Abstract
Background
Underweight defined as body mass index (BMI) < 18.5 is associated with negative health and quality of life outcomes including mortality. Yet, little is known about the socioeconomic differentials in underweight and its association with health and well-being among older adults in India. This study examined the socioeconomic differentials in underweight among respondents aged ≥50 in India. Consequently, three outcomes of the association of underweight were studied. These are poor self-rated health, cognition and quality of life.
Methods
Cross-sectional data on 6,372 older adults derived from the first wave of the WHO’s Study on global AGEing and adult health (SAGE), a nationally representative survey conducted in six states of India during 2007–8, were used. Bivariate and multivariate regression analyses were applied to fulfil the objectives.
Results
The overall prevalence of underweight was 38 percent in the study population. Further, socioeconomic status showed a significant and negative association with underweight. The association of underweight with poor self-rated health (OR = 1.60; p < .001), cognition (β = –0.95; p < .001) and quality of life (β = –1.90; p < .001) were remained statistically significant after adjusting for age, sex, place of residence, marital status, years of schooling, wealth quintile, sleep problems, chronic diseases, low back pain and state/province.
Conclusion
The results indicated significant socioeconomic differentials in underweight and its association with poor self-rated health, cognition and quality of life outcomes. Interventions focussing on underweight older adults are important to enhance the overall wellbeing of the growing older population in India.
Introduction
Despite rapid economic growth in India in recent decades, the nutritional status of the adult population remains poor [1]. On the one hand India is experiencing a steady rise in the prevalence of overweight and obesity [2], which is globally recognized as a strong predictor of all-cause mortality and chronic diseases such as diabetes, cardiovascular diseases and hypertension. On the other hand, India has the highest number of underweight adults in the share of global adult underweight [2, 3]. The coexistence of undernutrition and overnutrition is referred as the dual burden of malnutrition, which commonly occurs in developing countries and poses a major public health challenge [4, 5, 6, 7, 8, 9].
Both underweight and overweight are associated with increased risks of poor health and mortality. The potential health risks owing to overweight and obesity in western countries are well documented. Further, in recent years, many studies have investigated the health risks of over-nutrition across the globe. Overweight and obesity are strongly associated with all-cause mortality [10, 11, 12], chronic diseases [13, 14], disabilities, poor self-rated health [15, 16, 17, 18, 19, 20], poor health-related quality of life and cognition [21, 22, 23].
Little is known about the nature and patterns of underweight and its subsequent health implications. Globally, undernutrition contributes 16 percent to the disability-adjusted life year (DALY) [24]. Further, the impact of lower body mass index on health is stronger in developing countries. Lower body mass index is associated with increased risk of excess mortality [11, 25, 26], poor cognition [27], poor self-rated health and health-related quality of life [28, 29]. While 35 percent older adults aged 50 and above in India are underweight [30], the association between underweight and subsequent health outcomes is less known in India. A few studies examined the association of low body mass index with mortality [25, 26], poor self-rated health [31] and morbidities [32]. However, these studies used micro-level, hospital-based, region-specific data sets. Also, most of them examined the pattern of undernutrition in India focussing on the younger population (15–49). The extent of undernutrition among the older population has been less documented. Therefore, it is less clear to what extent underweight differs by socioeconomic status among older adults in the country.
Using the SAGE data, the present study examined the socioeconomic pattern of underweight among older adults in India. Further, the association of underweight was examined with three outcomes: self-rated health, cognition and quality of life. Previous literature from India among middle-aged and older populations demonstrates that underweight is concentrated in adults of low socioeconomic status [9, 32, 33, 34, 35, 36, 37] and rural residents [38, 39, 40]. Despite the fact that overweight and obesity are strong predictors of mortality in other parts of the world, in Asian countries such as India and China excess mortality owing to underweight is seen higher [25, 41, 42, 43].
In this context, understanding the association of underweight with self-rated health and other outcomes will provide a better insight into the health implications of underweight and will be useful in policy perspectives. To our knowledge, there is no study which has examined the association of underweight with various health outcomes in India. The outcome variables—poor self-rated health, measures of cognition and quality of life—that were employed in the present study are frequently used in aging and epidemiological surveys. Existing literature strongly suggests that the measure of self-rated health is considered as a global measure of overall health which is strongly associated with other health outcomes such as disability and mortality [28, 44, 45]. Further, measures of cognition and quality of life are important components of physical well-being and major contributors to the overall well-being of the older population [46, 47, 48, 49].
We used nationally representative data on 6,372 Indians aged 50 and above for this analysis which was collected during 2007–8 from six states of India. As the share of the older population in India is growing faster, mainly due to the decline in fertility and mortality, it is necessary to understand the contribution of nutrition-related health implications, which will help policymakers to identify and improve the overall health and well-being of the growing older population in India.
Materials and methods
Data
The present study used the first wave data set of the SAGE survey conducted between the years 2007 and 2008. This was the first longitudinal study carried out by the WHO on health and ageing in multiple low- and middle-income countries including India. The target population in the SAGE survey was individuals above 18 years of age. A multistage, stratified clustered sample design was used homogeneously in all countries included in the SAGE. The survey comprised nationally representative samples and yielded results that are comparable to those of similar ageing surveys in high-income countries. This is the latest available data which includes detailed information on health behaviour, use of health services and health outcomes along with a varied set of socioeconomic items from a nationally representative household population aged above 50 in India.
The total sample for the SAGE survey was 12,198. However, we considered the data of 6,372 older adults aged ≥50 in India. The survey encompassed a wide range of geographic and socioeconomic variabilities. Face-to-face interviews were conducted to collect information about the physical characteristics of the dwelling/household as well as to develop a household roster, including sex, age, education, marital status and care needs of each household member. The health status of individuals was also assessed with cognition, quality of life and other tests. A short set of cognition tests measured concentration, attention and memory, which provides an estimate of cognitive ability and its impact on health status (for example, dementia). In biomarker components, various tests were conducted to assess the prevalence of chronic diseases. Further, physical tests and biomarker measures such as walking speed, lung function test and grip strength were conducted. A detailed description about the survey is given in Kowal et al. 2012 [50].
Ethics and consent
The SAGE study was approved by the Ethics Review Committee, World Health Organization, Geneva, Switzerland and the Institutional Review Board, International Institute of Population Sciences, Mumbai, India. Informed written consent was obtained from each participant, who were ensured that data would remain confidential and used for research purposes only.
Methods
Body mass index (BMI)
BMI Calculation: BMI was calculated as weight in kilograms divided by height in metres squared. In this study, objectively measured height and weight were used to calculate BMI. Further, it was categorized as < 18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), 30.0+ kg/m2 (obesity).
