Abstract
Dementia is a risk factor for unsafe driving. Therefore, an assessment strategy has recently been developed for the prediction of fitness to drive in patients with the Alzheimer disease (AD). The aim of this study was to investigate whether this strategy is also predictive of fitness to drive in patients with non-AD dementia, that is, vascular dementia, frontotemporal dementia, and dementia with Lewy bodies. Predictors were derived from 3 types of assessment: clinical interviews, neuropsychological tests, and driving simulator rides. The criterion was the pass-fail outcome of an official on-road driving assessment. About half of the patients with non-AD dementia (n=34) failed the on-road driving assessment. Neuropsychological assessment [area under the curve (AUC)=0.786] was significantly predictive of fitness to drive in patients with non-AD dementia, however, clinical interviews (AUC=0.559) and driving simulator rides (AUC=0.404) were not. The fitness-to-drive assessment strategy with the 3 types of assessment combined (AUC=0.635) was not found to significantly predict fitness to drive in non-AD dementia. Different types of dementia require different measures and assessment strategies.
Key Words: vascular dementia, frontotemporal dementia, dementia with Lewy bodies, car driving, fitness-to-drive assessment
The most common types of dementia are Alzheimer disease (AD), vascular dementia (VaD), frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB).1 In early stages, different patterns of cognitive dysfunctions may be present in patients with different types of dementia. Initial impairments of AD usually lie in the cognitive domain of memory, whereas, VaD often starts with cognitive slowing, FTD with behavioral or language impairments and DLB with visuospatial impairments. These different impairments may have different effects on activities of daily living such as driving.2,3
Many patients with different types of dementia continue driving,4 but dementia is a risk factor for traffic accidents. There is consensus that patients with moderate to severe dementia should not drive anymore.5 However, in the early stages of dementia, some patients still drive safely, whereas others do not.5 In order to advise patients with mild dementia about driving, patients should be assessed on fitness to drive.2,6,7 On-road driving assessments are the “gold standard” because of a high face validity, but it is not feasible to assess all drivers with dementia on the road. A reliable and validated fitness-to-drive assessment strategy for clinical application would therefore be useful.8 However, it seems crucial to validate fitness-to-drive assessment strategies for patients with different types of dementia separately, because they may vary in symptoms and in the effects of symptoms on driving behavior.2,9,10
Studies on driving with non-AD dementia are scarce. There is only 1 study on driving with VaD,6 which showed that patients with VaD made more driving errors on the road than healthy participants.6 Patients with VaD might not operate a car quickly enough and may not perceive other road users or signs in time as a consequence of cognitive slowing.2 Nonetheless, some patients with VaD have mild symptoms for a long time and these patients may be safe drivers for several years after diagnosis.
Driving with FTD was investigated using interviews and driving simulators, but no on-road driving assessments were reported yet.9,11–13 Antisocial behavior, agitation, impulsivity, and distraction due to FTD may lead to speeding, ignoring road signs, running red lights, and not recognizing pedestrians at intersections, all having the clear potential to cause accidents.9,11–13 Moreover, impairment of judgment may cause difficulty estimating distances between vehicles,9 and result in a lack of understanding that particular driving behavior is inappropriate and risky.11 On the basis of the moderately progressive course and early behavioral symptoms, it has been suggested that patients with FTD should cease driving soon after diagnosis.9,11,14
There is only 1 study on driving with DLB.15 In this driving simulator study, patients with DLB were regularly speeding, swerving, running red lights, and causing accidents.15 DLB has a slowly progressive course, but the initial symptoms, that is, visual hallucinations, visuospatial impairments, fluctuations in attention, and parkinsonism, may already impede safe driving at the time of diagnosis.14
To address the need for validated fitness-to-drive assessment strategies, an assessment strategy was developed recently for patients with AD.7 The assessment strategy consisted of clinical interviews, a neuropsychological assessment, and driving simulator rides, because these 3 types of assessments were shown to provide nonredundant information for the prediction of fitness to drive in patients with AD. The aim of the present study is to investigate whether the suggested assessment strategy is also predictive for fitness to drive in patients with VaD, FTD, and DLB. We hypothesize that the proposed strategy will aid the prediction of fitness to drive, because cognitive and functional aspects important for driving are assessed. However, the differences in clinical syndromes of VaD, FTD, and DLB may result in a considerable drop in predictive accuracy compared with the original study on patients with AD. The measures of the 3 types of assessments may differ in how disease-specific they are in predicting fitness to drive, therefore the different types of assessments will also be evaluated separately.
