Abstract
Background: Paracetamol exposure has been positively associated with asthma development. The relative importance of prenatal vs infant exposure and confounding by indication remains elusive. We examined the association of prenatal and infant (first 6 months) paracetamol exposure with asthma development while addressing confounding by indication.
Methods: We used information from the Norwegian Mother and Child Cohort Study, including 53169 children for evaluation of current asthma at 3 years, 25394 for current asthma at 7 years and 45607 for dispensed asthma medications at 7 years in the Norwegian Prescription Database. We calculated adjusted relative risks (adj. RR) and 95% confidence intervals (CI) using log-binomial regression.
Results: There were independent modest associations between asthma at 3 years with prenatal paracetamol exposure (adj. RR 1.13; 95% CI: 1.02–1.25) and use of paracetamol during infancy (adj. RR 1.29; 95% CI: 1.16–1.45). The results were consistent for asthma at 7 years. The associations with prenatal paracetamol exposure were seen for different indications (pain, respiratory tract infections/influenza and fever). Maternal pain during pregnancy was the only indication that showed an association both with and without paracetamol use. Maternal paracetamol use outside pregnancy and paternal paracetamol use were not associated with asthma development. In a secondary analysis, prenatal ibuprofen exposure was positively associated with asthma at 3 years but not asthma at 7 years.
Conclusions: This study provides evidence that prenatal and infant paracetamol exposure have independent associations with asthma development. Our findings suggest that the associations could not be fully explained by confounding by indication.
Keywords: Asthma, epidemiology, ibuprofen, paracetamol
Key Messages
Prenatal and infant paracetamol exposures were independently associated with asthma development after adjusting for common indications.
We compared associations between several conditions during pregnancy, with and without the use of paracetamol, and asthma development in the offspring. Our findings suggest that the associations could not be fully explained by confounding by indication.
Maternal use of paracetamol outside pregnancy and paternal use of paracetamol did not show associations with asthma development in the offspring, indicating little influence by unmeasured characteristics reflected in the propensity for using paracetamol.
Introduction
Asthma is the most common chronic disease during childhood. 1,2 Prenatal and infant paracetamol exposures are proposed to be positively associated with asthma. 3–5 However, few previous studies were able to evaluate the relative importance of prenatal vs infant paracetamol exposure. 6–8 Paracetamol is the recommended analgesic/antipyretic for pregnant women and infants. 9 If the observed associations reflect true underlying effects, this is a public health concern. A limited number of previous studies of prenatal paracetamol exposure and asthma development considered confounding by indication. 7,10,11
An inherent limitation in observational studies is the possibility of unmeasured confounding. One approach to examine whether associations in observational studies might reflect unmeasured characteristics influencing the propensity for exposure is to use negative controls. 12,13 Examples of negative controls include maternal use of paracetamol outside pregnancy and the father’s use of paracetamol. If the associations are similar for maternal paracetamol use outside pregnancy and paternal paracetamol use, it is likely that confounding by genetic or shared environmental characteristics is present. 12,13
The objective of the study was thus to examine the association of prenatal and infant paracetamol exposure with asthma development while addressing confounding by indication. To further assess whether the previously reported associations reflected unmeasured confounding, we evaluated maternal paracetamol use outside pregnancy and paternal paracetamol use. Since ibuprofen is used for many of the same indications as paracetamol, we also evaluated the association between prenatal ibuprofen exposure and asthma development.
Methods
Study population
We used data from the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health. 14,15 MoBa recruited pregnant women between 1999 and 2008, at approximately 18 weeks of gestation. The participation rate of invited pregnant women was 40.6%. Mothers could participate with more than one pregnancy, resulting in approximately 95 200 mothers and 114500 children. All participants gave written informed consent. In May 2014, the 114761 children participating in MoBa were linked to the Medical Birth Registry of Norway (henceforth called ‘birth registry’) and the Norwegian Prescription Database (henceforth called ‘prescription registry’). Children not linked to the birth registry ( n = 499) and children from multiple births ( n = 3,971) were not eligible for the current study. We used information from the MoBa questionnaires completed at 18 and 30 gestational weeks and when the child was 6 months, 3 years and 7 years old. The Norwegian Data Inspectorate and the Regional Ethics Committee for Medical Research of South/East Norway approved this study.
