Abstract
Background
Borderline personality disorder (BPD) and avoidant personality disorder (AvPD) are characterized by hyper-reactivity to negatively-perceived interpersonal cues, yet they differ in degree of affective instability. Recent work has begun to elucidate the neural (structural and functional) and cognitive-behavioral underpinnings of BPD, although some initial studies of brain structure have reached divergent conclusions. AvPD, however, has been almost unexamined in the cognitive neuroscience literature.
Methods
In the present study we investigated group differences among 29 BPD patients, 27 AvPD patients, and 29 healthy controls (HC) in structural brain volumes using voxel-based morphometry (VBM) in five anatomically-defined regions of interest: amygdala, hippocampus, medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC). We also examined the relationship between individual differences in brain structure and self-reported anxiety and affective instability in each group.
Results
We observed reductions in MPFC and ACC volume in BPD relative to HC, with no significant difference among patient groups. No group differences in amygdala volume were found. However, BPD and AvPD patients each showed a positive relationship between right amygdala volume and state-related anxiety. By contrast, in HC there was an inverse relationship between MPFC volume and state and trait-related anxiety as well as between bilateral DLPFC volume and affective instability.
Limitations
Current sample sizes did not permit examination of gender effects upon structure-symptom correlations.
Conclusions
These results shed light on potentially protective, or compensatory, aspects of brain structure in these populations–namely, relatively reduced amygdala volume or relatively enhanced MPFC and DLPFC volume.
Keywords: borderline personality disorder, avoidant personality disorder, voxel-based morphometry, anxiety, affective instability
Introduction
Borderline personality disorder (BPD) and avoidant personality disorder (AvPD) are two serious and prevalent disorders marked by great interpersonal sensitivity, anxiety, and affective instability. BPD is a severe, pernicious mental disorder present in an estimated 2.7 percent of the population (Tomko et al., 2014) and characterized by heightened anxiety, unstable interpersonal relationships, significant affective instability, and a 10% suicide rate (Koenigsberg et al., 2014; Koenigsberg et al., 2001; Lieb et al., 2004; Skodol et al., 2002a). In recent years, the underlying neural mechanisms of BPD have begun to be explored using structural and functional neuroimaging modalities (Carpenter and Trull, 2013; Koenigsberg et al., 2014; Krause-Utz et al., 2014; Schmahl and Bremner, 2006; Schulze et al., 2016). However, significant questions remain, including the nature of the relationship between inter-individual variation in brain structure and disordered patterns of emotional, cognitive, and behavioral response (Kanai and Rees, 2011).
AvPD, a personality disorder with a prevalence rate comparable to BPD (American_Psychiatric_Association, 2013), is characterized by fears of criticism, rejection, disapproval and embarrassment in interpersonal situations, leading to social avoidance and inhibition. Thus, like BPD, it is marked by interpersonal hypersensitivity and anxiety, but unlike BPD, affective instability, anger and impulsivity are less prominent. The structural features and structural-affective correlates of AvPD have been unexamined. Comparing and contrasting these two disorders, which have important areas of phenomenological overlap and difference, affords a unique opportunity to identify the structural correlates of distinct affective and interpersonal symptoms.
Prior work has identified structural neural anomalies in BPD patients relative to healthy controls (HC), though evidence has been mixed in key frontolimbic regions, including those importantly related to negative emotional experience (e.g. amygdala) and episodic memory (e.g. hippocampus) and those that represent nodes of a network underlying the cognitive control of emotion, including medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC) (Krause-Utz et al., 2014; Ochsner et al., 2012; Schulze et al., 2016). The amygdala is a medial temporal lobe region consistently implicated in processing negative affect in BPD (Krause-Utz et al., 2014), AvPD (Denny et al., 2015), and many other psychiatric illnesses (Denny et al., 2009), as well as in HC given its importance in representing the threat value of a stimulus (LeDoux, 1996; Ochsner et al., 2012). Further, hippocampus, noted for its crucial role in episodic and autobiographical memory consolidation (Squire, 1992), has been shown to differentiate BPD patients from healthy controls in terms of structural volume (Schulze et al., 2016). MPFC, DLPFC, and ACC, by contrast, are associated with diverse cognitive control functions including mental state attribution (MPFC; Ochsner et al., 2012), selective attention and working memory (DLPFC; Curtis and D’Esposito, 2003; Ochsner et al., 2012) and attentional control (ACC; Fan et al., 2005) that each contribute to modulations of emotional intensity (Ochsner et al., 2012).
Several prior studies have indicated anomalous concentrations of gray matter in BPD relative to healthy controls in amygdala (Krause-Utz et al., 2014; Schulze et al., 2016), though there is mixed evidence as to the direction of the anomaly. Reduced (Depping et al., 2015; Niedtfeld et al., 2013; Soloff et al., 2008) and greater amygdala volume in BPD patients relative to healthy controls (Minzenberg et al., 2008) have both been reported. Still other work has examined structural differences between BPD patients and healthy controls with amygdala as a particular region-of-interest and reported no group difference in amygdala volume (Brambilla et al., 2004; Kuhlmann et al., 2013; Zetzsche et al., 2006). Importantly, some of this work has shown that greater BPD amygdala volumes predict less BPD symptom severity (Niedtfeld et al., 2013), while somewhat more evidence exists showing that greater amygdala volumes predict greater symptom severity (Depping et al., 2015; Zetzsche et al., 2006). Thus, the question of volumetric differences in amygdala between BPD patients and healthy controls—and, crucially, the clinical significance of such differences—is unresolved.
Hippocampal evidence, however, has been more consistent in implicating reduced structural volume in BPD patients relative to healthy controls (Driessen et al., 2000; Sala et al., 2011; Schmahl et al., 2009; Schulze et al., 2016). However, the relationship between individual differences in hippocampal volume and self-reported anxiety and affective instability is less clear, although some evidence has shown that, among BPD patients, relatively reduced hippocampal volume predicts greater aggressiveness (Sala et al., 2011; Zetzsche et al., 2007). Thus, we were motivated to examine hippocampus as a region-of-interest in this study.