Measures of socioeconomic status
In this study years of schooling and wealth quintile have been included as the measures of socioeconomic status to examine their association with underweight. Years of schooling was categorized as ‘no schooling’, ‘1–5 years’, ‘6–9 years’ and ‘10 years or above’. A composite wealth index was generated based on household ownership of assets. Principal component analysis was used to create the composite index and categorized as first (lowest), second, third, fourth and fifth (highest) with cut-off points of 20% quintile each [51]. List of variables used to construct wealth quintile is provided in the supplementary file (see S1 File).
Self-rated health (SRH)
This study used self-rated health as one of the outcome variables. In SAGE, self-rated health was assessed on a five-point scale with the following question: In general, how would you rate your health today? The response categories were: ‘very good’, ‘good’, ‘moderate’, ‘bad’ and ‘very bad’. In the analysis, ‘bad’ and ‘very bad’ health categories were combined to represent poor self-rated health.
Cognitive score index
To understand the composite effect of cognition we made a cognitive index combining four variables: verbal fluency, verbal recall, digit span forward and digit span backward.
Different cognition tests and procedure used in the survey are;
Verbal recall: Interviewer read out a list of 10 commonly used words to the respondents and asked them to repeat again in some time.
Digit span (forward and backward): Participants were read a series of digits and asked to immediately repeat them back. In the backward test, the person must repeat the numbers in reverse order. These tests measure concentration, attention, and immediate memory.
Verbal fluency: Participants were asked to produce as many animal names as possible in one-minute time span. This test assessed retrieval of information from semantic memory.
The composite index was derived using Principal Components Analysis (PCA), a mathematical tool which helps in creating a composite index using uncorrelated components, where each component captures the largest possible variation in the original variables. Selected raw scores for cognitive tasks were bundled into three domains (digit span, memory and executive functioning) to yield compound cognitive scores. This was done to condense the number of cognitive variables while refining the robustness of the underlying cognitive construct [52]. We followed two steps to make a cognitive index:
Step 1: All four variables were in different scales. So first, we standardized these variables. A standardized variable (sometimes called a z-score or a standard score) is a variable that has been rescaled to have a mean of zero and a standard deviation of one. Each case's value on the standardized variable designates its difference from the mean of the primary variable in some standard deviations (of the original variable).
Step 2: PCA is a multivariate statistical technique used for extracting from a set of variables those few orthogonal linear combinations that capture the common information most successfully [53]. Further, this index comprises both values, positive and negative. So we converted this index into a 0–100 scale which facilitates easier interpretation of the data. Higher scores indicate better cognitive abilities.
Quality of life index (WHO-QoL)
We used the quality of life questionnaire (S-QoL 30) from the WHO-SAGE data set, which was a particular, self-administered and multidimensional QoL questionnaire designed for people. It included 30 items describing five dimensions: physical health, psychological health, level of independence, social relation and environment. It also included a total score (Index). The five dimensions and the Index score ranged from 0 to 100; higher scores indicated better quality of life [49, 54].
Covariates
The selected demographic and health risk factors were: age (50–59, 60–64, 65–69, 70–79, and ≥80), sex (male and female), place of residence (urban and rural), marital status (currently married and otherwise). We have also included self-reported sleep problems, low back pain, chronic diseases such as hypertension, diabetes, angina, stroke, arthritis and asthma as risk factors of general health and quality of life [18, 28, 55, 56, 57].
Sleep problems: Presence of insomnia symptoms such as difficulty in falling asleep, difficulty staying asleep, or early wakening were assessed in WHO-SAGE survey with the following question. ‘Overall in the last 30 days, how much of a problem did you have with sleeping, such as falling asleep, waking up frequently during the night or waking up too early in the morning?’ The responses were: ‘none’, ‘mild’, ‘moderate’, ‘severe’ and ‘extreme/cannot do’. We combined ‘severe’ and ‘extreme/cannot do’ to represent sleep problems.
In SAGE Survey, the self-reported prevalence of chronic diseases and back pain were assessed through following questions;
Have you ever been diagnosed with high blood pressure (hypertension)? (Yes, No)
Have you ever been diagnosed with diabetes (high blood sugar)? (Yes, No)
Have you ever been diagnosed with angina or angina pectoris (a heart disease)? (Yes, No)
Have you ever been told by a health professional that you have had a stroke? (Yes, No)
Have you ever been diagnosed with/told you have arthritis (a disease of the joints, or by other names rheumatism or osteoarthritis)? (Yes, No)
Have you ever been diagnosed with asthma (an allergic respiratory disease)? (Yes, No)
Have you experienced back pain during the last 30 days? (Yes, No)
We also included state/province variable as India is experiencing regional variations in socioeconomic development, demographic and health transition [58, 59, 60]. In SAGE survey, six states were included which consists of Assam, Karnataka, Maharashtra, Rajasthan, Uttar Pradesh and West Bengal.
Statistical analysis
First, descriptive statistics were calculated by different BMI categories, demographic and socioeconomic variables (Table 1). Second, bivariate analysis was carried out to understand the prevalence of poor self-rated health, mean cognition score and quality of life score by body mass index category.
Table 1. Sociodemographic characteristics of the study population by body mass index category (Weighted %).