METHODS
Participants
Participants were recruited and assessed according to the study protocol described by Piersma et al.7 The study was approved by the Medical Ethical Committee at the University Medical Center Groningen, the Netherlands. Inclusion criteria for patients were an age above 30, a valid driving license, a wish to continue driving, and a diagnosis of dementia in very mild to mild stages (clinical dementia rating <2). Exclusion criteria were the diagnosis of neurological or psychiatric conditions unrelated to dementia that may influence driving performance and usage of medications legally incompatible with driving (ICADTS category III drugs). In addition, patients were screened on visual functions according to legal limits for driving, that is, a minimum visual acuity of 0.5 and a minimum horizontal field of view of 120 degrees.
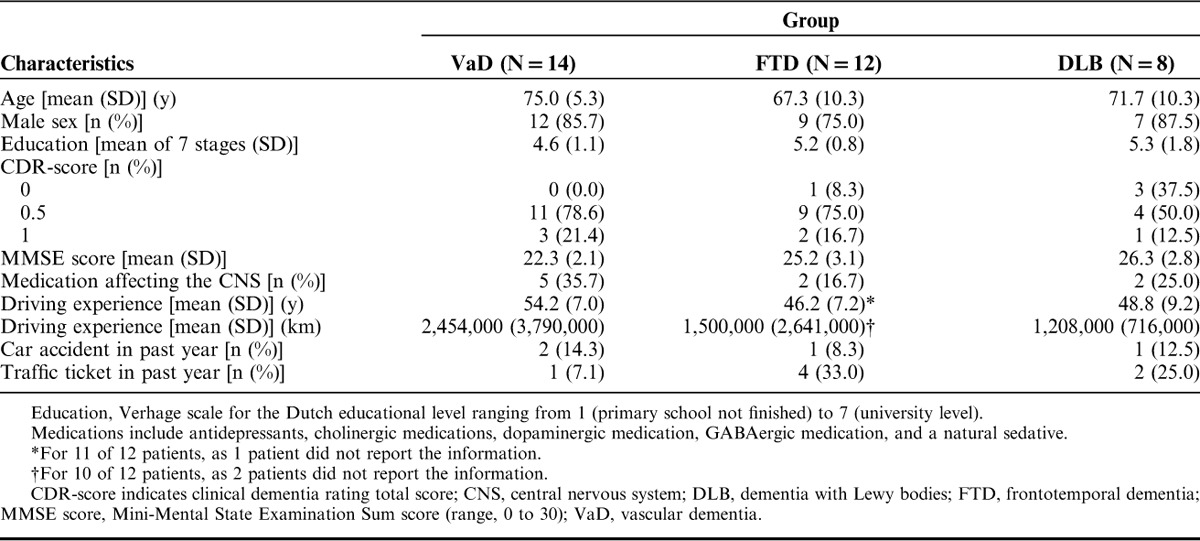
Referring physicians established the diagnosis of VaD with the NINDS-AIREN criteria,16 the diagnosis of FTD and its variants by the criteria of the International bvFTD Criteria Consortium17 and the International PPA Consortium,18 and the diagnosis of DLB using the criteria of the DLB consortium.19 Two patients with VaD had to be excluded because they did not fulfill the visual requirement of a minimum horizontal visual field of 120 degrees, resulting in 14 patients with VaD who completed the study. Moreover, 2 patients with FTD had to be excluded because their visual acuity was below the requirement of 0.5. Two additional patients with FTD were excluded because they did not perform the on-road assessment. Hence, 12 patients with FTD completed the study. The behavioral variant of FTD was diagnosed in 7 cases, the semantic variant in 2 cases and primary progressive aphasia in 1 case. One case was diagnosed with both the behavioral and semantic variant of FTD. In 1 case, the diagnosis of FTD was not specified as a particular variant. Finally, 8 patients with DLB participated in this study. Table 1 shows characteristics of the 3 patient groups.