Further, evidence has been mixed with regard to volumetric differences between BPD patients and healthy controls in MPFC, DLPFC, and ACC. In MPFC, evidence for or against volume differences is scarce. One study has shown ventral MPFC gray matter reductions in BPD patients relative to healthy controls (contiguous with volumetric reductions in ACC), though the same study also reported greater gray matter volumes in BPD patients relative to healthy controls in dorsal MPFC (Soloff et al., 2008). In DLPFC, a recent multimodal meta-analysis has shown evidence of increased gray matter volume in BPD patients relative to HC (Schulze et al., 2016), though some individual studies have found no significant group difference in gray matter volume (Brambilla et al., 2004; Sala et al., 2011) as well as an inverse relationship between DLPFC volume and impulsiveness in BPD patients (Sala et al., 2011). In ACC, several studies have indicated that BPD patients show reduced volumes relative to healthy controls (Goodman et al., 2011; Hazlett et al., 2005; Minzenberg et al., 2008; Niedtfeld et al., 2013; Soloff et al., 2008), including one study where greater ACC volume in BPD patients predicted less BPD symptom severity (Niedtfeld et al., 2013). However, other recent reports have specifically examined volumetric differences in ACC and have not found evidence for a difference between BPD patients and healthy controls (Depping et al., 2015; Kuhlmann et al., 2013; Labudda et al., 2013). Thus, similar to amygdala, studies examining structural abnormalities in MPFC, DLPFC, and ACC in BPD relative to healthy controls have reached varying conclusions.
With regard to AvPD patients, to our knowledge there are only two published neuroimaging reports in the literature, both of which were functional neuroimaging studies (Denny et al., 2015; Koenigsberg et al., 2014). Denny and colleagues (2015) found evidence for amygdala hyper-reactivity in AvPD patients relative to healthy controls when anticipating engaging in explicit emotion regulation via cognitive reappraisal, whereas Koenigsberg and colleagues (2014) did not find evidence of greater amygdala recruitment in AvPD patients relative to healthy controls when repeatedly viewing negative emotional pictures. However, structural differences in AvPD have remained unexplored. Moreover, a direct comparison between the neural structure of BPD and AvPD, two personality disorders with different phenotypic presentations, could help to better understand the relationship between brain structural volume and personality disorder symptoms.
Thus, the present study sought to examine structural differences in gray matter volume among BPD patients, AvPD patients, and healthy controls using voxel-based morphometry (VBM) in a priori-motivated, unbiased, anatomically-defined regions-of-interest. Importantly, we further investigated the relationship between observed structural differences and inter-individual variation in disorder-relevant measures of anxiety and affective instability. Given the varied structural neuroimaging evidence reviewed above in a priori regions of interest including the amygdala, hippocampus, MPFC, DLPFC, and ACC, we did not make predictions regarding the direction of volumetric main effects. However, greater gray matter volume in regions strongly linked to processing negative affect (i.e. amygdala) was expected to predict greater self-reports of negative affect within and across groups, and greater gray matter volume in regions linked to cognitive control (i.e. MPFC, DLPFC, and ACC) was expected to predict diminished self-reports of negative affect within and across groups.
Methods and Materials
Participants
Structural neuroimaging data were obtained from 29 BPD patients, 27 AvPD patients, and 29 HC’s. Participants were recruited from outpatient clinics at the Mount Sinai Medical Center and the James J Peters VA Medical Center in New York City as well as from newspaper and online advertisements. All participants provided written informed consent after study procedures were fully explained to them according to the regulations of the Institutional Review Board at the Icahn School of Medicine at Mount Sinai. BPD patients met DSM-IV criteria for borderline personality disorder as the primary personality disorder, including the affective instability criterion, and did not meet criteria for schizotypal personality disorder. AvPD patients met DSM-IV criteria for avoidant personality disorder but not criteria for borderline or schizotypal personality disorder. Participants had to be free of psychotropic medications for 2 weeks (6 weeks in the case of fluoxetine). Comorbidities, prior medication use, and treatment experiences are indicated in the Supplemental Information. BPD’s and AvPD’s were excluded if they met DSM-IV criteria for current PTSD, bipolar I disorder, schizoaffective disorder, substance dependence, organic mental syndromes, head trauma, CNS neurological disease, seizure disorder, substance abuse disorder in the previous 6 months or current major depressive disorder. Participants with significant medical illness, contraindications to magnetic resonance imaging (MRI), pregnant women and those with current active suicidal ideation were excluded. HC’s were lifetime-free of any axis I or axis II disorder diagnosis. Diagnostic assessments were obtained using the Structured Clinical Interview for DSM-IV–Patient Edition and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Our group has achieved an inter-rater reliability of κ = 0.81 for diagnosing borderline personality disorder (Koenigsberg et al., 2014). Sample characteristics are presented in Table 1. As shown, groups did not significantly differ in age or gender (p>0.10, two-tailed, for all pair-wise differences; see Table 1).
Table 1.