| Characteristics | BMI (Kg/M2) Category | |||
|---|---|---|---|---|
| <18.5 (Underweight) | 18.5–24.9 (Normal Weight) | 25.0–29.9 (Overweight) | >30.0 (Obesity) | |
| Age group | ||||
| 50–59 | 33.7 | 50.3 | 13.0 | 2.8 |
| 60–64 | 39.6 | 48.1 | 9.8 | 2.3 |
| 65–69 | 39.3 | 50.0 | 7.8 | 2.7 |
| 70–79 | 49.0 | 41.0 | 7.7 | 2.1 |
| 80+ | 55.8 | 36.0 | 4.4 | 3.6 |
| Sex | ||||
| Male | 39.9 | 50.1 | 8.0 | 1.8 |
| Female | 37.6 | 45.5 | 13.2 | 3.6 |
| Residence | ||||
| Urban | 28.6 | 49.1 | 18.4 | 3.7 |
| Rural | 42.8 | 47.3 | 7.4 | 2.3 |
| Marital status | ||||
| Currently married | 37.0 | 49.7 | 10.7 | 2.6 |
| Otherwise | 45.2 | 41.8 | 10.0 | 3.0 |
| Wealth quintile | ||||
| Lowest | 56.1 | 40.2 | 2.5 | 1.1 |
| Second | 46.6 | 44.0 | 8.1 | 1.1 |
| Middle | 42.0 | 46.6 | 9.3 | 1.8 |
| Fourth | 32.9 | 54.7 | 10.3 | 1.9 |
| Highest | 21.9 | 51.8 | 19.8 | 6.3 |
| Schooling (Years) | ||||
| No schooling | 46.3 | 44.5 | 6.8 | 2.3 |
| 1–5 years | 36.8 | 47.2 | 13.6 | 2.2 |
| 6–9 years | 29.4 | 56.3 | 12.5 | 1.6 |
| 10 years or above | 22.2 | 54.9 | 17.1 | 5.6 |
| State | ||||
| Assam | 41.0 | 50.9 | 6.2 | 1.8 |
| Karnataka | 28.6 | 50.8 | 16.4 | 4.1 |
| Maharashtra | 31.5 | 55.3 | 11.2 | 1.7 |
| Rajasthan | 33.7 | 49.9 | 12.5 | 3.7 |
| Uttar Pradesh | 46.9 | 42.1 | 8.4 | 2.4 |
| West Bengal | 42.2 | 45.3 | 9.5 | 2.9 |
| Total | 38.2 | 48.2 | 10.7 | 2.7 |
Multivariate regression analysis
The study was carried out with a multinomial logistic regression for simultaneous examination of differentials and determinants of body mass index by demographic and socioeconomic characteristics. Multinomial logistic regression is an often used standard statistical tool, particularly when more than four discrete outcomes are needed (such as normal weight, underweight, overweight and obesity). In the first stage of this model, we estimated beta coefficients for the four discrete outcomes by taking ‘normal weight’ as the reference category. The mathematical form of multinomial regression models can be written as:
Where
β01 to β03: Constant
β11 to β36: Multinomial Regression Coefficient and ref = Reference
Further, binary logistic regression was used to assess the association of underweight with poor self-rated health with covariates on socio-demographic characteristics, health markers, low back pain, sleep problems and state/province. The binary response (0 = good health and 1 = poor health) for each was related to a set of categorical predictors, X (BMI, age group, sex, residence, marital status, years of schooling, wealth quintile, sleep problems, health markers and state/province).
The logit model was formulated for analysis as:
We used different models to better understand the association of each covariate with poor self-rated health (see S2 File). Model 1, includes only BMI; model 2, includes demographic variables; model 3, includes socioeconomic variables; model 4, includes self-reported sleep problems, chronic diseases and low back pain; model 5, includes state/province variable.
Finally, we used the linear regression model [61] to examine the association of underweight with cognition and quality of life, which can be specified as:
Where
β0: Constant, β1 to β10 regression coefficient and i = stand for the individual; also Ai = BMI, Bi = age group, Ci = sex, Di = residence, Ei = marital status, Fi = education level, Gi = wealth quintile, Hi = sleep problems and Ji = states and Ki = health markers
We used five different models to better understand the association of each covariate with cognition and quality of life as described previously. All the statistical analysis of this study was performed using STATA version 12.0 (Stata Corp LP, College Station, TX, USA).
Results
Table 1 presents the characteristics of the study population by BMI category. This study used data on respondents of 50 years and above with a total sample of 6,372. The overall prevalence of underweight was 38.2 percent. The percentages of overweight and obesity in the study population were 10.7 and 2.7 respectively. The sample distribution among different age groups by BMI category indicated that a higher share of older adults were underweight in the 80+ category. More than half of the respondents in the age group 50–59 were in the normal weight category. Compared to women (37.6 percent), a higher proportion of men were in the underweight category (39.9 percent). On the other hand, the distribution of women respondents was higher in the overweight and obesity categories compared to men respondents. In rural areas 42.8 percent older adults were underweight compared to 28.6 percent in urban areas. A higher proportion of older adults in the overweight and obesity categories was from urban areas. The distribution of underweight varied considerably by marital status. Increase in years of schooling and wealth associated with lower prevalence of underweight.
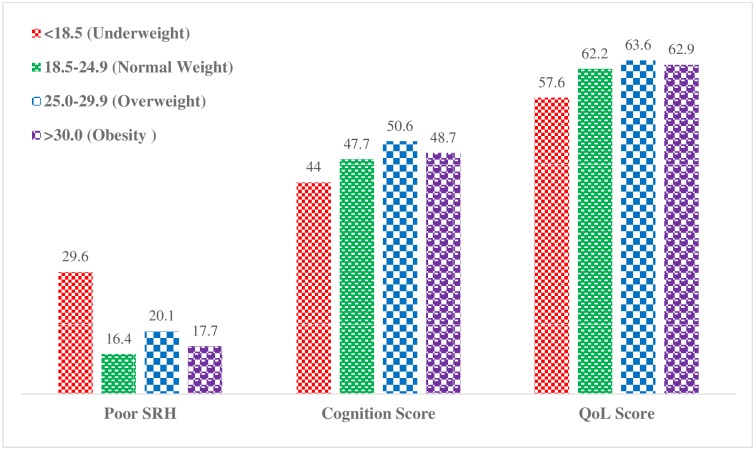
Fig 1 presents the prevalence of poor self-rated health, mean cognition score and quality of life score by BMI category. The prevalence of poor self-rated health was higher among older adults in the underweight category (29.6 percent) than normal weight (16.4 percent), overweight (20.1 percent) and obesity categories (17.7 percent). Similarly, the mean cognition and quality of life scores were lower among underweight older adults than normal, overweight and obese older adults.
Fig 1. Weighted prevalence of poor self-rated health (SRH), mean cognition and quality of life (QoL) scores by body mass index category.
The results of multinomial regression analysis is shown in Table 2 by coefficient and level of significance. According to the table, the probability of being underweight or obese compared to normal weight increases with age. Compared to men, older women were more likely to be obese (β = 3.66; p < .001). Further, older adults in rural areas were more likely to be underweight than the urban counterparts (β = 1.44; p < .005). It was also evident that the older adults belonging to highest wealth quintile were less likely to be underweight (p < .001) compared to those from the lowest wealth quintile (p < .001). Further, years of schooling was positively associated with overweight and obesity. Older adults in Uttar Pradesh were more likely to be underweight as compared to the reference group Assam, whereas older adults in Rajasthan were more likely to be obese.