Exposures
Participating mothers reported the use of medications for different indications during pregnancy through questionnaires completed at 18 gestational weeks, 30 gestational weeks and when the child was 6 months old. The mother was asked if she had experienced the disease/symptom of interest, and to list the medications she had used for that specific disease/symptom. The mother also listed the child’s use of medications during the first 6 months of life. Medications were coded using the Anatomical Therapeutic Chemical classification system (ATC). Medications containing paracetamol (N02BE01, N02AA59, N02AX52 and N02BE51) were extracted. Three of the medications contained other active ingredients, namely codeine (N02AA59), tramadol (N02AX52) and caffeine (N02BE51). To distinguish between prenatal vs infant paracetamol exposure, we classified mutually exclusive exposure categories: no exposure, prenatal exposure only, infant exposure only, both prenatal and infant exposure.
Outcomes
We examined two asthma outcomes based on the MoBa questionnaires and one outcome based on dispensed asthma medications. (i) We defined current asthma at 3 years as a ‘yes’ in response to current asthma in combination with use of short- or long-acting beta2-agonists (R03AC) and/or inhaled corticosteroids (R03BA) in the past year reported in the 3-year questionnaire. (ii) Current asthma at 7 years was defined according to three positive criteria reported in the 7-year questionnaire: a positive report of ever asthma, experiencing asthma symptoms during the past year and using medications for asthma in the past year. (iii) A separate asthma definition at age 7 was based on information from the prescription registry. A child was regarded as having asthma if he/she had a dispensed asthma medication during the past year in addition to a second dispensed prescription within 1 year after the first. Asthma medications included R03AC, R03BA, R03AK and R03DC.
Other variables
Maternal characteristics included age, parity, education, pre-pregnancy body mass index (weight in kilograms divided by measured height in metres squared), smoking during pregnancy, asthma, respiratory tract infections/influenza during pregnancy, pain during pregnancy, fever during pregnancy and use of antibiotics during pregnancy. Child characteristics included the child’s gender, birthweight, breastfeeding in the first 6 months, respiratory tract infections in the first 6 months, body mass index at 6 months and use of antibiotics during the first 6 months. We classified antibiotics for systemic use based on ATC codes J01A-J01X.
Statistical analyses
The analysis consisted of several steps. First, we examined the associations between prenatal and infant paracetamol exposure with asthma development using log-linear regression, reporting relative risks (RR) and 95% confidence intervals (CI). The multivariable analyses adjusted for all the potential confounding factors as in Table 1 . We used cluster variance estimation to account for siblings in the analyses. In order to evaluate whether maternal history of asthma was an effect modifier on the multiplicative scale, we included product terms in the multivariable regression models.
Table 1.
Characteristics by prenatal and infant paracetamol exposure ( N = 53169)