TABLE 1.
Characteristics of Patients With VaD, FTD, and DLB
Measures
The following description of methods entails only the measures used in the prediction equations as derived from the original study.7 Measures of clinical interviews included 2 subscores of the clinical dementia rating, that is, orientation and judgment as well as problem solving,20 the patients’ judgments of their own driving safety, and recent driving experience. The neuropsychological assessment comprised the Mini-Mental State Examination (MMSE),21,22 the reaction time S2,23 the hazard perception test,24 and a traffic theory test (see Piersma et al7 for details). Fixed-based Jentig50 driving simulators of ST Software were used. Driving simulator measures included the minimum speed when approaching an intersection with traffic lights, the number of collisions in a ride with intersections and 2 measures concerning a merging maneuver, that is, the deceleration of the rear car after merging and the time headway directly after merging (see Piersma et al7 for details).
The on-road driving assessments were carried out by approved experts on practical fitness to drive of the Dutch driving test organization (CBR). Experts were blind to the participants’ diagnoses and test results. They rated driving behavior of patients on the Test Ride Investigating Practical fitness to drive forms.25,26 Finally, a pass, doubtful or fail outcome was given by the expert. This outcome was recoded into a dichotomous item which indicates whether or not a participant is fit to drive, that is, pass outcomes indicated that participants could retain their driving license, whereas doubtful or fail outcomes indicated that participants would have lost their driving license if this was an official relicensing assessment.
Statistical Analyses
Missing Data
The traffic theory test measure of 1 patient with VaD was missing. Because of simulator sickness, 7 (50.0%) patients with VaD, 3 (25.0%) patients with FTD, and 2 (25.0%) patients with DLB were excluded entirely from analyses that involved driving simulator rides. Because of technical problems, driving simulator measures of 1 patient with VaD and of 1 patient with FTD were missing. In addition, 1 driving simulator measure, that is, the deceleration of the rear car after merging, was missing of 1 patient with VaD and 1 patient with DLB, because these participants merged onto the motorway after all cars had passed. As these 2 patients did complete the driving simulator rides, it was decided to impute the 2 missing values using an imputation model (including all complete variables of the specific patient group) that was estimated by maximum likelihood, providing a singly imputed data set.
Evaluation of the Prediction Model for Fitness to Drive
The goal of the analysis was to evaluate whether fitness to drive of patients with non-AD dementia can be predicted with a prediction model that has been developed using data of patients with AD.7 The previously proposed prediction equations were applied using data of 34 patients with non-AD dementia: 14 VaD, 12 FTD, and 8 DLB. Receiver operating characteristic (ROC) analyses were used to evaluate the predictive accuracy of the model. The area under the curve (AUC) was used as a classification measure with larger areas indicating better predictive accuracy. The 3 groups of predictor variables, that is, clinical interviews, neuropsychological assessment and driving simulator rides, and the complete approach (ie, variables from all groups of predictors) were evaluated in separate ROC analyses to explore the accuracy of each set of variables in predicting fitness to drive for non-AD dementia.
RESULTS
Four of 14 patients with VaD, 5 of 12 patients with FTD, and 5 of 8 patients with DLB passed the on-road driving assessment. Overall, 14 (41.2%) patients passed and 20 (58.8%) patients failed the on-road driving assessment. Results of patients who passed and failed the on-road assessment are presented in Table 2.