Table 2. Multinomial logistic regression coefficient and confidence interval of BMI by socioeconomic and demographic characteristics.
| Background | 18.5–24.9(ref) (Normal Weight) | <18.5 (Underweight) | 25.0–29.9 (Overweight) | >30.0 (Obesity) | |||
|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | ||
| Age group | |||||||
| 50-59(ref) | |||||||
| 60–64 | 1.14** | (0.97, 1.34) | 0.75 | (0.59, 0.95) | 0.84* | (0.56, 1.26) | |
| 65–69 | 1.26** | (1.06, 1.48) | 0.68** | (0.53, 0.88) | 0.79* | (0.50, 1.23) | |
| 70–79 | 1.59*** | (1.36, 1.88) | 0.75 | (0.58, 0.96) | 0.94** | (0.59, 1.46) | |
| 80+ | 2.08*** | (1.58, 2.74) | 0.56 | (0.33, 0.96) | 1.28** | (0.64, 2.53) | |
| Sex | |||||||
| Male(ref) | |||||||
| Female | 0.91 | (0.80, 1.03) | 2.07** | (1.69, 2.52) | 3.66*** | (2.48, 5.39) | |
| Residence | |||||||
| Urban(ref) | |||||||
| Rural | 1.44** | (1.24, 1.68) | 0.65 | (0.54, 0.78) | 0.73* | (0.11, 0.99) | |
| Years of schooling | |||||||
| No schooling(ref) | |||||||
| 1–5 years | 0.84** | (0.73, 0.97) | 1.71 | (1.36, 2.14) | 1.67** | (1.11, 2.49) | |
| 6–9 years | 0.62* | (0.48, 0.77) | 1.53* | (1.12, 2.07) | 0.96* | (0.49, 1.86) | |
| 10 years or above | 0.52*** | (0.40, 0.64) | 1.78** | (1.33, 2.37) | 2.59** | (1.56, 4.27) | |
| Wealth quintile | |||||||
| Lowest(ref) | |||||||
| Second | 0.73*** | (0.61, 0.87) | 1.25 | (0.80, 1.94) | 0.86*** | (0.42, 1.75) | |
| Middle | 0.62* | (0.51, 0.74) | 1.97 | (1.30, 2.96) | 1.29** | (0.67, 2.45) | |
| Fourth | 0.46*** | (0.38, 0.55) | 2.02** | (1.34, 3.02) | 1.08** | (0.57, 2.02) | |
| Highest | 0.34*** | (0.41, 0.64) | 3.16*** | (2.11, 4.71) | 2.31*** | (1.26, 4.23) | |
| State | |||||||
| Assam(ref) | |||||||
| Karnataka | 0.73*** | (0.57, 0.92) | 2.20*** | (1.49, 3.25) | 2.38 | (1.16, 4.84) | |
| Maharashtra | 0.72** | (0.57, 0.90) | 1.89 | (1.29, 2.78) | 1.62 | (0.78, 3.33) | |
| Rajasthan | 0.75* | (0.60, 0.93) | 2.11 | (1.43, 3.09) | 2.61*** | (1.29, 5.27) | |
| Uttar Pradesh | 1.22** | (0.98, 1.51) | 1.72 | (1.15, 2.55) | 2.53* | (1.24, 5.15) | |
| West Bengal | 0.91** | (0.72, 1.12) | 1.76 | (1.19, 2.58) | 1.81* | (0.88, 3.73) | |
| Sample Size = 6332 | Pseudo R2 = 0.0844 | ||||||
β = coefficient value; ref = reference;
*** Significant at p < .001,
** Significant at p < .005,
* Significant at p < .01
Table 3 presents the association of underweight with poor self-rated health, cognition and quality of life after adjusting for relevant covariates. The association of underweight with all outcomes were significant. Compared with normal weight, older adults in the underweight category were 1.60 (p < .001) times more likely to report poor self-rated health. Similarly, underweight older adults experienced reduced cognition (β = -0.95; p < .001) and quality of life scores (β = -1.90; p < .001) compared to normal weight counterparts. Further, the association between education and self-rated health was significant. Demographic factors such as age, female gender and rural residence had negative association with the cognition score. Older adults in currently not married category had lower cognition and quality of life scores. The association of socioeconomic status and cognition were stronger; especially older adults who had 10 or above years of schooling had a score 13.97 (p < .001) points higher than older adults with no formal schooling. Similarly, wealth quintile was strongly associated with quality of life; older adults in the highest wealth quintile category had a score 8.41 (p < .001) points higher than older adults in the poorest wealth quintile category. Self-reported chronic diseases and low back pain were strongly associated with poor self-rated health and quality of life. The negative association of sleep problems with all three outcomes was significant and stronger.
Table 3. Multivariable regression analysis of the association of underweight with poor self-rated health, cognition and quality of life.
| Background | Poor self-rated health | Cognition | Quality of Life | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | β | 95% CI | β | 95% CI | |
| BMI | ||||||
| Underweight | 1.60*** | (1.37, 1.87) | -0.95*** | (-1.46, -0.45) | -1.90*** | (-2.52, -1.29) |
| Normal(ref) | ||||||
| Overweight | 1.01 | (0.77, 1.31) | 1.30*** | (0.56, 2.04) | 0.33 | (-0.56, 1.24) |
| Obese | 1.06 | (0.69, 1.63) | 0.60 | (-0.67, 1.89) | -0.06 | (-1.63, 1.49) |
| Age group | ||||||
| 50-59(ref) | ||||||
| 60–64 | 1.22** | (1.00, 1.50) | -1.09*** | (-1.71, -0.47) | -0.95** | (-1.71, -0.20) |
| 65–69 | 1.51*** | (1.23, 1.85) | -1.31*** | (-1.96, -0.65) | -1.80*** | (-2.60, -1.00) |
| 70–79 | 2.19*** | (1.79, 2.68) | -2.86*** | (-3.54, -2.19) | -2.91*** | (-3.72, -2.09) |
| 80+ | 2.81*** | (2.07, 3.82) | -4.86*** | (-6.02, -3.69) | -4.74*** | (-6.11, -3.37) |
| Sex | ||||||
| Male(ref) | ||||||
| Female | 0.99 | (0.84, 1.18) | -3.43*** | (-3.96, -2.89) | -0.54 | (-1.19, 0.10) |
| Residence | ||||||
| Urban(ref) | ||||||
| Rural | 1.07 | (0.88, 1.29) | -0.48* | (-1.05, 0.07) | 0.67* | (-0.01, 1.36) |
| Marital status | ||||||
| Currently married(ref) | ||||||
| Otherwise | 1.13 | (0.95, 1.33) | -1.11*** | (-1.67, -0.54) | -1.21*** | (-1.89, -0.52) |
| Years of schooling | ||||||
| No schooling(ref) | ||||||
| 1–5 years | 0.78*** | (0.65, 0.94) | 6.25*** | (5.66, 6.84) | 1.25*** | (0.53, 1.97) |
| 6–9 years | 0.61*** | (0.45, 0.82) | 9.86*** | (9.00, 10.7) | 2.98*** | (1.93, 4.03) |
| 10 years or above | 0.54*** | (0.40, 0.73) | 13.97*** | (13.1, 14.8) | 5.28*** | (4.27, 6.29) |
| Wealth quintile | ||||||
| Lowest(ref) | ||||||
| Second | 1.04 | (0.83, 1.29) | 1.12*** | (0.36, 1.89) | 1.91*** | (0.99, 2.83) |
| Middle | 1.04 | (0.83, 1.30) | 2.02*** | (1.25, 2.79) | 3.75*** | (2.81, 4.68) |
| Fourth | 0.93 | (0.73, 1.18) | 2.51*** | (1.74, 3.29) | 5.00*** | (4.06, 5.94) |
| Highest | 0.78* | (0.61, 1.01) | 3.92*** | (3.11, 4.74) | 8.41*** | (7.42, 9.40) |
| Health markers (Ref: No) | ||||||
| Sleep problems | 4.37*** | (3.68, 5.19) | -1.39*** | (-2.07, -0.71) | -7.09*** | (-7.91, -6.27) |
| Hypertension | 1.48*** | (1.23, 1.78) | -0.24 | (-0.87, 0.37) | -1.63*** | (-2.39, -0.87) |
| Diabetes | 1.31* | (0.99, 1.73) | 0.89** | (0.01, 1.78) | -1.08** | (-2.16, -0.003) |
| Angina | 1.19 | (0.88, 1.62) | 0.19 | (-0.85, 1.24) | -0.73 | (-2.02, 0.54) |
| Stroke | 1.28 | (0.84, 1.95) | 0.39 | (-1.18, 1.98) | -2.97*** | (-4.83, -1.10) |
