Abstract
Aims
Hybrid imaging provides a non-invasive assessment of coronary anatomy and myocardial perfusion. We sought to evaluate the added clinical value of hybrid imaging in a multi-centre multi-vendor setting.
Methods and results
Fourteen centres enrolled 252 patients with stable angina and intermediate (20-90%) pre-test likelihood of coronary artery disease (CAD) who underwent myocardial perfusion scintigraphy (MPS), CT coronary angiography (CTCA), and quantitative coronary angiography (QCA) with fractional flow reserve (FFR). Hybrid MPS/CTCA images were obtained by 3D image fusion. Blinded core-lab analyses were performed for CTCA, MPS, QCA and hybrid datasets. Hemodynamically significant CAD was ruled-in non-invasively in the presence of a matched finding (myocardial perfusion defect co-localized with stenosed coronary artery) and ruled-out with normal findings (both CTCA and MPS normal). Overall prevalence of significant CAD on QCA (>70% stenosis or 30-70% with FFR≤0.80) was 37%. Of 1004 pathological myocardial segments on MPS, 246 (25%) were reclassified from their standard coronary distribution to another territory by hybrid imaging. In this respect, in 45/252 (18%) patients, hybrid imaging reassigned an entire perfusion defect to another coronary territory, changing the final diagnosis in 42% of the cases. Hybrid imaging allowed non-invasive CAD rule-out in 41%, and rule-in in 24% of patients, with a negative and positive predictive value of 88% and 87%, respectively.
Conclusion
In patients at intermediate risk of CAD, hybrid imaging allows non-invasive co-localization of myocardial perfusion defects and subtending coronary arteries, impacting clinical decision-making in almost one every five subjects.
Keywords: Hybrid imaging, Myocardial perfusion scintigraphy, CT coronary angiography, Coronary artery disease
Introduction
The risk of patients with stable coronary artery disease (CAD) varies considerably based on the extent of anatomical involvement and of myocardial ischaemia.1 Unfortunately, there is disagreement between the angiographic severity of CAD and myocardial perfusion abnormalities.2,3 Thus, current guidelines recommend a comprehensive anatomo-functional assessment to decide on the most appropriate treatment, with patients at low-risk treated conservatively, while high-risk patients are generally referred for more aggressive therapies.1 Specifically, revascularization strategies should be guided by the presence of haemodynamically significant coronary stenosis, while non-significant coronary stenoses may be treated conservatively.4,5
Recently, hybrid cardiac imaging has emerged as a non-invasive way of assessing CAD by integration of myocardial perfusion images with individual coronary anatomy.6 Small studies have suggested superior diagnostic accuracy compared with the separate imaging modalities,7 whereas others have reported incremental prognostic value.8 While the technique is finding increasing acceptance in clinical practice, questions remain over the clinical role of hybrid imaging. Furthermore, the impact of the technique has never been tested in a multicentre, multi-device, real-world setting.
This study sought to assess the clinical role of hybrid cardiac imaging in a multicentre study using different equipment and practice, and to explore its value for the diagnosis of haemodynamically significant CAD.
Methods
Study design
The EVINCI (EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease) study is a ‘European Commission 7th Framework Program for Research and Innovation’-sponsored multimodality imaging project in 14 centres from 9 European countries.9 The characteristics of the study population have been already described in detail9 and are summarized in Table 1. Briefly, between March 2009 and June 2012, patients with symptoms suggestive of CAD and intermediate pre-test probability (20–90%)10,11 underwent a study of coronary anatomy by computed tomography coronary angiography (CTCA) and at least one coronary functional imaging test by myocardial perfusion scintigraphy (MPS), single-photon emission computed tomography (SPECT) or positron emission tomography (PET), and/or wall motion imaging (stress echocardiography or cardiac magnetic resonance), with the recommendation to perform invasive coronary angiography (ICA) with fractional flow reserve (FFR) in intermediate lesions. Each patient was followed-up for 30 days and the referral for coronary revascularization recorded. Ethical approval was provided by each centre, and all subjects gave written informed consent.
Table 1.
Patients' baseline characteristics
| Parameter | Overall population (n = 252) |
|---|---|
| Demographics, n (%) | |
| Age, years (mean ± SD) | 61 ± 9 |
| Male gender | 161 (64) |
| Clinical characteristics, n (%) | |
| Typical angina | 62 (25) |
| Atypical angina | 148 (59) |
| Non-anginal chest pain | 42 (17) |
| Pre-test probability of CAD | 59 ± 23 |
| Left ventricular ejection fraction | 59 ± 9 |
| Cardiovascular risk factors, n (%) | |
| Family history of CAD | 75 (30) |
| Diabetes mellitus | 68 (27) |
| Hypercholesterolemia | 161 (64) |
| Hypertension | 155 (62) |
| Smoking | 60 (24) |
| Obesity | 72 (29) |
| Invasive coronary angiography data, n (%) | |
| Normal coronaries or non-obstructive CAD | 158 (63) |
| Single-vessel disease | 60 (23) |
| Multi-vessel disease | 34 (14) |
| Myocardial perfusion imaging, n (%) | |
| Single-photon emission computed tomography | 180 (71) |
| 99mTc-Sestamibi | 103 (57) |
| 99mTc-Tetrofosmin | 77 (43) |
| Positron emission tomography | 72 (29) |
| 15O-Water | 63 (88) |
| 13N-Ammonia | 8 (11) |
| 82Rubidium | 1 (1) |
Data are given in absolute numbers and percentages (%), unless otherwise stated.