TABLE 2.
Comparison of Patients With Non-AD Dementia Who Passed and Who Failed the On-road Driving Assessment on Predictor Variables*
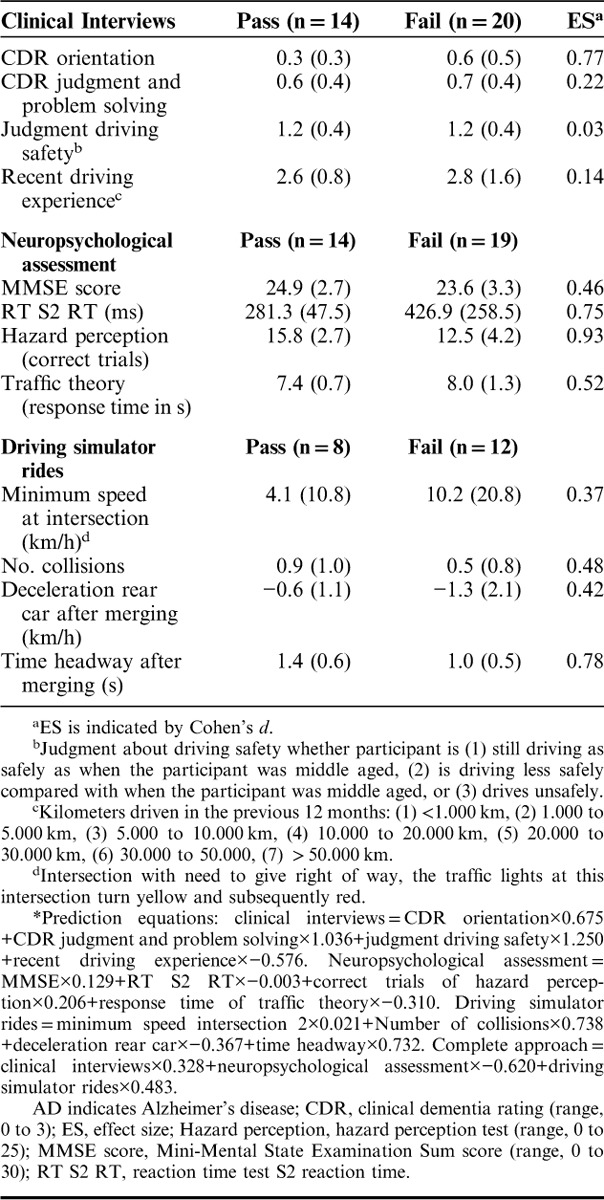
Prediction equations derived from the previous study on patients with AD were applied.7 ROC analysis showed that the clinical interviews (n=34) were not predictive of fitness to drive in patients with non-AD dementia with a nonsignificant AUC close to chance level (AUC=0.559, SE=0.104, P=0.564). In contrast, ROC analysis revealed that neuropsychological assessment (n=33) was predictive of fitness to drive in this patient group with a significant AUC of 0.786, SE=0.081, P=0.006. Similar to clinical interviews, driving simulator rides (n=20) were not found to aid the prediction of fitness to drive in patients with non-AD dementia (AUC=0.417, SE=0.130, P=0.537). The complete approach with the 3 types of assessments combined (n=20) was not useful for the prediction of fitness to drive in this sample of patients with non-AD dementia (AUC=0.635, SE=0.129, P=0.316).