| Arthritis | 1.71*** | (1.44, 2.04) | -1.28*** | (-1.88, -0.67) | -2.40*** | (-3.13, -1.66) |
| Asthma | 2.01*** | (1.58, 2.54) | -0.07 | (-0.96, 0.81) | -4.65*** | (-5.72, -3.58) |
| Back pain | 1.76*** | (1.52, 2.04) | 0.01 | (-0.46, 0.49) | -2.85*** | (-3.43, -2.27) |
| State | ||||||
| Assam(ref) | ||||||
| Karnataka | 0.16*** | (0.11, 0.21) | 0.71 | (-0.23, 1.66) | 5.25*** | (4.09, 6.41) |
| Maharashtra | 0.31*** | (0.23, 0.40) | 1.75*** | (0.85, 2.65) | 5.43*** | (4.33, 6.54) |
| Rajasthan | 0.29*** | (0.22, 0.38) | 1.42*** | (0.54, 2.30) | 5.03*** | (3.96, 6.10) |
| Uttar Pradesh | 0.55*** | (0.43, 0.71) | 0.96** | (0.09, 1.83) | 7.08*** | (6.02, 8.14) |
| West Bengal | 0.97 | (0.76, 1.23) | 0.06 | (-0.81, 0.95) | -2.89*** | (-3.97, -1.81) |
| Pseudo R2 | 0.1909 | |||||
| Adjusted R2 | 0.4086 | 0.3257 | ||||
| Sample Size | 6330 | 6164 | 6330 | |||
OR = Odds Ratio; β = coefficient value; CI = Confidence Interval; ref = reference;
*** Significant at p < .001,
** Significant at p < .005,
* Significant at p < .01
Discussion and conclusion
This study examined the socioeconomic patterns of underweight among older adults aged ≥50 in India. Subsequently, we studied the association of underweight with poor self-rated health, cognition and quality of life outcomes. From this nationally representative and cross-sectional data, we found a strong socioeconomic differential in underweight among older adults aged ≥50. Although, socioeconomic improvement is evident in India in recent decades, a larger proportion of older adults is underweight, especially those of low socioeconomic status. Underweight older adults were more likely to report poor self-rated health and had poor cognition and quality of life scores after adjusting for age, sex, place of residence, marital status, years of schooling, wealth quintile, sleep problems, low back pain, chronic diseases and state.
In this study, a large proportion (38 percent) of older adults aged 50 years and above are underweight. The result is consistent with previous literature which showed higher prevalence of nutritional deficiency among older population in South India [62]. Further, socioeconomic status was found to be strongly associated with underweight, as shown in previous studies conducted among younger and older adults [9, 32, 33, 34, 35, 36, 37]. A higher prevalence of underweight was found among less educated and older adults in the lower wealth quintile category. On the other hand, overweight and obesity were concentrated in wealthier and educated older adults, which suggested a dual burden of malnutrition among older adults characterized by socioeconomic status. Literature shows that higher economic status plays an important role in energy intake along with sedentary behaviours causing a higher body mass index [63, 64]. On the other hand, individuals in poor socioeconomic status are exposed to food insecurity, limited food choice, poor life course socioeconomic status and health risk behaviours, which are strongly associated with lower body mass index [65, 66, 67, 68, 69].
Furthermore, age is strongly associated with underweight which is consistent with prior literature [70]. Existing studies show that increasing age is correlated with various physiological and biological changes such as loss of muscle mass, absorption of iron and vitamin, height and body shape, which in turn lead to lower body mass index [71, 72, 73]. In addition, studies also found age as an important risk factor of change in dietary habits and reduced smell, taste and appetite which further may lead to lower food intake and body mass index [62, 74, 75]. Further, poor oral health condition in old age was found to be significant determinant of involuntary weight loss and being underweight [76, 77].
Most of the studies conducted in India and other Asian countries found a strong and positive association of underweight with excess mortality [11, 25, 26]. This study also supported the hypothesis by showing a strong association of underweight with all three outcomes (poor self-rated health, cognition and quality of life). Prior studies have found U shaped relationship between body mass index and poor self-rated health in United States [18]. In this study, being underweight was consistently associated with poor self-rated health across different models which is consistent with prior literature [28] whereas, overweight and obesity were not associated with poor self-rated health among older adults in India.
The association between lower body weight and quality of life was observed in the present study. Existing literature found similar findings. For example, Zhu et al. (2015) showed significantly lower quality of life scores among underweight adult population in China [29]. However, this study found no significant association between obesity and quality of life contrary to the previous literature [78]. Also, underweight older adults had poor cognitive abilities [27]. Similar to this result, Qizilbash et al. (2015) found that underweight during adulthood and old age is associated with increased risks of dementia and cognitive impairment [79]. On the other hand, older adults in overweight category had better cognitive abilities than normal weight counterparts as shown in other studies [80, 81].
Further, measures of socioeconomic status, such as years of schooling and wealth quintile strongly predicted self-rated health, cognition and quality of life. Previous studies conducted in India and other developing countries also had similar findings [82, 83, 84, 85]. Health markers such as sleep problems, chronic morbidities and low back pain strongly associated with self-rated health and quality of life [55, 56, 57].
While most of the developed and developing countries face the challenges of obesity and related health consequences. This study showing a higher prevalence of underweight in a developing country and its significant implications on poor self-rated health, cognition and quality of life is important to be considered from policy perspective. Also, in recent years, India is experiencing a growing problem of overweight and obesity leading to the dual burden of malnutrition. In addition to obesity-related interventions, special attention on diet and potential nutritional interventions should be given to underweight older adults to improve the overall health and quality of life.