| Characteristics | n | No exposure (%) | Prenatal exposure only (%) | Infant exposure only (%) | Both prenatal and infant exposure (%) |
|---|---|---|---|---|---|
| Maternal age, years | |||||
| < 25 | 4758 | 36.0 | 30.1 | 15.2 | 18.8 |
| 25–29 | 17725 | 36.8 | 27.8 | 15.7 | 19.7 |
| 30–34 | 21137 | 37.0 | 27.8 | 15.7 | 19.5 |
| > = 35 | 9549 | 40.4 | 27.3 | 14.9 | 17.4 |
| Maternal parity | |||||
| Primiparous | 25486 | 40.6 | 27.3 | 15.5 | 16.6 |
| 1 | 18158 | 33.6 | 27.9 | 16.1 | 22.4 |
| 2 | 7488 | 35.4 | 29.8 | 14.5 | 20.3 |
| 3 or more | 2037 | 38.9 | 28.8 | 14.3 | 17.9 |
| Maternal education | |||||
| Less than high school | 2910 | 38.2 | 27.9 | 14.2 | 19.8 |
| High school | 14175 | 37.6 | 29.9 | 14.1 | 18.4 |
| Up to 4 years of college | 22841 | 36.1 | 27.7 | 15.9 | 20.3 |
| More than 4 years of college | 13046 | 39.5 | 26.1 | 16.7 | 17.8 |
| Missing | 197 | 37.6 | 29.4 | 16.2 | 16.8 |
| Maternal pre-pregnancy BMI | |||||
| Underweight (< 18.5) | 1520 | 41.7 | 25.5 | 15.9 | 16.9 |
| Normal weight (18.5–24.9) | 34625 | 39.2 | 26.8 | 16.0 | 18.0 |
| Overweight (25–29.9) | 11247 | 33.8 | 30.1 | 14.9 | 21.2 |
| Obese ( > = 30) | 4626 | 30.7 | 32.1 | 13.3 | 23.9 |
| Missing | 1151 | 42.1 | 26.2 | 14.9 | 16.9 |
| Maternal asthma | |||||
| No | 49338 | 38.2 | 27.6 | 15.5 | 18.7 |
| Yes | 3831 | 28.5 | 31.9 | 15.2 | 24.4 |
| Maternal RTI/influenza during pregnancy | |||||
| No | 21248 | 45.8 | 22.8 | 17.7 | 13.8 |
| Yes | 31921 | 31.9 | 31.3 | 14.1 | 22.7 |
| Maternal fever during pregnancy | |||||
| No | 44359 | 41.1 | 25.3 | 16.9 | 16.7 |
| Yes | 8810 | 18.9 | 41.0 | 8.6 | 31.5 |
| Maternal pain during pregnancy | |||||
| No | 12329 | 53.7 | 17.5 | 18.9 | 10.0 |
| Yes | 40840 | 32.6 | 31.1 | 14.5 | 21.9 |
| Maternal smoking during pregnancy | |||||
| No | 48947 | 37.8 | 27.6 | 15.6 | 19.0 |
| Yes | 3970 | 32.6 | 31.8 | 14.1 | 21.4 |
| Missing | 252 | 38.5 | 31.8 | 11.1 | 18.7 |
| Child gender | |||||
| Male | 27193 | 37.1 | 26.6 | 16.5 | 19.8 |
| Female | 25976 | 37.8 | 29.3 | 14.5 | 18.4 |
| Child birthweight, grams | |||||
| <2500 | 1332 | 38.5 | 28.8 | 12.8 | 19.9 |
| 2500–2999 | 4505 | 39.6 | 26.3 | 16.0 | 18.1 |
| 3000–3499 | 15523 | 38.3 | 27.6 | 15.6 | 18.5 |
| 3500–4000 | 20023 | 37.1 | 27.9 | 15.7 | 19.3 |
| 4000 | 11764 | 36.0 | 28.8 | 15.2 | 20.0 |
| Missing | 22 | 31.8 | 27.3 | 13.6 | 27.3 |
| Child breastfeeding the first 6 months | |||||
| None | 1360 | 34.6 | 29.8 | 13.4 | 22.2 |
| Partial | 28191 | 35.3 | 28.5 | 15.6 | 20.7 |
| Exclusive | 23618 | 40.2 | 27.1 | 15.6 | 17.1 |
| Child RTI the first 6 months | |||||
| No | 11441 | 47.4 | 32.1 | 10.0 | 10.6 |
| Yes | 40343 | 34.3 | 26.6 | 17.2 | 21.8 |
| Missing | 1385 | 46.8 | 30.8 | 11.7 | 10.8 |
| Child BMI at 6 months | |||||
| < 16.1 | 12573 | 38.4 | 28.0 | 15.1 | 18.6 |
| 16.1–17.0 | 12559 | 37.2 | 28.1 | 15.5 | 19.2 |
| 17.1–18.0 | 12743 | 37.1 | 28.4 | 15.4 | 19.1 |
| 18.1 | 12520 | 36.8 | 27.1 | 16.2 | 20.0 |
| Missing | 2774 | 39.3 | 28.2 | 14.8 | 17.7 |
| Prenatal and infant antibiotic exposure | |||||
| None | 44143 | 39.1 | 27.2 | 15.7 | 18.0 |
| Prenatal only | 6387 | 28.8 | 33.7 | 12.9 | 24.6 |
| Infant only | 2188 | 31.8 | 25.3 | 19.2 | 23.7 |
| Prenatal and infant | 451 | 23.7 | 31.0 | 14.2 | 31.0 |
The frequencies in this table are based on the study sample used to examine current asthma at 3 years.