Image acquisition
Acquisition protocols were agreed on for each technique based on best available clinical practice. Individual core-labs were responsible for harmonization and quality control of imaging protocols. Details on imaging procedures and protocols can be found in the EVINCI publication.9 All EVINCI subjects in whom core-lab analyses of CTCA, MPS, and ICA were available were selected for the present hybrid sub-study (Figure 1). Accordingly, patients submitted to wall motion imaging modalities were not included in the analysis, because their format precludes formation of 3D hybrid data sets with CTCA. No further exclusion criterion was considered.
Figure 1.
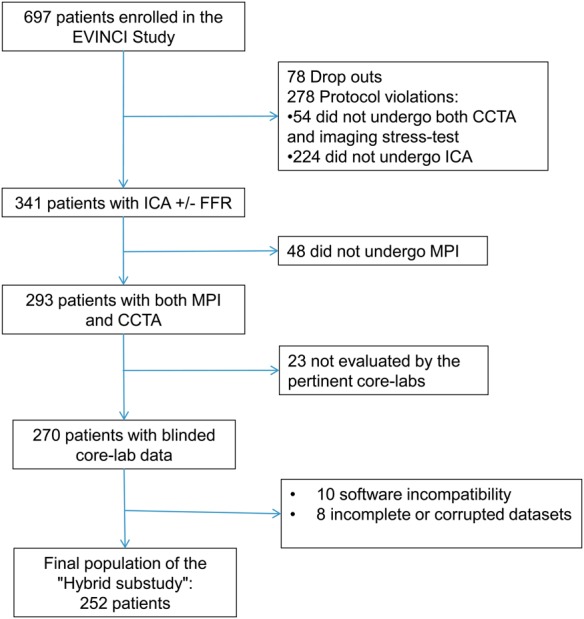
Patient flow chart. CTCA, coronary CT angiography; ICA, invasive coronary angiography; FFR, fractional flow reserve; MPS, myocardial perfusion imaging.
Image fusion
MPS and CTCA data sets were transferred to a dedicated hybrid core-lab blinded to clinical history and imaging findings (Cardiac Imaging, University Hospital Zurich, Switzerland). Image fusion of MPS and CTCA data sets was performed on a dedicated workstation (Advantage Workstation 4.4, GE Healthcare) using the CardIQ Fusion software package (GE Healthcare) as previously described.12 In case of H215O-PET images, parametric myocardial blood flow data sets, showing flows on a segmental level, were generated based on quantitative analysis performed using a commercially available software, PMOD 3.6 software package (PMOD Technologies Ltd, Zurich, Switzerland).
Hybrid analysis was performed using an optimized alignment tool, allowing projection of the MPS image on the left ventricular epicardial surface obtained from the CTCA, allowing a panoramic view of the coronary artery tree projected onto the left ventricular myocardial perfusion territories. In all patients, the image fusion procedure (including image generation and reading) was performed by two independent and blinded operators. Disagreement with regard to allocation of myocardial perfusion defects was resolved by consensus reading.
Image interpretation and definitions
Image interpretation was performed in dedicated core-labs as follows.
Computed tomography coronary angiography
CTCA was assessed using a modified 16-segment system13 and considered abnormal if at least one coronary segment had a diameter stenosis >50%. Significant left main stem stenoses were assigned to both left anterior descending (LAD) and left circumflex (LCX) coronary arteries. To limit any selection bias, any non-diagnostic segment was considered abnormal.
Myocardial perfusion scintigraphy
Perfusion in each of 17 segments14 was visually classified as 0 = normal, 1 = mild reduction, 2 = moderate reduction, 3 = severe reduction, or 4 = absent perfusion, and the segmental scores were summed for the stress (SSS) and rest (SRS) images. 15O-H2O PET data were processed and parametric perfusion images were scored similarly. The difference between SSS and SRS was calculated as the summed difference score (SDS). On per-patient analysis, a reversible perfusion defect (ischaemia) was defined as a SDS ≥2, either from a score ≥1 in at least two contiguous segments or ≥2 in at least one segment. Myocardial scar was defined similarly as a SRS ≥2. Accordingly, MPS studies were considered pathological in the presence of significant myocardial ischaemia and/or scar.
For per-vessel analysis, a reversible perfusion defect (ischaemia) was defined as a territorial difference score ≥1, and a scar as a rest score ≥1. Each perfusion defect was assigned to one or more coronary territories according to the standardized myocardial segmentation model.14 Similarly to CTCA analysis, any non-diagnostic segment was considered abnormal.
Invasive coronary angiography
Coronary angiograms were subdivided using the previously mentioned segmentation model13 and analysed using quantitative coronary angiography (QCA). A stenosis was considered haemodynamically significant if causing a >50% diameter reduction in the left main stem or >70% elsewhere, or between 30 and 70% with an FFR ≤0.80.