Sample Characteristics
| BPD | AvPD | HC | Statistic
|
|||
|---|---|---|---|---|---|---|
| BPD vs. HC | AvPD vs. HC | BPD vs. AvPD | ||||
| N | 29 | 27 | 29 | |||
| Age | 31.3 (9.7) |
27.9 (7.0) |
28.0 (6.5) |
t(56) = 1.54, n.s. | t(54) = 0.02, n.s. | t(54) = 1.51, n.s. |
| Gender (F/M) | 19/10 | 15/12 | 14/15 | χ2=1.76, n.s. | χ2=0.30, n.s. | χ2=0.58, n.s. |
| State Anxiety (STAI-State) |
42.0 (14.6) |
33.3 (10.0) |
27.4 (6.1) |
t(51) = 4.58** | t(50) = 2.57* | t(51) = 2.52* |
| Trait Anxiety (STAI-Trait) |
47.5 (11.3) |
40.4 (9.6) |
29.6 (5.9) |
t(54) = 7.10** | t(52) = 4.89** | t(54) = 2.52* |
| Affective Instability (ALS) |
70.2 (28.5) |
49.2 (21.1) |
29.5 (21.4) |
t(56) = 6.09** | t(51) = 3.36* | t(51) = 3.08* |
BPD – Borderline Personality Disorder; AvPD – Avoidant Personality Disorder; HC – Healthy Controls; STAI – State-Trait Anxiety Inventory; ALS – Affective Lability Scale. Average values are shown, with standard deviations given in parentheses.
p < 0.001, two-tailed
p < 0.02, two-tailed
Materials and Procedure
Participants first completed the questionnaires shown below prior to undergoing structural MRI.
Questionnaire Measures of Anxiety and Affective Instability
Individual differences in self-reported anxiety were assessed using the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1970), with separate assessments of state-related and trait-related anxiety. Further, affective instability was assessed using the Affective Lability Scale (ALS; Harvey et al., 1989). STAI-State scores were not available for 2 BPD’s, 1 AvPD, and 3 HC’s. STAI-Trait scores were not available for 2 HC’s. ALS scores were not available for 3 AvPD’s.
Structural Image Acquisition
High-resolution T2-weighted anatomical images were obtained using a 3.0 T Siemens Allegra scanner as part of separate functional neuroimaging experiments that have been described previously (Denny et al., 2015; Koenigsberg et al., 2014; Koenigsberg et al., 2009). T2-weighted images were acquired on an axis plane parallel to the AC-PC line using a turbo spin-echo pulse sequence and a voxel size of 0.41 mm × 0.41 mm × 3.3 mm. Importantly, T2-weighted image VBM has been shown to achieve results that are at least comparable to T1-weighted image VBM (Diaz-de-Grenu et al., 2011). In the present study, T1-weighted images were not available.
Voxel-Based Morphometry Analysis
The present study used a unified segmentation and spatial normalization approach (Ashburner and Friston, 2005) as implemented in SPM8 (Wellcome Trust Centre for Neuroimaging; www.fil.ion.ucl.ac.uk/spm/software/spm8). T2-weighted images were automatically segmented into gray matter, white matter, and cerebrospinal fluid, and normalized to the standard SPM MNI template and resampled to 2 mm isotropic voxels. Segmented and normalized tissue maps were then modulated using an affine plus non-linear transformation to correct voxel signal intensities by multiplying the voxel values by the Jacobian determinant derived from the spatial normalization, permitting inter-subject inferences pertaining to gray matter volume (Good et al., 2001). A montage showing one slice per participant of the resulting modulated, normalized gray matter images (mwc1*.nii) is shown in Figure S1. These modulated, normalized images were then spatially smoothed with a 10 mm FWHM Gaussian kernel (smwc1*.nii).
Given our a priori interest in amygdala, hippocampus, MPFC, DLPFC, and ACC volumes, region-of-interest (ROI) analyses were then carried out using unbiased, anatomical ROI definitions derived from the WFU Pickatlas version 3.0.4 (fmri.wfubmc.edu/software/pickatlas; Maldjian et al., 2003) and the Talairach Daemon within the WFU Pickatlas (Lancaster et al., 2000). In particular, ROI’s for right amygdala (158 voxels, center at [24, −4, −19]) and left amygdala (161 voxels, center at [−23, −4, −19]) were created using the TD Brodmann atlas within the WFU Pickatlas. ROI’s for hippocampus (right hippocampus: 946 voxels, center at [29, −21, −12]; left hippocampus: 932 voxels, center at [−25, −22, −11]), MPFC (6701 voxels, center at [1, 49, 20]) and ACC (2712 voxels, center at [2, 35, 14]) were created using the AAL atlas within the WFU Pickatlas. ROI’s for DLPFC (right DLPFC: 8,865 voxels, center at [33, 31, 25]; left DLPFC: 8,722 voxels, center at [−32, 28, 26]) were created using the Hammers_mith atlas n30r83, labels 29 and 28, respectively (Hammers et al., 2003).
Estimates of total gray matter were derived for each participant by summing the values of the modulated, normalized gray matter images (mwc1*.nii), masked by a whole-brain mask excluding brainstem and cerebellum, and multiplying by the volume of each voxel. The whole-brain mask was created using the TD Hemispheres atlas within the WFU Pickatlas and excluded bilateral brainstem and cerebellum in order to eliminate variance attributable to incomplete cerebellar acquisition during the T2-weighted scan. Average smoothed, modulated, normalized gray matter estimates (i.e. using smwc1*.nii images) were then extracted for each ROI described above (i.e. amygdala, hippocampus, MPFC, DLPFC, ACC) using MarsBaR version 0.43 (marsbar.sourceforge.net) for each participant. These volumes were then proportionally-corrected using the total gray matter estimates (i.e. extracted average smwc1 volume estimates divided by total gray matter estimate) for each ROI for each participant in order to correct for inter-individual variation in global volume of gray matter. Resulting proportionally-corrected gray matter volume indices for each ROI were then compared between groups and submitted to correlations with questionnaire reports of anxiety and affective instability (i.e. STAI-State, STAI-Trait, and ALS scores). Gender was included as a covariate in univariate ANOVAs performed using SPSS Statistics (Version 20) for all group comparisons of self-reported anxiety, affective instability, and gray matter volume in all ROI’s.
Finally, as a companion exploratory analysis, a whole-brain analysis was performed in SPM8 using the smoothed, modulated, normalized gray matter images (i.e. smwc1*.nii) and global proportional normalization using the total gray matter volume estimates defined above. Whole-brain analysis and multiple comparison correction details are provided in the Supplemental Information.