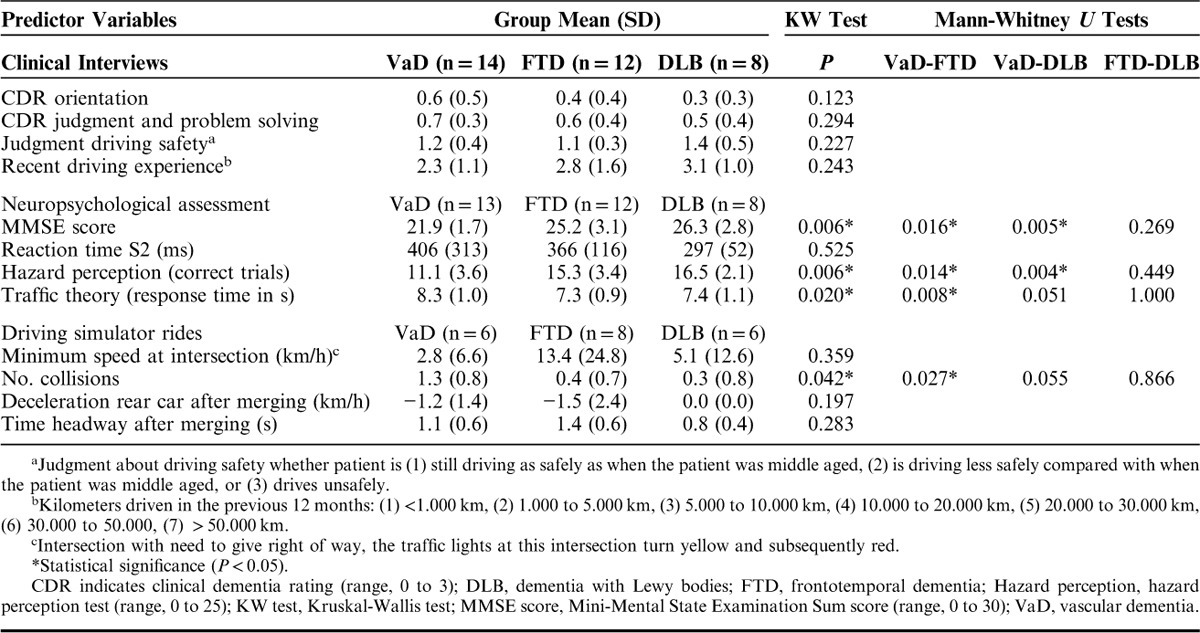
The patient groups were too small to evaluate the prediction model for the 3 types of dementia separately, however, to get an idea whether the results from the 3 different types of non-AD dementia diverge, their mean scores on the predictor variables were explored (Table 3). Patients with VaD had poorer mean scores on the predictor variables of clinical interviews and neuropsychological assessment than patients with FTD and patients with DLB, which was particularly evident for the scores on the MMSE and the hazard perception test. In general, patients with DLB had “safer” mean scores on the predictor variables than the other 2 patient groups, for example an adequate reaction time S2 score. Notably, patients with FTD judged their own driving safety as safe, but approached an intersection with traffic lights with a high speed compared with the other 2 patient groups. Nonparametric comparisons using Kruskal-Wallis tests showed statistically significant differences between the patient groups in MMSE score, χ2(2)=10.228, P=0.006; hazard perception (correct trials), χ2(2)=10.198, P=0.006; traffic theory (response time), χ2(2)=7.852, P=0.020; and the number of collisions in the driving simulator, χ2(2)=6.358, P=0.042. Mann-Whitney post hoc tests indicated worse performance of patients with VaD compared with the other 2 groups of patients in the majority of comparisons (Table 3). In conclusion, the 3 patient groups seemed to differ in their scores on the predictor variables.
TABLE 3.
Predictor Variables of Patients With VaD, FTD, and DLB
DISCUSSION
About half of the patients failed the on-road driving assessment suggesting that VaD, FTD, and DLB are risk factors for unsafe driving. This is in line with previous studies showing that patients with VaD make more driving errors on the road and that patients with FTD and DLB make more driving errors in driving simulation, in comparison with healthy drivers.6,12,15 Nevertheless, a considerable proportion of patients of each type of dementia passed the on-road driving assessment. Likewise, Fitten et al6 showed a large variation in on-road driving performance among patients with VaD indicating that some patients with VaD are fit to drive, whereas, others are unfit to drive. Although research including on-road driving of patients with FTD and DLB was lacking, it has been argued that patients with FTD and DLB should cease driving very soon after the diagnosis is established.9,11,14 In a study by Seiler et al,4 only 9 of 16 patients with FTD had ceased driving (a rate comparable with patients with AD and VaD), whereas as many as 10 of 11 patients with DLB had ceased driving. The current study suggests that not all patients with FTD and DLB are unfit to drive. Consequently, all patients with dementia who wish to continue driving should be assessed on fitness to drive.