Hybrid images
All hybrid MPS/CTCA images were analysed by consensus of two independent readers with regard to the presence of matched, mismatched, or normal findings. A matched finding was defined as a perfusion defect in a territory subtended by a stenotic coronary. All other combinations of pathological findings were classified as mismatched. In the absence of pathological findings on both CTCA and MPS, hybrid images were considered normal. Finally, all pathological MPS segments were assigned to the pertinent vascular territory by spatial co-registration according to individual coronary anatomy by both operators to determine inter-observer agreement and repeatability of hybrid-based co-registration.
Statistical analysis
Statistical analysis was performed using the SPSS software. Continuous variables were expressed as mean ± SD, and categorical variables as percentages. Numerical values were compared using the Mann–Whitney U test or Student's t-test, and categorical values using the χ2 test. Inter-observer agreement was assessed using Cohen's kappa statistic. Sensitivity, specificity, and accuracy were calculated for each imaging method (MPS, CTCA, and hybrid imaging) on a per-vessel and per-patient basis. The McNemar test was performed to compare the accuracy of the different imaging methods against QCA ± FFR. A value of P < 0.05 was considered significant.
Results
Patient population
A total of 252 patients underwent CTCA, MPS, and ICA and were included in the analysis (Figure 1). The characteristics of the study populations are shown in Table 1. Compared with the overall EVINCI population,9 there were no significant differences in baseline characteristics except for a slightly higher CAD prevalence in our patient population (37 vs. 30%, P = 0.05) (Supplementary data online, Table SA).
Interestingly, as in the case of the main EVINCI population, also in the present study, traditional criteria for calculating pre-test probability11 overestimated the prevalence of haemodynamically significant CAD, which was 37% at QCA ± FFR. FFR was performed in 58/252 patients (23% of all patients and 66% of patients with intermediate coronary stenoses) and was abnormal (≤0.80) in 19 patients.
Imaging results: MPS and CTCA
A total of 180 (71%) patients were submitted to SPECT while 72 (29%) underwent PET (Table 2). Overall, 104 (41%) patients presented myocardial perfusion abnormalities in one (8%), two (41%), or three (51%) vascular territories. At core-lab analysis, MPS images were judged of non-diagnostic quality (having at least one non-diagnostic segment) in 11 patients.
Table 2.
Non-invasive imaging data
| Parameter | Overall population (n = 252) | SPECT (n = 180) | PET (n = 72) | P-value |
|---|---|---|---|---|
| Myocardial perfusion imaging data | ||||
| Normal perfusion | 148 (59) | 111 (62) | 37 (58) | 0.175 |
| Scar | 41 (16) | 35 (19) | 6 (8) | 0.037 |
| Inducible ischaemia | 88 (35) | 54 (30) | 34 (47) | 0.013 |
| Computed tomography data | 0.599 | |||
| One-vessel disease | 48 (19) | 38 (21) | 10 (14) | |
| Two-vessel disease | 41 (16) | 29 (16) | 12 (17) | |
| Three-vessel disease | 22 (9) | 15 (8) | 7 (10) | |
| Hybrid imaging | 0.054 | |||
| Hybrid match | 61 (24) | 39 (22) | 22 (31) | |
| Hybrid mismatch | 88 (35) | 68 (38) | 20 (28) | |
| MPS positive and CT negative | 39 (15) | 26 (14) | 13 (18) | |
| MPS negative and CT positive | 49 (19) | 42 (23) | 7 (10) | |
| Normal hybrid | 103 (41) | 73 (41) | 30 (42) | |
Data are given as numbers and percentages, n (%).
On CTCA, 111 (44%) patients presented significant CAD in one (48/111, 43%), two (41/111, 37%), or three (22/111, 20%) vessels (Table 2) with no significant difference between patients submitted to SPECT or PET. At core-lab analysis, CT images were judged of non-diagnostic quality (having at least one non-diagnostic segment) in 8 patients.
Hybrid imaging: feasibility and repeatability
In 18/270 (7%) patients originally submitted to CTCA and MPS, hybrid imaging could not be accomplished due to corruption of original data sets (8 patients) or software incompatibility (10 patients).
Inter-rater agreement of hybrid-based co-registration was good (κ = 0.75, 95% CI 0.70–0.80) with both observers agreeing in the classification of 92% of all pathological myocardial segments.