RTI/influenza during pregnancy included maternal report of upper respiratory tract infections (ear, throat, sinus infections and/or colds) lower respiratory tract infections (pneumonia and/or bronchitis) and/or influenza.
Pain during pregnancy included maternal report of pelvic prolapse, back pain, neck/shoulder pain, migraine/headache, fibromyalgia and/or unspecified muscle pain.
RTI, respiratory tract infections.
Second, we evaluated the likelihood of confounding by indication by focusing on three common indications for use of paracetamol in pregnancy: pain, fever and respiratory tract infections/influenza. There was little a priori evidence that maternal pain during pregnancy might be associated with asthma development in the offspring. However, we wanted to evaluate whether maternal use of paracetamol for different/partly unrelated indications yielded similar associations with asthma development in the offspring. We categorized mutually exclusive exposure categories of mothers who had experienced the different indications with and without the use of paracetamol. In order to evaluate whether these maternal indications acted as effect modifiers on the multiplicative scale, we included product terms in the multivariable regression models.
Third, we conducted sensitivity analyses to explore unmeasured confounding by background factors influencing the propensity for paracetamol exposure by examining maternal use of paracetamol the past 6 months before pregnancy, maternal use of paracetamol during the first 6 months after pregnancy and the father’s paracetamol use.
Fourth, since paracetamol and ibuprofen are used for similar indications, information about maternal use of ibuprofen during pregnancy was extracted (M01AE01). We subsequently evaluated prenatal exposure to paracetamol and ibuprofen in combination (neither, only ibuprofen, only paracetamol, both ibuprofen and paracetamol), to evaluate whether maternal use of these two medications during pregnancy yielded the same association with asthma development.
There were approximately 10-15% of observations with missing information in the multivariable analyses. We therefore conducted multiple imputation by chained equations, imputing a total of 20 datasets.
All of the P -values presented are two-sided. We conducted the analyses using Stata version 13 (Statacorp, TX).
Results
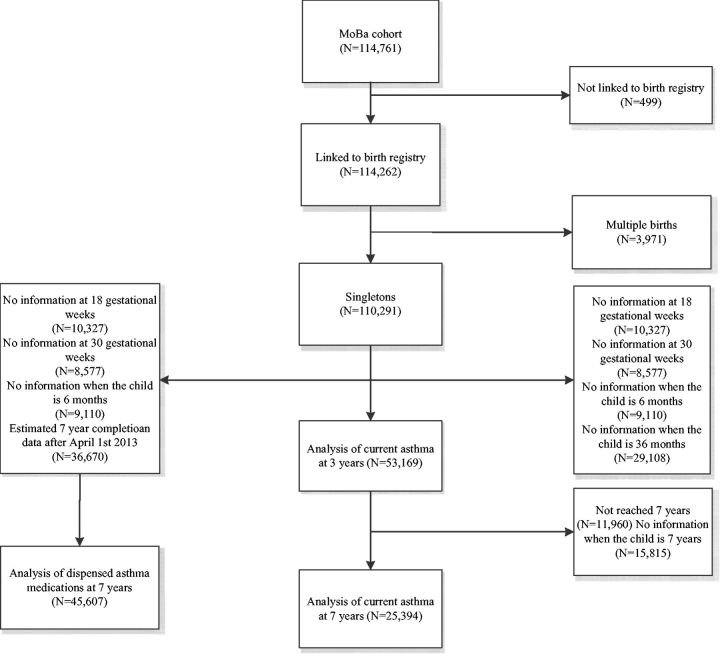
A total of 53169 children were included in the analysis of current asthma at 3 years, 25394 in the evaluation of current asthma at 7 years and 45607 in the evaluation of dispensed asthma medications at 7 years ( Figure 1 ). The characteristics among individuals with and without the necessary follow-up information are given in Supplementary Table 1 (available as Supplementary data at IJE online). A total of 27.9% of children were only exposed to paracetamol during pregnancy, 15.5% only during infancy and 19.1% were exposed both during pregnancy and infancy. The distribution of characteristics by prenatal and infant paracetamol exposure is given in Table 1 .
Figure 1.
Illustration of sample selection.