Results
Group Differences in Self-Reported Anxiety and Affective Instability
As shown in Table 1, participants showed the expected pattern of self-reported anxiety and affective instability across groups (BPD > AvPD > HC) for all measures, resulting in all pair-wise differences being significant (all p<0.02, two-tailed). These reports suggest a graded pattern transdiagnostically, such that AvPD patients evidenced an intermediate level of anxiety and affective instability relative to the more severely impaired BPD patients, as has been reported previously (Koenigsberg et al., 2014). No significant effects of gender (or significant group-by-gender interactions) were present.
Group Differences in Gray Matter Volume and Correlations with Self-Reported Anxiety and Affective Instability
VBM results are presented below for each of our five a priori ROI’s: amygdala, hippocampus, MPFC, DLPFC, and ACC. As described above, each region was independently defined using an unbiased, anatomically-defined ROI. For each ROI, group differences in volume are presented first, followed by correlations between VBM results and measures of self-reported anxiety and affective instability.
Amygdala
Figure 1(a) shows average gray matter volume by group for right amygdala. There was no main effect of group, and no pair-wise group differences were significant (all p>0.16, two-tailed). However, as shown in Figure 1(b), gray matter volume in both BPD patients and AvPD patients positively correlated with state anxiety (STAI-State) scores (r=0.33, p<0.05, one-tailed, and r=0.37, p<0.05, one-tailed, for BPD and AvPD patients, respectively). However, in HC’s, there was no association between right amygdala gray matter volume and state anxiety (r= −0.05, n.s.). However, an ANOVA using group as a categorical predictor, gray matter volume as a continuous predictor, and an interaction term, revealed a significant effect of increasing right amygdala gray matter volume predicting increasing state anxiety across all participants, F(1,73)=5.56, p<0.03. The group-by-gray matter volume interaction was not significant across all participants, F(2,73)=1.86, n.s. However, the group-by-gray matter volume interaction in predicting state anxiety levels was marginally significant between BPD patients and healthy controls alone, F(1,49)=3.18, p<0.09. Right amygdala volume correlations with trait anxiety and affective instability were not significant in any group.
Figure 1.
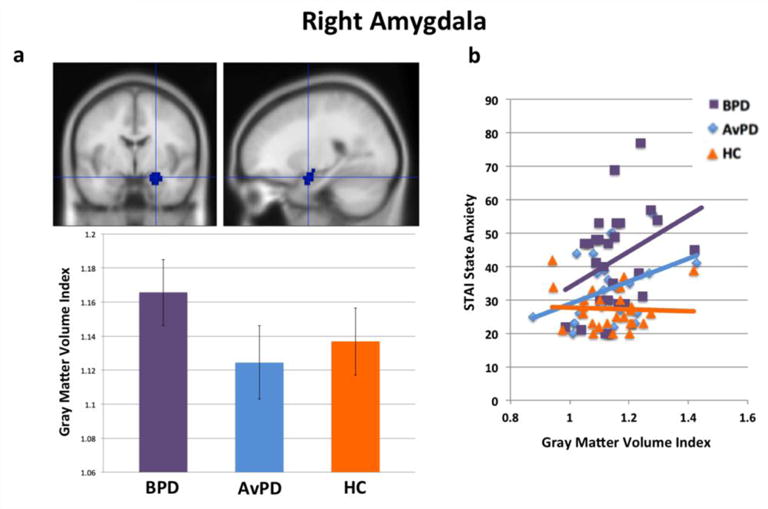
(a) Right amygdala volumes by group and (b) gray matter volume correlations with STAI-State scores.
In left amygdala (Figure 2(a)), there was again no main effect of group and no significant pair-wise differences between groups (all p>0.37, two-tailed). Further, no significant correlations between left amygdala volume and state or trait anxiety were found. However, among healthy controls, there was a correlation between increasing left amygdala volume and increasing affective instability (r=0.33, p<0.05, one-tailed). This association was not significant among BPD’s (r=0, n.s.) or AvPD’s (r=0.14, n.s.; Figure 2(b)). The group-by-gray matter volume interaction predicting affective instability scores was not significant between BPD patients and healthy controls, nor between AvPD patients and healthy controls, nor among all groups (all F<1.40, n.s.).
Figure 2.
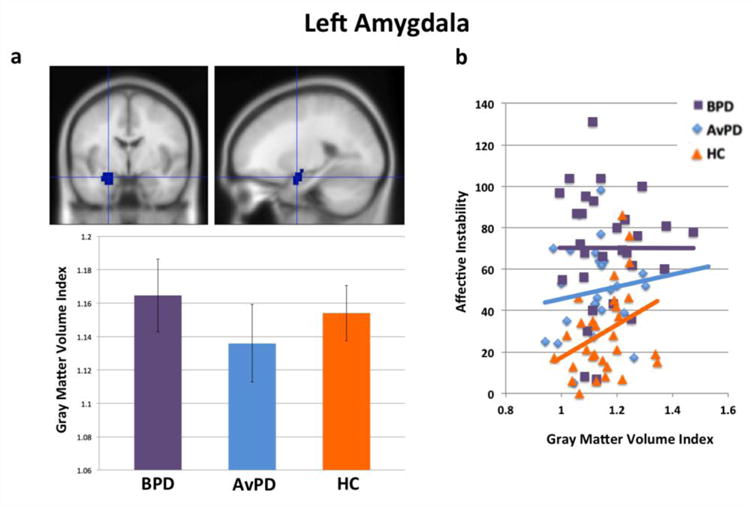
(a) Left amygdala volumes by group and (b) gray matter volume correlations with affective instability (ALS) scores.
Hippocampus
Right and left hippocampal volumes are shown in Figure S2. No main effects of group (or pair-wise differences) were present, nor were any significant correlations observed with self-reported anxiety or affective instability.