Strengths and limitations
The strength of this study is that it used nationally representative data and therefore the findings can be generalized at the national level. To our knowledge, this is the first study to examine the association of underweight with various health outcomes among older adults in India. The association of underweight with poor self-rated health, cognition and quality of life were statistically significant. Furthermore, this study used a standardized questionnaire in assessing cognition and quality of life, which provides a comparable estimation of developing and developed countries. While most studies used self-reported height and weight to examine mortality and other health consequences of BMI, this study collected height and weight measurements by trained investigators, which is considered as a standard measure.
The results of this study must be interpreted considering a few limitations. Since the study used cross-sectional data, any causal relationship cannot be established. Further, we used self-reported health assessment such as self-rated health and other measures used in the quality of life scale. Self-reported measures are subject to reporting and cultural bias, especially in the context of developing countries [86]. Furthermore, the size of the effect is relatively small (e.g. see R2 values) compared to other predictors of self-rated health, quality of life and cognition. Therefore, it is important to note the role of socioeconomic status and health markers in predicting subjective health and quality of life of the older population in India.
In conclusion, the results of this study suggest a significant socioeconomic disparities in body mass index among older population in India. A significant association of underweight with all three outcomes of poor self-rated health, cognition and quality of life were observed, which needs to be considered to improve the quality of aging in India.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to the World Health Organization (WHO) for providing data access. We are also thankful to Dr. Uma Vasudevan for her help in language editing.
Data Availability
For this study, we have used the secondary data set, collected by the World Health Organization in collaboration with country research organizations. The authors did not have any special access privileges that others would not have. The data can be requested at: http://apps.who.int/healthinfo/systems/surveydata/index.php/catalog/sage.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Deaton A, Drèze J. Food and nutrition in India: facts and interpretations. Economic and political weekly. 2009:42–65. [Google Scholar]
- 2.NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. The Lancet. 2016;387(10026):1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razak F, Corsi DJ, Slutsky AS, Kurpad A, Berkman L, Laupacis A, et al. Prevalence of body mass index lower than 16 among women in low-and middle-income countries. Jama. 2015;314(20):2164–71. 10.1001/jama.2015.15666 [DOI] [PubMed] [Google Scholar]
- 4.Kapoor SK, Anand K. Nutritional transition: a public health challenge in developing countries; the double burden of undernutrition and overnutriton in developing countries is a public health challenge.(Nutrition). Journal of Epidemiology & Community Health. 2002;56(11):804–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold F, Parasuraman S, Arokiasamy P, Kothari M. Nutrition in India. National Family Health Survey (NFHS-3), India. 2005;6:59 http://pdf.usaid.gov/pdf_docs/Pnadq632.pdf [Google Scholar]
- 6.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. International journal of obesity. 2005;29(1):129–136. 10.1038/sj.ijo.0802824 [DOI] [PubMed] [Google Scholar]
- 7.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. The American journal of clinical nutrition. 2005;81(3):714–21. [DOI] [PubMed] [Google Scholar]
- 8.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. New England journal of medicine. 2007;356(3):213–5. 10.1056/NEJMp068177 [DOI] [PubMed] [Google Scholar]
- 9.Neuman M, Finlay JE, Smith GD, Subramanian SV. The poor stay thinner: stable socioeconomic gradients in BMI among women in lower-and middle-income countries. The American journal of clinical nutrition. 2011:ajcn-018127. 10.3945/ajcn.111.018127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. The Lancet. 2009;373(9669):1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Yi SW, Sull JW, Hong JS, Jee SH, Ohrr H. Body mass index and mortality among Korean elderly in rural communities: Kangwha Cohort Study. PloS one. 2015;10(2):e0117731 10.1371/journal.pone.0117731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. bmj. 2016;353:i2156 10.1136/bmj.i2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S, Millett C. Social Epidemiology of Hypertension in Middle-Income Countries. Hypertension. 2013; 62(1), 18–26. 10.1161/HYPERTENSIONAHA.113.01374 [DOI] [PubMed] [Google Scholar]
- 14.Shukla A, Kumar K, Singh A. Association between obesity and selected morbidities: a study of BRICS countries. PloS one. 2014;9(4):e94433 10.1371/journal.pone.0094433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. American Journal of Public Health. 2002;92(5):834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrieu S, Peres K, Letenneur L, Berr C, Dartigues JF, Ritchie K, et al. Relationship between body mass index and different domains of disability in older persons: the 3C study. International journal of obesity. 2004;28(12):1555 10.1038/sj.ijo.0802755 [DOI] [PubMed] [Google Scholar]
- 17.Myint PK, Welch AA, Luben RN, Wainwright NW, Surtees PG, Bingham SA, et al. Obesity Indices and Self-Reported Functional Health in Men and Women in the EPIC-Norfolk. Obesity. 2006;14(5):884–93. 10.1038/oby.2006.102 [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Gregg EW, Chen YJ, Zhang P, Rekeneire N, Williamson DF. The Association of BMI With Functional Status and Self-rated Health in US Adults. Obesity. 2008;16(2):402–8. 10.1038/oby.2007.70 [DOI] [PubMed] [Google Scholar]
- 19.Koyanagi A, Moneta MV, Garin N, Olaya B, Ayuso-Mateos JL, Chatterji S, et al. The association between obesity and severe disability among adults aged 50 or over in nine high-income, middle-income and low-income countries: a cross-sectional study. BMJ open. 2015;5(4):e007313 10.1136/bmjopen-2014-007313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang A, Arah OA. Body Mass Index and Poor Self-Rated Health in 49 Low-Income and Middle-Income Countries, By Sex, 2002–2004. Preventing chronic disease. 2015;12 10.5888/pcd12.150070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson U, Karlsson J, Sullivan M. Impact of overweight and obesity on health-related quality of life—a Swedish population study. International journal of obesity. 2002;26(3):417 10.1038/sj.ijo.0801919 [DOI] [PubMed] [Google Scholar]
- 22.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International journal of obesity. 2003;27(2):260 10.1038/sj.ijo.802225 [DOI] [PubMed] [Google Scholar]
- 23.Huang IC, Frangakis C, Wu AW. The relationship of excess body weight and health-related quality of life: evidence from a population study in Taiwan. International journal of obesity. 2006;30(8):1250 10.1038/sj.ijo.0803250 [DOI] [PubMed] [Google Scholar]
- 24.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. The lancet. 1997;349(9063):1436–42. 10.1016/S0140-6736(96)07495-8 [DOI] [PubMed] [Google Scholar]
- 25.Pednekar MS, Hakama M, Hebert JR, Gupta PC. Association of body mass index with all-cause and cause-specific mortality: findings from a prospective cohort study in Mumbai (Bombay), India. International journal of epidemiology. 2008;37(3):524–35. 10.1093/ije/dyn001 [DOI] [PubMed] [Google Scholar]