A total 5.7% of children had current asthma at 3 years, 5.1% had current asthma at 7 years and 4.8% had dispensed asthma medications at 7 years. There were independent associations between asthma at 3 years with prenatal paracetamol exposure (adj. RR 1.13; 95% CI: 1.02–1.25) and use of paracetamol during infancy (adj. RR 1.29; 95% CI: 1.16–1.45) ( Table 2 ). Similar results were observed for current asthma at 7 years and dispensed asthma medications at 7 years ( Table 2 ).
Table 2.
The association of prenatal and infant paracetamol exposures with development of asthma
| Current asthma at 3 years ( N = 53 169) | n | % case | Unadjusted RR (95% CI) | Adjusted RR (95% CI) a |
|---|---|---|---|---|
| Prenatal and infant paracetamol exposures | ||||
| No exposure | 19912 | 4.4 | 1 | 1 |
| Prenatal exposure only | 14837 | 5.9 | 1.33 (1.21–1.46) | 1.13 (1.02–1.25) |
| Infant exposure only | 8246 | 6.5 | 1.47 (1.32–1.63) | 1.29 (1.16– 1.45) |
| Both prenatal and infant exposures | 10174 | 7.5 | 1.71 (1.55–1.88) | 1.27 (1.14–1.41) |
| Current asthma at 7 years ( N = 25 394) | ||||
| Prenatal and infant paracetamol exposure | ||||
| No exposure | 9905 | 3.9 | 1 | 1 |
| Prenatal exposure only | 7240 | 5.6 | 1.44 (1.26–1.66) | 1.27 (1.09–1.47) |
| Infant exposure only | 3763 | 5.2 | 1.35 (1.14–1.60) | 1.24 (1.03–1.48) |
| Both prenatal and infant exposures | 4486 | 6.9 | 1.78 (1.54–2.06) | 1.49 (1.27–1.75) |
| Dispensed asthma medications at 7 years ( N = 45 607) | ||||
| Prenatal and infant paracetamol exposure | ||||
| No exposure | 17840 | 3.9 | 1 | 1 |
| Prenatal exposure only | 12974 | 5.2 | 1.33 (1.20–1.48) | 1.17 (1.04–1.31) |
| Infant exposure only | 6708 | 5.2 | 1.33 (1.17–1.51) | 1.27 (1.11–1.46) |
| Both prenatal and infant exposure | 8085 | 5.8 | 1.50 (1.33–1.68) | 1.26 (1.10–1.43) |
RTI/influenza during pregnancy included maternal report of upper respiratory tract infections (ear, throat, sinus infections and/or colds) lower respiratory tract infections (pneumonia and/or bronchitis) and/or influenza.
Pain during pregnancy included maternal report of pelvic prolapse, back pain, neck/shoulder pain, migraine/head ache, fibromyalgia and/or unspecified muscle pain.
Infant paracetamol exposure reflects the child’s use of paracetamol the first 6 months of life.
The results presented are based on a complete case analysis. A total of 47 173 children were included in the multivariable analysis of current asthma at 36 months, 22 102 children in the analysis of current asthma at 7 years and 38 677 in the analysis of dispensed asthma medications at 7 years.
a Associations adjusted for maternal age, parity, education, pre-pregnancy body-mass index, smoking during pregnancy, asthma, respiratory tract infections/influenza during pregnancy, fever during pregnancy, pain during pregnancy and antibiotic use during pregnancy, in addition to the child’s gender, birth weight, breastfeeding the first 6 months of life, respiratory tract infections by 6 months, body mass index at 6 months and use of antibiotics by 6 months.
There was no strong evidence of multiplicative interaction between prenatal and infant paracetamol exposure on asthma development ( P -values interaction > 0.07). Separate analyses of prenatal and infant paracetamol exposure with asthma development are included in Supplementary Table 2 (available as Supplementary data at IJE online). The results indicated a slightly stronger association between children exposed to paracetamol during two time periods of pregnancy with asthma development ( Supplementary Table 2 ).
Our findings indicated evidence of an interaction by maternal history of asthma on the association between infant paracetamol exposure and asthma at 3 years ( P -value interaction < 0.01) ( Supplementary Table 3 , available as Supplementary data at IJE online). However, there was no strong evidence of an interaction by maternal history of asthma for the association between prenatal paracetamol and asthma development, or the association between infant paracetamol exposure and the asthma outcomes at 7 years ( P -values interaction > 0.1) ( Supplementary Table 3 ).