MPFC
Figure 3(a) shows average gray matter volume by group in MPFC. In MPFC, a marginal main effect of group was observed, with gray matter volume greatest for healthy controls, and a decreasing gradiential pattern for AvPD’s and BPD’s, F(2,81)=2.99, p<0.06, with no significant impact of gender. In addition, BPD patients showed significantly less gray matter volume relative to healthy controls (t(56)=2.39, p<0.03, two-tailed), and AvPD patients additionally showed marginally less gray matter volume relative to healthy controls (t(54)=1.68, p<0.10, two-tailed). Volume did not differ significantly between patient groups (t(54)=0.62, n.s.).
Figure 3.
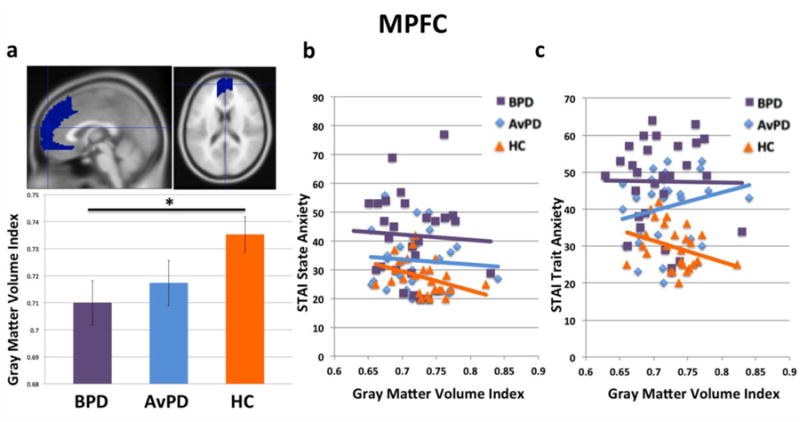
(a) MPFC volumes by group. (b) Gray matter volume correlations with STAI-State scores. (c) Gray matter volume correlations with STAI-Trait scores. * reflects a significant pair-wise difference, p<0.05, two-tailed.
Further, healthy controls showed a negative correlation between MPFC gray matter volume and both state and trait anxiety (r= −0.37, p<0.05, one-tailed, for state anxiety, Figure 3(b); and r= −0.32, p<0.05, one-tailed, for trait anxiety, Figure 3(c)). This suggests that, in healthy controls, greater gray matter volume in MPFC is adaptive and associated with lower self-reported anxiety. These correlations were not significant in BPD and AvPD patients (r= −0.05, n.s. and r= −0.01, n.s. for state and trait anxiety, respectively, in BPD patients; r= −0.08, n.s. and r=0.22, n.s. for state and trait anxiety, respectively, in AvPD patients). Among AvPD patients and healthy controls, there was a marginal group-by-gray matter volume interaction in predicting trait anxiety levels, F(1,50)=3.36, p<0.08, and a marginally-significant difference between group correlation coefficients, z=1.95, p<0.06, two-tailed. However, no other group-by-gray matter volume interactions predicting state or trait anxiety levels were significant between BPD patients and healthy controls, nor between AvPD patients and and healthy controls, nor among all groups (all F<1.23, n.s.). No significant correlations with affective instability were observed in any group.
DLPFC
Figure 4(a) shows average gray matter volume by group in right DLPFC. No main effect of group (nor any pair-wise differences) were present. However, among HC’s, increasing right DLPFC gray matter volume predicted reduced affective instability (r= −0.40, p<0.02, one-tailed; Figure 4(b)). This association was not present in BPD’s (r= −0.05, n.s.) or AvPD’s (r= −0.13, n.s.). This same pattern also held for left DLPFC (Figure 4(c–d)), with increasing DLPFC gray matter volume again predicting reduced affective instability among HC’s (r= −0.37, p<0.03, one-tailed), but not BPD’s (r= −0.04, n.s.) or AvPD’s (r=0.04, n.s.). In left DLPFC, while no main effect of group on volume was present, there was a significant effect of gender, F(1,81)=4.50, p<0.04, with female participants showing greater gray matter volume overall. However, there was no group-by-gender interaction. No significant correlations of gray matter volume with state and trait anxiety were observed in any group in right and left DLPFC.
Figure 4.
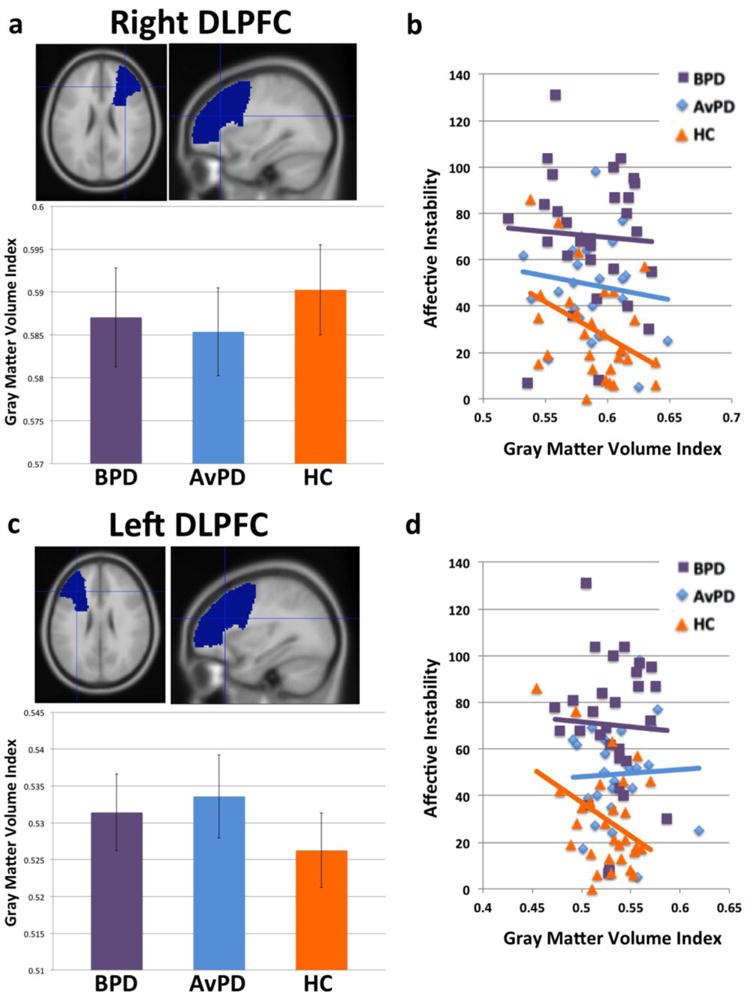
(a) Right DLPFC volumes by group. (b) Right DLPFC gray matter volume correlations with affective instability (ALS) scores. (c) Left DLPFC volumes by group. (d) Left DLPFC gray matter volume correlations with affective instability (ALS) scores.