Hybrid imaging: segment reclassification
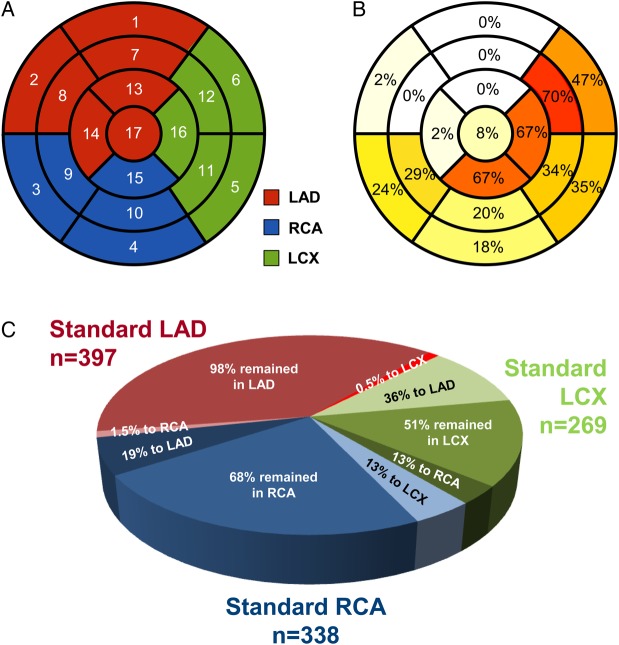
A total of 4284 myocardial segments were analysed, of which 1004 (23%) were pathological. According to the standard myocardial segmentation model, 397 (39%), 269 (27%), and 338 (34%) abnormal segments were allocated to the LAD, LCX, and right coronary artery (RCA) vascular territory, respectively. After image fusion, 246 (25%) of the 1004 abnormal myocardial segments were reclassified from their standard coronary distribution to another territory (Table 3). Segment reclassification was highest for the standard LCX (49%) and RCA (32%) segments, while it was very low for standard LAD segments (2%; P < 0.001 vs. both LCX and RCA). Figure 2 shows the proportion of pathological segments reassigned by hybrid imaging.
Table 3.
Hybrid-based reclassification of myocardial perfusion abnormalities
| Standard coronary distribution | Myocardial segments (17 segments LV model)a | Perfusion abnormality, n | Abnormal segment reclassified, n (%) | To LAD, n (%) | To LCX, n (%) | To RCA, n (%) |
|---|---|---|---|---|---|---|
| LAD | Segment 1 | 50 | 0 (0) | – | 0 (0) | 0 (0) |
| Segment 2 | 51 | 1 (2) | – | 0 (0) | 1 (100) | |
| Segment 7 | 56 | 0 (0) | – | 0 (0) | 0 (0) | |
| Segment 8 | 48 | 0 (0) | – | 0 (0) | 0 (0) | |
| Segment 13 | 62 | 0 (0) | – | 0 (0) | 0 (0) | |
| Segment 14 | 51 | 1 (2) | – | 0 (0) | 1 (100) | |
| Segment 17 | 79 | 6 (8) | – | 2 (33) | 4 (67) | |
| LCX | Segment 5 | 72 | 25 (35) | 0 (0) | – | 25 (100) |
| Segment 6 | 43 | 20 (47) | 19 (95) | – | 1 (5) | |
| Segment 11 | 58 | 20 (34) | 17 (85) | – | 3 (15) | |
| Segment 12 | 44 | 31 (70) | 30 (97) | – | 1 (3) | |
| Segment 16 | 52 | 35 (67) | 30 (86) | – | 5 (14) | |
| RCA | Segment 3 | 55 | 13 (24) | 10 (77) | 3 (23) | – |
| Segment 4 | 82 | 15 (18) | 0 (0) | 15 (100) | – | |
| Segment 9 | 52 | 15 (29) | 12 (80) | 3 (20) | – | |
| Segment 10 | 76 | 15 (20) | 0 (0) | 15 (100) | – | |
| Segment 15 | 73 | 49 (67) | 42 (86) | 7 (14) | – |
LV, left ventricle; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
aFor exact location of perfusion segment within the LV see Figure 2A.
Figure 2.
(A) Standardized myocardial segmentation model used in this study with number codes for each segment (see Table 3).14 (B) Reassignment rates by hybrid imaging for the 1004 pathological segments (the intensity of colours in each segment indicates the frequency of reassignment of that segment when pathological). (C) Pie chart indicating proportion of reassignment and reassignment fate for pathological segments in each standard coronary territory. Shades of red indicate standard LAD, of green standard LCX, and of blue standard RCA territories. Standard LCX segments were most often reassigned to LAD (36%), while standard RCA segments were equally distributed between LAD and LCX.