We evaluated respiratory tract infections/influenza, fever and pain during pregnancy as common indications for using paracetamol. Prenatal paracetamol exposure for these three indications yielded similar associations with asthma development ( Table 3 ). Furthermore, pain showed a positive association with asthma development without the use of paracetamol ( Table 3 ). The strongest association was seen if the mother used paracetamol during pregnancy for more than one indication, adj. RR 1.94 (95% CI: 1.58–2.37) for current asthma at 3 years ( Table 3 ). In order to further evaluate the potential role of confounding by indication, we conducted stratified analyses of the association between prenatal paracetamol exposure and asthma development by the three indications in turn ( Supplementary Table 4 , available as Supplementary data at IJE online). The results indicated no strong evidence of multiplicative interaction ( P -values interaction > 0.3). Likewise, we also conducted a stratified analysis of the association between infant paracetamol exposure and asthma development by the child’s experience of respiratory tract infections during the first 6 months of life ( Supplementary Table 5 , available as Supplementary data at IJE online). The results indicated no evidence of multiplicative interaction ( P -values interaction > 0.5).
Table 3.
The association of maternal indications and use of paracetamol during pregnancy with development of asthma in the offspring
| Exposure | |||||
|---|---|---|---|---|---|
| Current asthma at 3 years ( N = 45641) | n | % cases | Unadjusted RR (95% CI) | Adjusted RR (95% CI) a | |
| Did not use paracetamol | Did not experience indications | 4660 | 3.4 | 1 | 1 |
| Experienced RTI/influenza only | 3623 | 3.8 | 1.12 (0.90–1.41) | 1.07 (0.85–1.34) | |
| Experienced fever b | 659 | 3.1 | 0.91 (0.58–1.44) | 0.88 (0.56–1.40) | |
| Experienced pain only | 8499 | 5.5 | 1.63 (1.36–1.95) | 1.47 (1.23–1.76) | |
| Experienced more than one indication | 10717 | 5.9 | 1.74 (1.47–2.07) | 1.60 (1.35–1.91) | |
| Used paracetamol | For RTI/influenza only | 1817 | 5.7 | 1.70 (1.33–2.17) | 1.51 (1.18–1.93) |
| For fever b | 3234 | 6.5 | 1.91 (1.56–2.35) | 1.58 (1.28–1.94) | |
| For pain only | 9575 | 6.9 | 2.06 (1.74–2.45) | 1.73 (1.46–2.06) | |
| For more than one indication | 2857 | 7.8 | 2.32 (1.90–2.84) | 1.94 (1.58–2.37) | |
| Current asthma at 7 years ( N = 21910) | |||||
| Did not use paracetamol | Did not experience indications | 2377 | 3.5 | 1 | 1 |
| Experienced RTI/influenza only | 1810 | 4.3 | 1.23 (0.90–1.68) | 1.15 (0.83–1.58) | |
| Experienced fever b | 316 | 2.2 | 0.64 (0.30–1.38) | 0.65 (0.30–1.38) | |
| Experienced pain only | 4075 | 4.5 | 1.29 (1.00–1.67) | 1.18 (0.90–1.53) | |
| Experienced more than one indication | 5090 | 4.6 | 1.32 (1.03–1.70) | 1.23 (0.95–1.58) | |
| Used paracetamol | For RTI/influenza only | 877 | 5.4 | 1.55 (1.09–2.21) | 1.39 (0.97–1.99) |
| For fever b | 1418 | 5.5 | 1.58 (1.16–2.15) | 1.34 (0.98–1.85) | |
| For pain only | 4623 | 6.2 | 1.81 (1.42–2.30) | 1.53 (1.19–1.96) | |
| For more than one indication | 1324 | 7.8 | 2.25 (1.69–2.99) | 1.91 (1.43–2.55) | |
| Dispensed asthma medications at 7 years ( N = 39157) | |||||
| Did not use paracetamol | Did not experience indications | 4107 | 3.5 | 1 | 1 |
| Experienced RTI/influenza only | 3067 | 3.0 | 0.87 (0.67–1.13) | 0.83 (0.64–1.08) | |
| Experienced fever b | 558 | 2.2 | 0.62 (0.34–1.11) | 0.63 (0.35–1.14) | |
| Experienced pain only | 7548 | 4.5 | 1.28 (1.06–1.55) | 1.21 (0.99–1.47) | |
| Experienced more than one indication | 9267 | 5.0 | 1.42 (1.18–1.71) | 1.36 (1.13–1.65) | |
| Used paracetamol | For respiratory RTI/influenza only | 1452 | 4.6 | 1.33 (1.00–1.76) | 1.24 (0.93–1.66) |
| For fever b | 2509 | 5.6 | 1.60 (1.28–2.01) | 1.41 (1.12–1.79) | |