ACC
Figure 5(a) shows average gray matter volume by group in ACC. While there was no main effect of group, BPD patients showed marginally less gray matter volume relative to healthy controls (t(56)=1.75, p<0.09, two-tailed).
Figure 5.
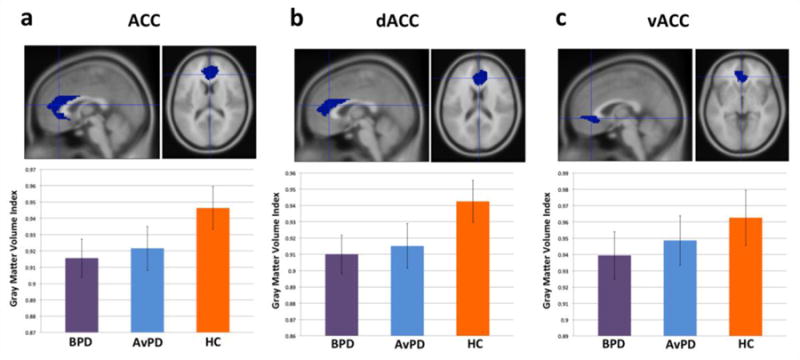
(a) ACC, (b) dACC, and (c) vACC volumes by group.
Given that prior meta-analyses have proposed a dorsal-ventral functional distinction in ACC (Bush et al., 2000), with dorsal ACC (dACC) being more strongly associated with cognitive control, and ventral ACC (vACC) being more strongly associated with attending to emotional information, as an exploratory analysis we split our ACC ROI above into separate dACC (z > 0 mm) and vACC (z ≤ 0 mm) ROI’s and examined volumetric differences across groups (Figure 5(b–c)). In dACC (Figure 5(b)), while there was no significant main effect of group, BPD patients again showed marginally less gray matter volume relative to healthy controls (t(56)=1.86, p<0.07, two-tailed). However, in vACC (Figure 5(c)), there was no main effect of group, and neither were any pair-wise volumetric differences significant (all p>0.31, two-tailed, n.s.). No significant correlations were found between ACC, dACC, or vACC gray matter volume and self-reported anxiety or affective instability in any group.
Whole-Brain Analysis
An exploratory whole-brain analysis of group differences in gray matter volume (proportionally-corrected by total gray matter volume) did not yield any results that met multiple comparison correction thresholds (height = p<0.002, two-tailed, uncorr., extent = 59 voxels [3 mm], FWE-corrected p<0.05) for comparisons of BPD to HC participants nor of BPD to AvPD participants. However, AvPD participants showed significantly reduced gray matter volume in ventral MPFC relative to HC (66 voxels [3mm], peak at [3, 63, −15], max t= −4.39, mean t= −3.58; Figure S3). This was the only region meeting FWE correction.
Discussion
Both borderline and avoidant personality disorder have been associated with heightened, clinically-significant amounts of anxiety and affective instability (Lieb et al., 2004; Sanislow et al., 2012; Skodol et al., 2002a; Skodol et al., 2002b), with some evidence for a gradiential relationship in increasing negative affectivity among healthy controls, AvPD patients, and BPD patients (Koenigsberg et al., 2014, as well as the current results). However, the neurobiological basis of this symptomatology has been unclear, both in terms of group differences in structural gray matter volumes in affectively-relevant ROI’s as well as in the relationship between individual differences in gray matter volume and disorder-relevant measures of anxiety and affective instability.
With regard to our first question concerning group differences in gray matter volume, we did not find significant evidence for a group structural difference in either right or left amygdala. While some recent work has provided evidence for decreased amygdala volume in BPD patients relative to healthy adults (Depping et al., 2015; Driessen et al., 2000; Niedtfeld et al., 2013; Soloff et al., 2008) and other work has indicated increased amygdala volume in BPD patients relative to healthy adults (Minzenberg et al., 2008), we examined 29 unmedicated BPD patients and 29 healthy adults and did not find a group difference, consistent with another recent study of 30 BPD patients and 33 healthy adults (Kuhlmann et al., 2013). Indeed, the relatively low sample sizes, comorbidities, and inclusion of patients using psychotropic medications in prior VBM work may have contributed to some discrepancies (Kuhlmann et al., 2013). Overall, the present work substantiates that amygdala volume is comparable among BPD patients, healthy adults, and AvPD patients. Further, while prior work has found evidence for reduced hippocampal volume in BPD patients relative to HC (Schulze et al., 2016), we did not observe significant group differences in the present study.