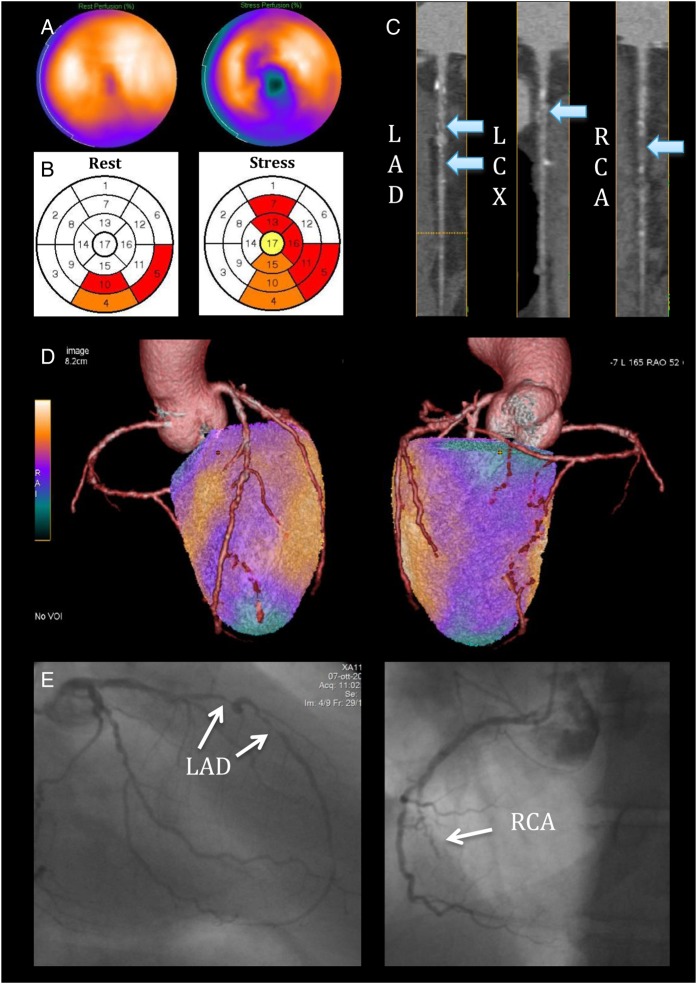
In 45/252 (18%) patients, hybrid imaging reassigned an entire perfusion defect to another coronary territory, changing the final diagnosis in 19 cases (from a mismatched to a matched finding in 16 patients, and the opposite in 3). Interestingly, in 16 (84%) of those patients, the myocardial perfusion abnormality was correctly assigned to a territory subtended by a haemodynamically significant stenosis at QCA ± FFR. The role of hybrid analysis in the anatomo-functional characterization of patients and in identifying significant CAD is exemplified in Figure 3.
Figure 3.
A 55-year-old gentleman with atypical chest pain. (A) SPECT shows a reversible perfusion defect inferiorly with lateral extension, and in addition, there is a separate reversible perfusion defect involving the apical region and the mid-ventricular anteroseptal wall. (B) The perfusion polar maps show the SPECT core-lab interpretation (white = normal, yellow = mildly reduced, orange = moderately reduced, and red = severely reduced radiotracer uptake) with pathological segments assigned to all three coronary territories. (C) CTCA reveals two 70–90% mid LAD stenoses, a 50% proximal LCX stenosis, and a probable occlusion of the mid RCA (arrows). (D) On hybrid imaging, the entire inferolateral perfusion defect is reassigned to the RCA, effectively changing the diagnosis from three-vessel to two-vessel disease. (E) Imaging findings were confirmed on QCA showing two high-grade lesions in the mid LAD, diffuse non-significant disease in the LCX, and a chronic total occlusion of the mid RCA.
‘Rule-in/rule-out’ clinical algorithm
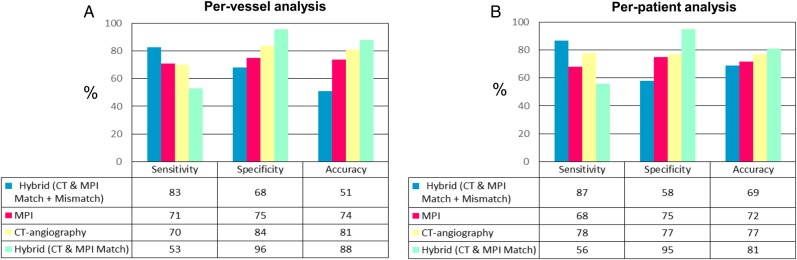
The diagnostic accuracy of hybrid imaging and of stand-alone imaging modalities in detecting significant CAD (QCA ± FFR) is reported in Figure 4.
Figure 4.
Accuracy analysis of stand-alone and hybrid protocols for the diagnosis of significant CAD (by QCA ± FFR) on per-vessel (A) and per-patient (B) analysis. On a per-vessel basis, when positivity was defined by the presence of at least one positive test (either matched or mismatched findings), hybrid imaging had higher sensitivity than single modalities (P < 0.001 vs. MPS and CTCA), at the price of lower specificity (P < 0.001 vs. both MPS and CTCA) and accuracy (P < 0.001 vs. both MPS and CTCA). When only matched findings were considered positive, hybrid imaging increased accuracy (P < 0.001 vs. both MPS and CTCA) driven by higher specificity (P < 0.001 vs. both MPS and CTCA) but with lower sensitivity (P < 0.001 vs. MPS and CTCA).
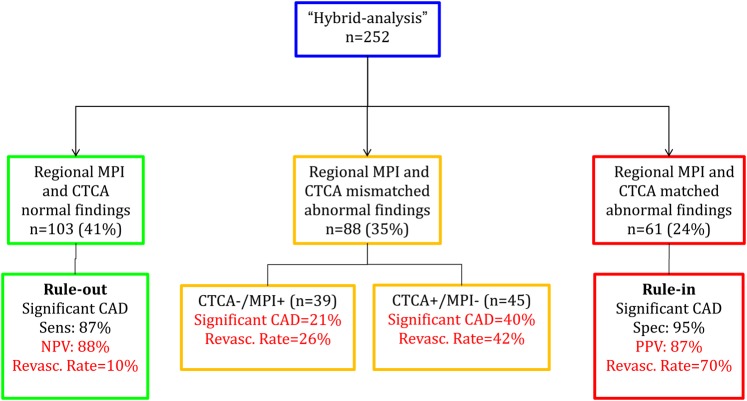
Specifically, a matched finding at hybrid imaging was found in 61 patients (24%), while 103 patients (41%) had normal hybrid findings. Of the remaining 88 patients with mismatched abnormal findings (35%), 45 presented a positive CTCA in the absence of perfusion abnormalities at MPS, while 39 showed a pathological MPS despite the absence of obstructive CAD at CTCA. Revascularization rates were 70% for matched hybrid images, 36% for mismatched findings, and 10% for normal findings (P < 0.001) (Figure 5).