| For pain only | 8263 | 5.9 | 1.71 (1.42–2.05) | 1.51 (1.25–1.83) | |
| For more than one indication | 2386 | 6.4 | 1.83 (1.46–2.29) | 1.59 (1.26–2.01) | |
RTI/influenza during pregnancy included maternal report of upper respiratory tract infections (ear, throat, sinus infections and/or colds) lower respiratory tract infections (pneumonia and/or bronchitis) and/or influenza. Pain during pregnancy included maternal report of pelvic prolapse, back pain, neck/shoulder pain, migraine/head ache, fibromyalgia and/or unspecified muscle pain. This analysis excluded individuals who used paracetamol for other/unspecified reason. This included 7528 children in the evaluation of current asthma at 3 years, 3484 children in the evaluation of current asthma at 7 years and 6450 children in the evaluation of dispensed asthma medications at 7 years. The results presented are based on a complete case analysis. A total of 43 789 children were included in the multivariable analysis of current asthma at 36 months, 20 800 children in the analysis of current asthma at 7 years and 37 783 in the analysis of dispensed asthma medications at 7 years.
RTI, respiratory tract infections.
a Associations adjusted for maternal age, parity, education, pre-pregnancy body mass index, smoking during pregnancy, asthma and antibiotic use during pregnancy
b The group who reported fever or use of paracetamol for fever were allowed to also report RTI/influenza or use of paracetamol for RTI/influenza. All other categories are mutually exclusive.
There was no strong evidence of an association between paternal paracetamol use and asthma in the offspring ( Supplementary Table 6 , available as Supplementary data at IJE online). Likewise, maternal paracetamol use during the past 6 months before pregnancy, or the first 6 months after delivery, showed no strong evidence of an association with asthma in the offspring ( Supplementary Table 6 ).
Prenatal exposure to ibuprofen was only reported for 5.8% of children. Prenatal exposure to ibuprofen only (no exposure to paracetamol) showed some evidence of an association with current asthma at 3 years, adj. RR 1.31 (95% CI: 1.00, 1.72), whereas there was weak evidence for a positive association with current asthma at 7 years and dispensed asthma medications at 7 years ( Table 4 ).
Table 4.
The association of maternal use of ibuprofen and paracetamol during pregnancy with development of asthma in the offspring
| Ibuprofen and paracetamol use during pregnancy | N | % case | Unadjusted RR (95% CI) | Adjusted RR (95% CI) a |
|---|---|---|---|---|
| Current asthma at 3 years ( N = 53169) | ||||
| Neither | 27392 | 5.0 | 1 | 1 |
| Only ibuprofen | 766 | 7.0 | 1.41 (1.08–1.84) | 1.31 (1.00–1.72) |
| Only paracetamol | 22675 | 6.5 | 1.32 (1.23–1.42) | 1.11 (1.02–1.19) |
| Both ibuprofen and paracetamol | 2336 | 6.6 | 1.34 (1.14–1.58) | 1.10 (0.93–1.30) |
| C urrent asthma at 7 years ( N = 25394) | ||||
| Neither | 13290 | 4.2 | 1 | 1 |
| Only ibuprofen | 378 | 4.9 | 1.16 (0.74–1.83) | 1.16 (0.73–1.83) |
| Only paracetamol | 10608 | 6.1 | 1.44 (1.29–1.61) | 1.26 (1.12–1.43) |
| Both ibuprofen and paracetamol | 1118 | 6.1 | 1.45 (1.14–1.86) | 1.28 (1.00–1.65) |
| Dispensed asthma medications at 7 years ( N = 45607) | ||||
| Neither | 23839 | 4.2 | 1 | 1 |
| Only ibuprofen | 709 | 4.8 | 1.13 (0.81–1.58) | 1.02 (0.73–1.44) |
| Only paracetamol | 19139 | 5.5 | 1.29 (1.19–1.40) | 1.12 (1.03–1.23) |
| Both ibuprofen and paracetamol | 1920 | 5.3 | 1.24 (1.02–1.52) | 1.03 (0.83–1.26) |
The results presented are based on a complete case analysis. A total of 51 011 children were included in the multivariable analysis of current asthma at 36 months, 24 114 children in the analysis of current asthma at 7 years and 44 009 in the analysis of dispensed asthma medications at 7 years.