In this study, it was found that the prediction model for fitness to drive in patients with AD was not predictive for fitness to drive in patients with non-AD dementia (AUC=0.635). Although the applied neuropsychological assessment battery was of significant value for the prediction of fitness to drive in patients with non-AD dementia (AUC=0.786), the selections of predictor variables from clinical interviews (AUC=0.559) and driving simulator rides (AUC=0.417) were not. Clinical interviews may be of limited utility for the prediction of fitness to drive in patients with dementia, because it requires insight of patients into their own abilities, and careful attention of informants to the patients’ behavior. In this study, patients with FTD estimated their driving safety as not being declined which is in accordance with a previous study stating that patients with FTD may not realize that their driving behavior is risky.11 It can be concluded that the primary use of clinical interviews is to discuss the impact of dementia on driving and to score the severity of dementia.
Furthermore, the selected measures from the driving simulator rides may not serve the prediction of fitness to drive in patients with non-AD dementia, because these measures do not represent all critical traffic situations, and patients with different types of dementia may have difficulties in different traffic situations. This would suggest that other driving simulator measures might be better predictors in patients with non-AD dementia. To start with, different measures from the current driving simulator rides could be investigated, for example, measures reflecting lane control. Another issue with driving simulation is that some measures are difficult to interpret in terms of “safe” or “unsafe” driving, as both a high and a low value may indicate poor driving performance. For example, 1 patient group might be too slow, whereas another patient group might be too fast in similar situations of simulated driving. A solution might be using measures differently for different patient groups, for example, driving slowly might predict unsafe driving in patients with VaD, whereas speeding could be a predictor for unsafe driving in patients with FTD and DLB. Currently, driving simulator rides provide a safe environment for subjective clinical evaluations of fitness to drive, but objective evidence-based measures with cutoffs still have to be defined for the prediction of fitness to drive in non-AD dementia.
The applied neuropsychological assessment was useful for fitness-to-drive evaluations in patients with non-AD dementia, especially specific traffic tests may have the potential to predict fitness to drive in multiple types of dementia. This fits with the promising results with DriveSafe/DriveAware in groups of patients with cognitive impairments related to a variety of diagnoses.27,28 When developing new assessment strategies, it should also be considered which symptoms and impairments are likely to result in unsafe driving per etiology and how these can be assessed. For example, cognitive slowing in VaD and visuospatial functions in DLB could be evaluated in a neuropsychological assessment.29 Patients with FTD show impairments of behavior (do) rather than of maximal performance (can do), which is difficult to measure with neuropsychological tests. As it is common for patients with FTD not to realize that their driving behavior is risky, inquiries with informants could be included when investigating fitness to drive in FTD. In brief, different algorithms using different measures may be needed to predict fitness to drive in patients with different types of dementia.
In future studies on fitness to drive in patients with non-AD dementia, dichotomized outcome scores might not always be feasible, therefore trichotomization may need to be considered.30 This means that outcome scores will be divided into 3 groups: safe, unsafe, and indeterminate. The latter group should be referred to additional fitness-to-drive assessments. Such an approach could improve the classification of driving safety.