- 26.Sauvaget C, Ramadas K, Thomas G, Vinoda J, Thara S, Sankaranarayanan R. Body mass index, weight change and mortality risk in a prospective study in India. International journal of epidemiology. 2008;37(5):990–1004. 10.1093/ije/dyn059 [DOI] [PubMed] [Google Scholar]
- 27.Xiang X, An R. Body weight status and onset of cognitive impairment among US middle-aged and older adults. Archives of gerontology and geriatrics. 2015;60(3):394–400. 10.1016/j.archger.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Wang R, Zhao Y, Ma X, Wu M, Yan X, et al. The relationship between self-rated health and objective health status: a population-based study. BMC public health. 2013;13(1):320 10.1186/1471-2458-13-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Wang Q, Pang G, Lin L, Origasa H, Wang Y, et al. Association between body mass index and health-related quality of life: The" Obesity Paradox" in 21,218 adults of the Chinese general population. PLoS One. 2015;10(6):e0130613 10.1371/journal.pone.0130613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samal S, Panigrahi P, Dutta A. Social epidemiology of excess weight and central adiposity in older Indians: analysis of Study on global AGEing and adult health (SAGE). BMJ open. 2015;5(11):e008608 10.1136/bmjopen-2015-008608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy KK, Reddy BK, Rao AP. Interaction among body composition, self-rated health and functional status of the elderly in an Indian population. Asia Pacific journal of clinical nutrition. 2004;13(1). [PubMed] [Google Scholar]
- 32.Khongsdier R. Body mass index and morbidity in adult males of the War Khasi in Northeast India. European Journal of Clinical Nutrition. 2002; 56(6):484 10.1038/sj.ejcn.1601281 [DOI] [PubMed] [Google Scholar]
- 33.Shukla HC, Gupta PC, Mehta HC, Hébert JR. Descriptive epidemiology of body mass index of an urban adult population in western India. Journal of Epidemiology & Community Health. 2002;56(11):876–80. 10.1136/jech.56.11.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian SV, Smith GD. Patterns, distribution, and determinants of under-and overnutrition: a population-based study of women in India. The American journal of clinical nutrition. 2006;84(3):633–40. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian SV, Kawachi I, Smith GD. Income inequality and the double burden of under-and overnutrition in India. Journal of Epidemiology & Community Health. 2007;61(9):802–9. 10.1136/jech.2006.053801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian SV, Perkins JM, Khan KT. Do burdens of underweight and overweight coexist among lower socioeconomic groups in India?. The American journal of clinical nutrition. 2009;90(2):369–76. 10.3945/ajcn.2009.27487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arokiasamy P, Kowal P, Chatterji S. Age and Socioeconomic Gradients of Health of Indian Adults: An Assessment of Self-Reported and Biological Measures of Health. Journal of cross-cultural gerontology. 2016;31(2):193–211. 10.1007/s10823-016-9283-3 [DOI] [PubMed] [Google Scholar]
- 38.Griffiths P, Bentley M. Women of higher socio-economic status are more likely to be overweight in Karnataka, India. European Journal of Clinical Nutrition. 2005;59(10):1217 10.1038/sj.ejcn.1602228 [DOI] [PubMed] [Google Scholar]
- 39.IIPS. ORC-Macro. National Family Health Survey, 2005–2006: India. Mumbai, India: International Institute for Population Sciences, 2007. http://dhsprogram.com/pubs/pdf/FRIND3/FRIND3-Vol1andVol2.pdf
- 40.Siddiqui ST, Kandala NB, Stranges S. Urbanisation and geographic variation of overweight and obesity in India: a cross-sectional analysis of the Indian Demographic Health Survey 2005–2006. International journal of public health. 2015;60(6):717–26. 10.1007/s00038-015-0720-9 [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. New England Journal of Medicine. 2011;364(8):719–29. 10.1056/NEJMoa1010679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CY, Chou YC, Huang N, Chou YJ, Hu HY, Li CP. Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PloS one. 2014;9(7):e102589 10.1371/journal.pone.0102589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JB, Gu MJ, Shen P, Huang QC, Bao CZ, Ye ZH, et al. Body Mass Index and Mortality: A 10-Year Prospective Study in China. Scientific reports. 2016;6 10.1038/srep31609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of health and social behavior. 1997:21–37. [PubMed] [Google Scholar]
- 45.Schnittker J, Bacak V. The increasing predictive validity of self-rated health. PloS one. 2014;9(1):e84933 10.1371/journal.pone.0084933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Netuveli G, Blane D. Quality of life in older ages. British medical bulletin. 2008;85(1):113–26. 10.1093/bmb/ldn003 [DOI] [PubMed] [Google Scholar]
- 47.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. The Lancet. 2009;374(9704):1821–30. 10.1016/S0140-6736(09)61829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dotchin CL, Paddick SM, Gray WK, Kisoli A, Orega G, Longdon AR, et al. The association between disability and cognitive impairment in an elderly Tanzanian population. Journal of epidemiology and global health. 2015;5(1):57–64. 10.1016/j.jegh.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh P, Govil D, Kumar V, Kumar J. Cognitive Impairment and Quality of Life among Elderly in India. Applied Research in Quality of Life.2016: 1–7.28392845 [Google Scholar]
- 50.Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, et al. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). International journal of epidemiology. 2012; 41(6):1639–49. 10.1093/ije/dys210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–32. [DOI] [PubMed] [Google Scholar]
- 52.Lezak MD. Neuropsychological assessment. Oxford University Press, USA; 2004. [Google Scholar]
- 53.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health policy and planning. 2006;21(6):459–68. 10.1093/heapol/czl029 [DOI] [PubMed] [Google Scholar]
- 54.Hirve S, Oud JH, Sambhudas S, Juvekar S, Blomstedt Y, Tollman S, et al. Unpacking self-rated health and quality of life in older adults and elderly in India: a structural equation modelling approach. Social indicators research. 2014; 117(1):105–19. [Google Scholar]
- 55.Mäntyselkä PT, Turunen JH, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. Jama. 2003; 290(18):2435–42. 10.1001/jama.290.18.2435 [DOI] [PubMed] [Google Scholar]
- 56.Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002; 25(8):48–52. [PubMed] [Google Scholar]
- 57.Arokiasamy P, Uttamacharya U, Jain K, Biritwum RB, Yawson AE, Wu F, et al. The impact of multimorbidity on adult physical and mental health in low-and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal?. BMC medicine. 2015;13(1):178 10.1186/s12916-015-0402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James KS. India’s demographic change: opportunities and challenges. Science. 2011; 333(6042):576–80. 10.1126/science.1207969 [DOI] [PubMed] [Google Scholar]
- 59.Lee J, Smith JP. Regional Disparities in Adult Height, Educational Attainment and Gender Difference in Late-Life Cognition: Findings from the Longitudinal Aging Study in India (LASI). The journal of the economics of ageing. 2014;4:26 10.1016/j.jeoa.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dandona L, Dandona R, Kumar GA, Shukla DK, Paul VK, Balakrishnan K, et al. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. The Lancet. 2017; 390(10111):2437–60. 10.1016/S0140-6736(17)32804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duraisamy P, Ajay Mahal. Health, Poverty and Economic Growth in India. National Commission on Macroeconomics and Health, Background Papers, Health Systems in India: Delivery and Financing of Services. New Delhi: Ministry of Health and Family Welfare, Government of lndia. 2005.