Figure 5.
Hybrid-based ‘rule-in/rule-out’ clinical protocol.
Interestingly, among the 41 ‘false-negative’ hybrid studies (either normal or mismatched findings in the presence of significant CAD at QCA), the majority (80%) showed negative MPSs, despite a stenotic vessels on CTCA in 64% of the cases. FFR was performed in 17/41 patients and was positive in 13 (76%) (Supplementary data online, Table SB). On the other hand, the ‘false-positive’ hybrid studies were almost exclusively associated with the presence of intermediate coronary lesions (>30 and ≤70%) on QCA mainly in the absence of an invasive assessment of the haemodynamic relevance of stenoses by FFR (Supplementary data online, Table SC).
Radiation burden of the non-invasive imaging protocol
Average radiation doses in the study population were 7.9 mSv (range 0.6–24 mSv) for CTCA, 10.4 mSv (range 3.2–17.5 mSv) for SPECT, and 1.8 mSv (range 1.7–3.5 mSv) for PET. The average radiation dose of hybrid imaging was 9.4 mSv (range 5.2–21 mSv) for PET/CTCA and 18.5 mSv (range 6–31 mSv) for SPECT/CTCA (P < 0.001).
Discussion
The EVINCI hybrid sub-study is one of the largest studies to assess the clinical value of non-invasive hybrid imaging in stable CAD. Several methodological advantages, including the use of dedicated blinded core-lab image analysis, the multicentre and multivendor design, and the use of an accepted invasive gold standard (QCA ± FFR), distinguish it from previously published reports and provide greater uniformity and generalizability of its results. The main findings of the study are (i) large variability of coronary anatomy leading to systematic errors of standardized myocardial segmentation in predicting culprit coronary vessels; (ii) hybrid imaging (by 3D co-registration of CTCA and MPS) is feasible and reproducible; and (iii) a hybrid anatomo-functional protocol allows non-invasive ‘rule-in/rule-out’ of haemodynamically significant CAD.
Standardized myocardial segmentation models are widely used to assign myocardial territories to subtending coronary arteries.14 However, coronary anatomy is highly variable, which may frequently lead to mistaken identification of culprit vessels by standard models. In this respect, it has been previously suggested that hybrid imaging may help in the individual co-localization of myocardial perfusion abnormalities and subtending coronary arteries.15–18
We identified systematic deviation from the standardized assignment of myocardial segments in 25% of pathological segments, localized almost exclusively in the standard LCX and RCA territories (i.e. the lateral and inferior myocardial wall). This turned out to be clinically significant in almost every fifth patient, in whom the entire perfusion defect was reassigned to another coronary artery, changing the final diagnosis in almost half of them. This result might be of particular relevance in patients considered for revascularization, where only haemodynamically significant lesions deserve treatment.5,19
Previous reports have shown the feasibility and reproducibility of 3D fusion of anatomical (CTCA) and functional (MPS) imaging.12 In this study, hybrid analysis was successfully performed in 93% of the EVINCI patients originally submitted to MPS and CTCA with good inter-observer repeatability, highlighting the robustness of the technique. In fact, technical image fusion failure occurred in only 7% of patients mainly in the case of early generation SPECT devices with incomplete or corrupted data sets or software incompatibility.
Given the heterogeneity of hybrid results (combining various anatomo-functional patterns), we considered that a binary diagnostic approach disregards the complexity of CAD. Conversely, a ‘rule-in/rule-out’ hybrid-based approach appears more clinically meaningful, since matched positive findings allow rule-in of CAD and matched normal findings CAD rule-out (Figure 5). Accordingly, although in the EVINCI study the clinical management of patients, including the decision for coronary revascularization, was entirely left to the judgement of the local clinician, possibly introducing a bias in the analysis of the data, a matched positive hybrid finding was still associated with a high early revascularization rate (70%). On the other hand, in patients with a completely negative hybrid report, the revascularization rate was extremely low (≈10%), making ICA theoretically superfluous. It should be emphasized that the majority of false-negative hybrid studies were due to negative MPS downstream of a stenotic coronary vessel at CTCA, which was confirmed by a >70% lumen diameter reduction at QCA (considered as haemodynamically significant). After the FAME study,2 published almost at the end of the EVINCI study, coronary stenoses between 70 and 90% should also be submitted to FFR since a considerable proportion of these lesions have a normal FFR. On the other hand, the false-positive hybrid imaging studies were essentially associated with the presence of intermediate coronary lesions (>30 and ≤70%) that did not undergo an invasive evaluation of their haemodynamic relevance through FFR and, thus, considered as not significant. It is conceivable that, if FFR would have been more extensively performed, the number of ‘false-negative’ and ‘false-positive’ results could have been considerably reduced. Interestingly, a consistent proportion of those patients were still submitted to coronary revascularization despite the absence of an objective proof of myocardial ischaemia (either by MPS or through FFR) (Supplementary data online, Tables SB and SC), further highlighting the existing gap between evidence-based patient management1,3,5,19 and everyday clinical conduct.20
Patients with mismatched findings (positive MPS/negative CTCA or negative MPS/positive CTCA) represent a heterogeneous group. In the absence of coronary stenoses on CTCA, myocardial perfusion defects may represent either artefacts or microvascular/endothelial dysfunction. Accordingly, in this group, CAD prevalence and revascularization rates were low (Figure 5). CTCA has a very high negative predictive value as demonstrated by a vast number of studies comparing it with the angiographical gold standard of ICA.21 The fact that we used a more comprehensive anatomo-functional gold standard (ICA + FFR) may explain to some extent the low sensitivity. Moreover, the sensitivity of CTCA by core-lab analysis in the main EVINCI trial was lower than by individual-centre analysis.9 As a result, some lesions may have been underestimated accounting for the small number of revascularizations in this group.