In MPFC, where prior findings in BPD and AvPD have been very limited, we have shown evidence for a significant volumetric reduction in BPD patients relative to healthy controls, and, consistent with the results of a whole-brain exploratory analysis, a marginally significant reduction in AvPD patients relative to healthy controls. This is again consistent with AvPD patients showing an intermediate level of abnormality relative to BPD patients and healthy controls. Given the MPFC’s role in cognitive control, including via cognitive reappraisal (Buhle et al., 2014; Ochsner et al., 2012), mentalizing (Denny et al., 2012; Northoff et al., 2006), and perspective taking (D’Argembeau et al., 2007), it is possible that this structural deficit forms part of the neurobiological basis of the dysregulated emotional processes typical of BPD. DLPFC volumes, however, did not show evidence of group differences in the present study, in contrast to prior work comparing BPD and HC (Schulze et al., 2016)
In ACC, we did find trend-level evidence of a volumetric reduction in BPD patients relative to healthy controls. This is consistent with several prior reports (Goodman et al., 2011; Hazlett et al., 2005; Minzenberg et al., 2008; Niedtfeld et al., 2013; Soloff et al., 2008). However, whereas several of these studies have reported a ventral focus of ACC volume decrease in BPD patients (Minzenberg et al., 2008; Soloff et al., 2008), or a reduction across ACC collapsing across vACC and dACC (Goodman et al., 2011; Hazlett et al., 2005), here we have reported both a reduction across the entire ACC as well as evidence that this is driven by a volumetric reduction in dorsal rather than ventral ACC, consistent with some prior work (Niedtfeld et al., 2013). Given the functional heterogeneity of ACC (Bush et al., 2000), this suggests that portions of the ACC most important for cognitive and attentional control, rather than emotional processing, may be most affected by the group difference in structural volume between BPD patients and healthy adults.
Relatedly, our second question specifically probed the association between individual differences in structural volume in these ROI’s and individual differences in self-reported anxiety and affective instability. Here, we found that, in right amygdala, greater gray matter volume was associated with greater state anxiety in both BPD patients and AvPD patients, but not in healthy controls. While a similar positive relationship between amygdala size and anxiety levels has been reported in major depressive disorder patients alone (MDD patients; Weniger et al., 2006), prior work involving BPD patients that also reported this positive correlation with amygdala size examined depressive symptom severity rather than anxiety (Depping et al., 2015; Zetzsche et al., 2006). Thus, to our knowledge this is the first demonstration of this relationship between increasing amygdala size and increasing anxiety levels in BPD patients alone, as well as the first in AvPD patients.
This group difference between BPD patients and healthy controls manifested itself in a marginal group-by-gray matter volume interaction in predicting state-related anxiety. Importantly, this interaction suggests that while gray matter volume in right amygdala is not associated with state-related anxiety in healthy controls—commensurate with the fact that healthy controls showed relatively low anxiety overall— having relatively low gray matter volume in right amygdala may represent a protective factor in BPD patients, mitigating the group tendency toward greater self-reported anxiety in BPD patients relative to healthy controls overall.
In left amygdala, however, a different pattern was observed, albeit in relation to affective instability rather than state-related anxiety. Here, greater left amygdala volume predicted greater affective instability in healthy controls alone, rather than in the two patient groups. While laterality differences in the functional specialization of the amygdala are not entirely clear, as both right and left amygdala are associated with negative affective experience (Buhle et al., 2014; Ochsner et al., 2012), there is some evidence to suggest that right amygdala may be more engaged than left amygdala in making quick, broad (Markowitsch, 1999) and situationally-relevant (Davidson, 2002) assessments of threat. Thus, this is consistent with the fact that right amygdala showed significant correlations with a situationally-relevant variable (state-related anxiety) in patients groups for whom anxiety is elevated. However, in left amygdala, reduction in structural volume in BPD patients was not shown to be associated with mitigation of affective instability, a personality variable consistently elevated in—and particularly associated with the phenomenology of—BPD patients.
Finally, we found that, in healthy controls, MPFC volume was inversely predictive of both state and trait-related anxiety, and bilateral DLPFC volume was inversely predictive of affective instability. However, these relationships were not observed in either BPD or AvPD patients. This result may further contextualize the volumetric group difference noted above wherein healthy controls showed larger MPFC volume overall. Indeed, in addition to showing greater gray matter volume overall relative to BPD and AvPD patients in a region associated with cognitive control, healthy controls further showed evidence of a more adaptive relationship between structure and function in this same region relative to BPD and AvPD patients. These results may reflect the fact that, even in the event that BPD patients or AvPD patients possess relatively large amounts of gray matter in MPFC or DLPFC, this neural architecture is not being effectively or efficiently utilized in the service of maintaining emotional equilibrium. In addition the capacity of healthy controls to engage MPFC and DLPFC in emotion regulation may explain the finding that state-related anxiety is independent of amygdala volume in healthy controls. A promising future direction involves the development of cognitive interventions that target more effective and efficient functional recruitment of MPFC and DLPFC, which may compensate for reduced gray matter to help alleviate deficits in emotion regulation.
Regarding limitations of the present results, current sample sizes precluded our ability to fully examine whether the present volume-symptom correlations are modulated by gender in each diagnostic group. If found among borderline patients, such effects may further clarify extant discrepancies across studies with different gender distributions. Further, several of the current within-group volume-symptom correlations did not yield significant differences in correlation coefficients across groups (or of significant group-by-gray matter volume interactions). While this does not diminish the within-group results, caution should be taken in extrapolating group differences. Future work and meta-analyses utilizing larger sample sizes may help resolve this ambiguity.
In summary, the neurobiological basis of BPD and AvPD involves abnormalities of brain structure as well as neural and behavioral function. In the present work we have provided evidence for what may constitute part of this basis: namely, abnormalities in MPFC and ACC volume as well as anomalous relationships between amygdala, MPFC, and DLPFC structure and disorder-specific symptoms. Our findings are consistent with the idea that in BPD and AvPD right amygdala volume is associated with state anxiety, with little downregulatory influence by the MPFC, whereas in healthy controls MPFC and DLPFC may play a role in compensating for the effect of amygdala volume upon state anxiety and affective instability. Ultimately, understanding the key nodes of an anomalous neural network in BPD and AvPD patients may lead to the development of more effective cognitive and behavioral treatments for these disorders.