This is the first study in which the prediction of fitness to drive in patients with 3 different types of non-AD dementia was investigated. Strengths of the study are that all patients were assessed according to the same protocol and that on-road driving evaluations were performed. In many studies on fitness to drive, patients with AD and other types of dementia were pooled into 1 group. In this study, it was found that the prediction equation with measures from clinical interviews, neuropsychological assessment, and driving simulator rides that predicted fitness to drive in patients with AD did not apply to patients with non-AD dementia. These findings may imply that it is not possible to predict fitness to drive for all patients with dementia with 1 assessment strategy. Moreover, patients with different types of non-AD dementia also seem to differ in fitness-to-drive assessment results based on the exploration of their mean scores, which indicates that fitness-to-drive assessment strategies require validation for each type of dementia separately. It is important to note that the differences in mean scores between the patient groups are likely to be affected by the severity of cognitive impairment (ie, the severity of cognitive impairment may have been worse in patients with VaD than in patients with FTD and DLB in this sample), in addition to the different types of dementia. The heterogeneity of the samples of patients with dementia may partially explain why predictive accuracies of fitness-to-drive assessment strategies were often low in previous studies.31
In the current study, 3 types of dementia were pooled into 1 non-AD dementia group, because of small sample sizes. As a consequence, the results do not reveal whether the proposed assessment strategy was, for example, predictive for 1 of the 3 types of dementia included. To investigate this, the number of correct classifications for each type of dementia was counted after application of cutoff −0.6 as suggested in the original study.7 For patients with VaD, the cutoff was too strict, because all 6 patients with VaD were classified as fail, whereas 2 of them passed the on-road assessment. For patients with DLB, the cutoff was too lenient since all 6 patients with DLB were classified as pass, whereas 3 of them failed the on-road assessment. In the FTD group, the classification accuracy was better, nonetheless, 2 of 8 patients were incorrectly classified as pass. These results confirm that the proposed strategy cannot predict fitness to drive in each group of patients with non-AD dementia.
In conclusion, the results of this study show that a valid assessment strategy for the prediction of fitness to drive in patients with AD7,32 is not useful for the prediction of fitness to drive in patients with non-AD dementia. This is in line with previously stated notions that each type of dementia has its own typical symptoms, resulting in different impairments and variations in driving behavior.2,9 The implication of the findings is that assessment strategies for the prediction of fitness to drive should be developed specifically tailored to VaD, FTD, and DLB.
ACKNOWLEDGMENTS
The authors thank all referring physicians, all participants for their participation, and the students and research assistant Anita C.M. van Oers for their role in data acquisition.
Footnotes
W.H.B. received a grant (#5000001470/31052108) from the Ministry of Infrastructure and the Environment (NL). The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Goodman RA, Lochner KA, Thambisetty M, et al. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement. 2016;13:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piersma D, de Waard D, Davidse R, et al. Car drivers with dementia: different complications due to different aetiologies? Traffic Inj Prev. 2016;17:9–23. [DOI] [PubMed] [Google Scholar]
- 3.Snyder CH. Dementia and driving: autonomy versus safety. J Am Acad Nurse Pract. 2005;17:393–402. [DOI] [PubMed] [Google Scholar]
- 4.Seiler S, Schmidt H, Lechner A, et al. Bayer A. Driving cessation and dementia: results of the prospective registry on dementia in austria (PRODEM). PLoS One. 2012;7:e52710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundberg C, Johansson K, Ball K, et al. Dementia and driving: an attempt at consensus. Alzheimer Dis Assoc Disord. 1997;11:28–37. [DOI] [PubMed] [Google Scholar]