Conversely, patients with significant coronary stenoses on CTCA but the absence of perfusion defects had a substantial CAD prevalence and revascularization rate (40 and 42%, respectively). This finding has several explanations. On one hand, the gold standard used in the present study was mainly anatomical (QCA), favouring agreement with CTCA rather than MPS. On the other hand, as already shown,22 the cut-off chosen for FFR (≤0.80)5,19 may overestimate the haemodynamic significance of CAD compared with non-invasive ischaemia testing. In line with this evidence, among the 19 patients with a pathological FFR evidenced in this study, only 21% had a matched finding on hybrid imaging. Interestingly, only 12/19 (63%) of those lesions presented a FFR ≤0.75, as a more stringent cut-off for positivity.3 However, the incomplete FFR penetration observed in the present study, mainly due to protocol violations, does not allow defining whether the use of a lower cut-off value of FFR would have better correlated with hybrid findings.
Such a ‘rule-in/rule-out’ protocol is supported by follow-up data, indicating low event rates in patients with normal hybrid findings, high event rates for pathological matched findings, and intermediate event rates with mismatched findings.8 Moreover, in selected cases, our integrated protocol may overcome the limitations of the more simplistic binary (i.e. either functional or anatomic) approach usually applied to CAD diagnostics, as recently reported.23
Limitations
Like the overall EVINCI population, our study had a significant dropout rate, as not every patient underwent all protocol-specified imaging studies. Additionally, data corruption and incomplete data sets accounted for further dropouts. Accordingly, 252 of the 697 patients originally enrolled in the EVINCI study were included in the present sub-study. However, those represented all the EVINCI patients that underwent MPS, CTCA, and ICA and in whom, thus, hybrid analysis could be practically performed. In fact, only a marginal portion of those patients (7%) was excluded because of technical reasons, confirming the overall robustness of 3D image fusion. Moreover, since the demographical, clinical, and angiographic characteristics of the present patients were almost superimposable to those of the main EVINCI population,9 the presence of a significant selection bias can be excluded (Supplementary data online, Table SA). Second, no long-term follow-up data were obtained precluding any analysis on the impact of hybrid imaging on downstream patient management and outcomes. Third, FFR rate was only 23%, and 34% of patients with intermediate lesions were not interrogated with FFR. Incomplete FFR penetration due to frequent protocol violations highlights the sub-optimal FFR use across Europe and may have been responsible for some of the ‘false-negative’ hybrid findings and prevents any conclusive analysis on the ‘false-positive’ studies (Supplementary data online, Tables SB and SC). In our study, the respective sensitivities of CTCA and MPS were lower than anticipated from small single-centre studies (particularly for CTCA: 78%). This may be explained by selecting higher risk patients who had additional MPS performed, as well as by the inclusion of patients with intermediate stenosis (30–70%) without invasive functional evaluation, and by the exclusive use of independent core-lab data for the present analysis. In fact, the accuracies of stand-alone imaging modalities reported were almost superimposable to those of the overall EVINCI study when only core-lab data were considered.9 Notably, on centre-based analysis, the diagnostic accuracy of the different non-invasive imaging modalities was generally improved compared with the core-lab data. Nevertheless, even when only individual-centre data were considered, hybrid imaging maintained significantly elevated specificity and overall diagnostic accuracy, at both per-patient and vessel-based analyses (Supplementary data online, Figure S1).
Moreover, in the accuracy analyses, MPS was considered pathological in the presence of ischaemia and/or scar. Interestingly, the presence of a matched hybrid finding showed comparable sensitivity, specificity, and accuracy if myocardial ischaemia (and not scar) was considered as the only positivity criteria (50, 96, and 79%, respectively).