Supplementary Material
Highlights.
We investigate structural brain volumes in personality disorder patients.
Borderline patients show relatively reduced medial prefrontal cortex (MPFC) volume.
Borderline and avoidant patients show positive amygdala volume-anxiety correlations.
Healthy controls but not patients show a negative MPFC volume-anxiety correlation.
The results suggest potentially compensatory aspects of structural brain volume.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American_Psychiatric_Association. Diagnostic and Statistical Manual of mental Disorders. Fifth. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry research. 2004;131:125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carpenter RW, Trull TJ. Components of emotion dysregulation in borderline personality disorder: a review. Current psychiatry reports. 2013;15:335. doi: 10.1007/s11920-012-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in cognitive sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of cognitive neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Denny BT, Fan J, Liu X, Ochsner KN, Guerreri S, Mayson SJ, Rimsky L, McMaster A, New AS, Goodman M, Siever LJ, Koenigsberg HW. Elevated amygdala activity during reappraisal anticipation predicts anxiety in avoidant personality disorder. Journal of affective disorders. 2015;172:1–7. doi: 10.1016/j.jad.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of cognitive neuroscience. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Silvers JA, Ochsner KN. How we heal what we don’t want to feel: The functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. Guilford Press; New York: 2009. pp. 59–87. [Google Scholar]
- Depping MS, Wolf ND, Vasic N, Sambataro F, Thomann PA, Christian Wolf R. Specificity of abnormal brain volume in major depressive disorder: A comparison with borderline personality disorder. Journal of affective disorders. 2015;174:650–657. doi: 10.1016/j.jad.2014.11.059. [DOI] [PubMed] [Google Scholar]
- Diaz-de-Grenu LZ, Acosta-Cabronero J, Pereira JM, Pengas G, Williams GB, Nestor PJ. MRI detection of tissue pathology beyond atrophy in Alzheimer’s disease: introducing T2-VBM. NeuroImage. 2011;56:1946–1953. doi: 10.1016/j.neuroimage.2011.03.082. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of general psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. Journal of psychiatric research. 2011;45:803–807. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human brain mapping. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. Journal of clinical psychology. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature reviews. Neuroscience. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, Rimsky L, New AS, Goodman M, Siever LJ. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. The American journal of psychiatry. 2014;171:82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, New A, Siever LJ. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, New AS, Goodman M, Silverman J, Serby M, Schopick F, Siever LJ. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? Journal of personality disorders. 2001;15:358–370. doi: 10.1521/pedi.15.4.358.19181. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Current psychiatry reports. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- Kuhlmann A, Bertsch K, Schmidinger I, Thomann PA, Herpertz SC. Morphometric differences in central stress-regulating structures between women with and without borderline personality disorder. Journal of psychiatry & neuroscience : JPN. 2013;38:129–137. doi: 10.1503/jpn.120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labudda K, Kreisel S, Beblo T, Mertens M, Kurlandchikov O, Bien CG, Driessen M, Woermann FG. Mesiotemporal volume loss associated with disorder severity: a VBM study in borderline personality disorder. PloS one. 2013;8:e83677. doi: 10.1371/journal.pone.0083677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human brain mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain. Simon & Schuster; New York: 1996. [Google Scholar]
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Differential contribution of right and left amygdala to affective information processing. Behavioural Neurology. 1999;11:233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic structural changes in borderline personality disorder. Journal of psychiatric research. 2008;42:727–733. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedtfeld I, Schulze L, Krause-Utz A, Demirakca T, Bohus M, Schmahl C. Voxel-based morphometry in women with borderline personality disorder with and without comorbid posttraumatic stress disorder. PloS one. 2013;8:e65824. doi: 10.1371/journal.pone.0065824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E, Gambini F, Isola M, De Bona M, Rambaldelli G, d’Allio G, Barale F, Zappoli F, Brambilla P. Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. Journal of affective disorders. 2011;131:417–421. doi: 10.1016/j.jad.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Bartolini E, Zoloth E. Avoidant Personality Disorder. In: Ramachandran V, editor. Encyclopedia of Human Behavior. 2nd. Academic Press; San Diego, CA: 2012. pp. 257–266. [Google Scholar]
- Schmahl C, Berne K, Krause A, Kleindienst N, Valerius G, Vermetten E, Bohus M. Hippocampus and amygdala volumes in patients with borderline personality disorder with or without posttraumatic stress disorder. Journal of psychiatry & neuroscience : JPN. 2009;34:289–295. [PMC free article] [PubMed] [Google Scholar]
- Schmahl C, Bremner JD. Neuroimaging in borderline personality disorder. Journal of psychiatric research. 2006;40:419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Schmahl C, Niedtfeld I. Neural Correlates of Disturbed Emotion Processing in Borderline Personality Disorder: A Multimodal Meta-Analysis. Biological psychiatry. 2016;79:97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological psychiatry. 2002a;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Siever LJ, Livesley WJ, Gunderson JG, Pfohl B, Widiger TA. The borderline diagnosis II: biology, genetics, and clinical course. Biological psychiatry. 2002b;51:951–963. doi: 10.1016/s0006-3223(02)01325-2. [DOI] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry research. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory (Self-Evaluation Questionnaire) Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of personality disorders. 2014;28:734–750. doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of affective disorders. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Frodl T, Preuss UW, Schmitt G, Seifert D, Leinsinger G, Born C, Reiser M, Moller HJ, Meisenzahl EM. Amygdala volume and depressive symptoms in patients with borderline personality disorder. Biological psychiatry. 2006;60:302–310. doi: 10.1016/j.biopsych.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Munchhausen E, Tabrizi S, Leinsinger G, Born C, Reiser M, Moller HJ, Meisenzahl EM. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry research. 2007;154:157–170. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