- 6.Fitten LJ, Perryman KM, Wilkinson CJ, et al. Alzheimer and vascular dementias and driving. A prospective road and laboratory study. JAMA. 1995;273:1360–1365. [PubMed] [Google Scholar]
- 7.Piersma D, Fuermaier ABM, de Waard D, et al. Prediction of fitness to drive in patients with Alzheimer’s dementia. PLoS One. 2016;11:e0149566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omer S, Dolan C, Dimitrov BD, et al. General practitioners’ opinions and attitudes towards medical assessment of fitness to drive of older adults in Ireland. Australas J Ageing. 2014;33:E33–E36. [DOI] [PubMed] [Google Scholar]
- 9.Fujito R, Kamimura N, Ikeda M, et al. Comparing the driving behaviours of individuals with frontotemporal lobar degeneration and those with Alzheimer’s disease. Psychogeriatrics. 2016;16:27–33. [DOI] [PubMed] [Google Scholar]
- 10.Martin AJ, Marottoli R, O’Neill D. Driving assessment for maintaining mobility and safety in drivers with dementia. Cochrane Database Syst Rev. 2013;8:CD006222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst J, Krapp S, Schuster T, et al. Fahrtauglichkeit bei Patienten mit frontotemporaler und Alzheimer-Demenz [Car driving ability of patients with frontotemporal lobar degeneration and Alzheimer’s disease]. Nervenarzt. 2010;81:79–85. [DOI] [PubMed] [Google Scholar]
- 12.De Simone V, Kaplan L, Patronas N, et al. Driving abilities in frontotemporal dementia patients. Dement Geriatr Cogn Disord. 2007;23:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Turk K, Dugan E. Research brief: a literature review of frontotemporal dementia and driving. Am J Alzheimers Dis Other Demen. 2014;29:404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson S, Pinner G. Driving and dementia: a clinician’s guide. Adv Psychiatr Treat. 2013;19:89–96. [Google Scholar]
- 15.Yamin S, Stinchcombe A, Gagnon S. Driving competence in mild dementia with Lewy bodies: in search of cognitive predictors using driving simulation. Int J Alzheimers Dis. 2015:806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 17.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 20.Morris J. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22.Kok R, Verhey F. Dutch translation of the Mini-Mental State Examination (Folstein et al, 1975). 2002.
- 23.Prieler J. Manual RT: Reaction test, version 31. 2008.
- 24.Vlakveld W. Hazard Anticipation of Young Novice Drivers; Assessing and Enhancing the Capabilities of Young Novice Drivers to Anticipate Latent Hazards in Road and Traffic Situations [Dissertation]. Groningen, Leidschendam: University of Groningen, SWOV Institute for Road Safety Research; 2011. [Google Scholar]
- 25.Tant MLM, Brouwer WH, Cornelissen FW, et al. Driving and visuospatial performance in people with hemianopia. Neuropsychol Rehabil. 2002;12:419–437. [Google Scholar]
- 26.Withaar FK, Brouwer WH, van Zomeren AH. Fitness to drive in older drivers with cognitive impairment. J Int Neuropsychol Soc. 2000;6:480–490. [DOI] [PubMed] [Google Scholar]
- 27.Kay LG, Bundy AC, Clemson LM. Predicting fitness to drive in people with cognitive impairments by using drivesafe and driveaware. Arch Phys Med Rehabil. 2009;90:1514–1522. [DOI] [PubMed] [Google Scholar]
- 28.Hines A, Bundy AC. Predicting driving ability using DriveSafe and DriveAware in people with cognitive impairments: a replication study. Aust Occup Ther J. 2014;61:224–229. [DOI] [PubMed] [Google Scholar]
- 29.Levy JA, Chelune GJ. Cognitive-behavioral profiles of neurodegenerative dementias: beyond Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2007;20:227–238. [DOI] [PubMed] [Google Scholar]
- 30.Molnar FJ, Patel A, Marshall SC, et al. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: a systematic review. J Am Geriatr Soc. 2006;54:1809–1824. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson AE. Screening and assessment tools for determining fitness to drive: a review of the literature for the pathways project. Occup Ther Heal Care. 2014;28:82–121. [DOI] [PubMed] [Google Scholar]
- 32.Fuermaier ABM, Piersma D, de Waard D, et al. Assessing fitness to drive—a validation study on patients with mild cognitive impairment. Traffic Inj Prev. 2017;18:145–149. [DOI] [PubMed] [Google Scholar]