Finally, the added radiation exposure from hybrid protocols must also be considered. In the present study, average radiation doses varied considerably, depending on the imaging technique (PET vs. SPECT) and on the acquisition protocol employed. Specifically, the theoretical risk related to the radiation exposure of a SPECT/CTCA hybrid protocol may appear rather high, particularly if compared with PET/CTCA imaging or other non-invasive imaging modalities.24 However, previous results suggest that the use of modern equipment and dose-optimization protocols (e.g. prospective ECG-triggering for CTCA, stress-only for SPECT) may consistently reduce the radiation burden of hybrid imaging,25 favouring its clinical application on a larger scale. Nevertheless, further long-term comparative studies are probably needed to conclusively define the cost-efficiency and quantitate the added radiation hazard that may be related to hybrid imaging, and to definitively assess its possible prognostic impact.
Conclusions
Hybrid imaging allows more reliable co-localization of myocardial perfusion defects with subtending coronary arteries than standardized myocardial segmentation models accounting for variations in individual coronary anatomy. In two-thirds of patients at intermediate pre-test probability of CAD, hybrid imaging may offer a non-invasive ‘rule-in/rule-out’ of patients with haemodynamically significant CAD.
Funding
This work was supported by European Union FP7-CP-FP506 2007 (grant no. 222915) and, in part, by Centre of Excellence in Cardiovascular and Metabolic Disease, Academy of Finland, Cardiovascular Biomedical Research Unit of the Royal Brompton & Harefield NHS Foundation Trust, NIHR Cardiovascular Biomedical Research Unit at St Bartholomew's Hospital, Ministry of Science and Higher Education, Poland, and unrestricted grant and products from General Electric Healthcare.
Supplementary Material
References
- 1. Task Force Members. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 2. Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H et al. . Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 2008;248:414–23. [DOI] [PubMed] [Google Scholar]
- 3. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN et al. . Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21. [DOI] [PubMed] [Google Scholar]
- 4. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM et al. . Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91. [DOI] [PubMed] [Google Scholar]
- 5. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z et al. . Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–17. [DOI] [PubMed] [Google Scholar]
- 6. Flotats A, Knuuti J, Gutberlet M, Marcassa C, Bengel FM, Kaufmann PA et al. . Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 2011;38:201–12. [DOI] [PubMed] [Google Scholar]
- 7. Gaemperli O, Bengel FM, Kaufmann PA. Cardiac hybrid imaging. Eur Heart J 2011;32:2100–8. [DOI] [PubMed] [Google Scholar]
- 8. Pazhenkottil AP, Nkoulou RN, Ghadri JR, Herzog BA, Buechel RR, Küest SM et al. . Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J 2011;32:1465–71. [DOI] [PubMed] [Google Scholar]
- 9. Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S et al. . Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8:pii:e002179. doi:11.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 10. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–8. [DOI] [PubMed] [Google Scholar]
- 11. Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F et al. . Guidelines on the management of stable angina pectoris: full text. The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341–81. [DOI] [PubMed] [Google Scholar]
- 12. Gaemperli O, Schepis T, Kalff V, Namdar M, Valenta I, Stefani L et al. . Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging 2007;34:1097–106. [DOI] [PubMed] [Google Scholar]
- 13. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS et al. . A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5–40. [DOI] [PubMed] [Google Scholar]
- 14. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al. . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 15. Javadi MS, Lautamäki R, Merrill J, Voicu C, Epley W, McBride G et al. . Definition of vascular territories on myocardial perfusion images by integration with true coronary anatomy: a hybrid PET/CT analysis. J Nucl Med 2010;51:198–203. [DOI] [PubMed] [Google Scholar]
- 16. Kajander S, Joutsiniemi E, Saraste M, Pietilä M, Ukkonen H, Saraste A et al. . Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 2010;122:603–13. [DOI] [PubMed] [Google Scholar]
- 17. Danad I, Raijmakers PG, Appelman YE, Harms HJ, de Haan S, van den Oever ML et al. . Hybrid imaging using quantitative H215O PET and CT-based coronary angiography for the detection of coronary artery disease. J Nucl Med 2013;54:55–63. [DOI] [PubMed] [Google Scholar]
- 18. Schaap J, Kauling RM, Boekholdt SM, Nieman K, Meijboom WB, Post MC et al. . Incremental diagnostic accuracy of hybrid SPECT/CT coronary angiography in a population with an intermediate to high pre-test likelihood of coronary artery disease. Eur Heart J Cardiovasc Imaging 2013;14:642–9. [DOI] [PubMed] [Google Scholar]
- 19. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M et al. . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 20. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV et al. . Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stein PD, Yaekoub AY, Matta F, Sostman HD. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med 2008;121:715–25. [DOI] [PubMed] [Google Scholar]
- 22. Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J et al. . Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv 2010;3:307–14. [DOI] [PubMed] [Google Scholar]
- 23. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B et al. . Outcomes of Anatomical versus Functional Testing of Coronary Artery Disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fazel R, Gerber TC, Balter S, Brenner DJ, Carr JJ, Cerqueira MD et al. . Approaches to enhancing radiation safety in cardiovascular imaging: a scinetific statement from the American Heart Association. Circulation 2014;130:1730–48. [DOI] [PubMed] [Google Scholar]
- 25. Benz DC, Templin C, Kaufmann PA, Buechel RR. Ultra-low-dose hybrid single photon emission computed tomography and coronary computed tomography angiography: a comprehensive and non-invasive diagnostic workup of suspected coronary artery disease. Eur Heart J 2015;36:3345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.