- 62.Vedantam A, Subramanian V, Rao NV, John KR. Malnutrition in free-living elderly in rural south India: prevalence and risk factors. Public health nutrition. 2010;13(9):1328–32. 10.1017/S1368980009991674 [DOI] [PubMed] [Google Scholar]
- 63.Bhan N, Millett C, Subramanian SV, Dias A, Alam D, Williams J, et al. Socioeconomic patterning of chronic conditions and behavioral risk factors in rural South Asia: a multi-site cross-sectional study. International Journal of Public Health. 2017:1–0. 10.1007/s00038-017-1019-9 [DOI] [PubMed] [Google Scholar]
- 64.Darmon N, Drewnowski A. Does social class predict diet quality?. The American journal of clinical nutrition. 2008;87(5):1107–17. [DOI] [PubMed] [Google Scholar]
- 65.Akinyemiju TF, Zhao X, Sakhuja S, Jolly P. Life-course socio-economic status and adult BMI in Ghana; analysis of the WHO study on global ageing and adult health (SAGE). International journal for equity in health. 2016;15(1):185 10.1186/s12939-016-0474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhan N, Karan A, Srivastava S, Selvaraj S, Subramanian SV, Millett C. Have socioeconomic inequalities in tobacco use in India increased over time? Trends from the national sample Surveys (2000–2012). Nicotine & Tobacco Research. 2016;18(8):1711–8. 10.1093/ntr/ntw092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nédó E, Paulik E. Association of smoking, physical activity, and dietary habits with socioeconomic variables: a cross-sectional study in adults on both sides of the Hungarian-Romanian border. BMC public health. 2012;12(1):60 10.1186/1471-2458-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pednekar MS, Gupta PC, Shukla HC, Hebert JR. Association between tobacco use and body mass index in urban Indian population: implications for public health in India. BMC Public Health. 2006;6(1):70 10.1186/1471-2458-6-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schrock JM, McClure HH, Snodgrass JJ, Liebert MA, Charlton KE, Arokiasamy P, et al. Food insecurity partially mediates associations between social disadvantage and body composition among older adults in india: Results from the study on global AGEing and adult health (SAGE). American Journal of Human Biology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fares D, Barbosa AR, Borgatto AF, da Silva Coqueiro R, Fernandes MH. Factors associated with nutritional status of the elderly in two regions of Brazil. Revista da Associação Médica Brasileira (English Edition). 2012;58(4):434–41. [PubMed] [Google Scholar]
- 71.Amarya S, Singh K, Sabharwal M. Changes during aging and their association with malnutrition. Journal of Clinical Gerontology and Geriatrics. 2015;6(3):78–84. 10.1016/j.jcgg.2015.05.003 [DOI] [Google Scholar]
- 72.Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC geriatrics. 2013;13(1):71 10.1186/1471-2318-13-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernihough A, McGovern ME. Physical stature decline and the health status of the elderly population in England. Economics & Human Biology. 2015;16:30–44. 10.1016/j.ehb.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.SJöGREN AN, öSTERBERG TO, STEEN B. Intake of energy, nutrients and food items in a ten-year cohort comparison and in a six-year longitudinal perspective: a population study of 70-and 76-year-old Swedish people. Age and Ageing. 1994;23(2):108–12. [DOI] [PubMed] [Google Scholar]
- 75.Giezenaar C, Chapman I, Luscombe-Marsh N, Feinle-Bisset C, Horowitz M, Soenen S. Ageing is associated with decreases in appetite and energy intake—a meta-analysis in healthy adults. Nutrients. 2016;8(1):28 10.3390/nu8010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheiham A, Steele JG, Marcenes W, Finch S, Walls AW. The relationship between oral health status and body mass index among older people: a national survey of older people in Great Britain. British dental journal. 2002;192(12):703–6. [DOI] [PubMed] [Google Scholar]
- 77.do Nascimento TL, Liberalesso NA, Balbinot HJ, Neves HF. Association between underweight and overweight/obesity with oral health among independently living Brazilian elderly. Nutrition. 2013;29(1):152–7. 10.1016/j.nut.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 78.Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health-related quality of life: a cross-sectional analysis of the US population. International journal of obesity. 2003; 27(10):1227–32. 10.1038/sj.ijo.0802396 [DOI] [PubMed] [Google Scholar]
- 79.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. The lancet Diabetes & endocrinology. 2015; 3(6):431–6. [DOI] [PubMed] [Google Scholar]
- 80.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. International journal of obesity. 2015;39(9):1383 10.1038/ijo.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S, Kim Y, Park SM. Body mass index and decline of cognitive function. PloS one. 2016; 11(2):e0148908 10.1371/journal.pone.0148908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goli S, Singh L, Jain K, Pou LM. Socioeconomic determinants of health inequalities among the older population in India: a decomposition analysis. Journal of cross-cultural gerontology. 2014;29(4):353–69. 10.1007/s10823-014-9251-8 [DOI] [PubMed] [Google Scholar]
- 83.Kumar K, Shukla A, Singh A, Ram F, Kowal P. Association between wealth and health among older adults in rural China and India. The Journal of the Economics of Ageing. 2016;7:43–52. 10.1016/j.jeoa.2016.02.002 [DOI] [Google Scholar]
- 84.Wu F, Guo Y, Zheng Y, Ma W, Kowal P, Chatterji S, et al. Social-Economic Status and Cognitive Performance among Chinese Aged 50 Years and Older. PloS one. 2016;11(11):e0166986 10.1371/journal.pone.0166986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selvamani Y, Arokiasamy P. Effects of Childhood and Current Socioeconomic Status on Health of Older Adults in India, China, Ghana, Mexico, Russia and South Africa: An Analysis of WHO-SAGE Data In Applied Demography and Public Health in the 21st Century 2017. (pp. 329–348). Springer International Publishing. [Google Scholar]
- 86.Sen A. Health: perception versus observation: self reported morbidity has severe limitations and can be extremely misleading. BMJ: British Medical Journal. 2002;324(7342):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
For this study, we have used the secondary data set, collected by the World Health Organization in collaboration with country research organizations. The authors did not have any special access privileges that others would not have. The data can be requested at: http://apps.who.int/healthinfo/systems/surveydata/index.php/catalog/sage.