc.gov/zika/pdfs/pediatric-evaluation-follow-up-tool.pdf. Accessed January 2, 2018.
- 32.Rasmussen SA, Hayes EB. Public health approach to emerging infections among pregnant women. Am J Public Health. 2005;95(11):1942–1944. doi: 10.2105/AJPH.2004.054957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nancarrow SA, Booth A, Ariss S, Smith T, Enderby P, Roots A. Ten principles of good interdisciplinary team work. Hum Resour Health. 2013;11:19. doi: 10.1186/1478-4491-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Early Childhood Technical Assistance Center. Early intervention program for infants and toddlers with disabilities (part C of IDEA) Available at: http://ectacenter.org/partc/partc.asp. Accessed January 2, 2018.
- 35.Individuals with Disabilities Education Improvement Act Partnership. Part B section 619 (ages 3 through 5). Available at: http://www.ideapartnership.org/topics-database/idea-2004/part-b-section-619-ages-3-through-5.html. Accessed January 2, 2018.
- 36.Patient-Centered Primary Care Collaborative. Defining the medical home. Available at: https://www.pcpcc.org/about/medical-home. Accessed January 2, 2018.
- 37.Family Voices. Family to Family Health Information Centers. Available at: http://www.familyvoices.org/page?id=0052. Accessed January 2, 2018.
- 38.Centers for Disease Control and Prevention. Zika and pregnancy: Additional resources for healthcare providers. Available at: https://www.cdc.gov/zika/hc-providers/infants-children/resources-hc-providers-caring-for-infants.html. Accessed January 2, 2018.
- 39.National Center for Education in Maternal and Child Health; Georgetown university. Bright Futures. Available at: https://www.brightfutures.org. Accessed January 2, 2018.
- 40.Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/actearly. Accessed January 2, 2018.