a Associations adjusted for maternal age, parity, education, pre-pregnancy body mass index, smoking during pregnancy, asthma, respiratory tract infections/influenza during pregnancy, pain during pregnancy, fever during pregnancy and antibiotic use during pregnancy.
The results from the multiple imputation analyses were similar to the complete case analyses ( Supplementary Table 7 , available as Supplementary data at IJE online). A sensitivity analysis of prenatal paracetamol exposure excluding mothers who had reported multiple medications and indicated multiple time periods (during and outside pregnancy), approximately 9% of mothers, also indicated similar associations (data not shown).
Discussion
This large-scale prospective observational study indicated that both prenatal and infant paracetamol exposure showed independent positive associations with asthma development. Furthermore, the association between prenatal paracetamol exposure and asthma development was similar if used for respiratory tract infections/influenza, fever or pain. Prenatal ibuprofen exposure was only associated with an early asthma phenotype.
Comparison with previous studies
Our finding that prenatal and infant paracetamol exposures are positively associated with asthma development is largely in accordance with results summarized in meta-analyses. 3–5 However, most studies in these meta-analyses did not address confounding by indication.
Of the three previous studies examining prenatal paracetamol exposure and asthma development that adjusted for infections/antibiotics during pregnancy, all reported a positive association. 7,10,11 The magnitude of the associations in these studies ranged between 1.15 and 1.29. 7,10,11 Among previous studies of infant paracetamol exposure and asthma development that adjusted for the child’s experience of respiratory tract infections, 6,8,16–19 three studies reported a positive association. 6,16,19 These studies reported that paracetamol exposure during infancy was associated with a doubling in the risk of asthma development. 6,16,19 However, one of these studies indicated that the association was restricted to girls. 6 Our study is the first to evaluate the relative importance of prenatal vs infant paracetamol exposure and able to account for common indications during both exposure periods. This allowed us to show that both prenatal and infant paracetamol exposures had independent positive associations with asthma development after adjustment for confounding by indication during both exposure periods.
Two previous studies examined prenatal ibuprofen exposure and asthma development. 8,11 One of these studies examined both prenatal and infant ibuprofen exposure, reporting a positive association with infant ibuprofen exposure, adjusted odds ratio 1.20 (95% confidence interval 1.02-1.40), but no strong evidence of an association with prenatal ibuprofen exposure, 1.17 (0.78–1.76). 8 Our results indicated weak evidence for a positive association between prenatal ibuprofen exposure with asthma at 3 years and no strong evidence for an association with asthma at 7 years. We could not evaluate infant ibuprofen exposure as too few were exposed (0.1%).
Interpretation of main findings
Pregnant women who have asthma might be more likely to choose paracetamol as an analgesic/antipyretic compared with acetylsalicylic acid and other non-steroidal anti-inflammatory drugs, due to sensitization. 20 However, our results provided no strong evidence of an effect modification of maternal history of asthma on the observed associations.
This is the first study to address maternal conditions in pregnancy with and without the use of paracetamol in relation to asthma in the offspring. Maternal paracetamol use for respiratory tract infections/influenza, fever or pain all showed a positive association with asthma in the offspring, wheras maternal report of pain was the only indication that was positively associated with asthma in the offspring if the mother did not use paracetamol. Pain is highly subjective and likely influenced by a number of factors, and these findings therefore need to be replicated and explored in depth. One might speculate that maternal pain during pregnancy is a potential source of stress. A possible explanation for our finding is therefore the association between prenatal exposure to stress and asthma development. 21–24 However, our observed associations of prenatal and infant paracetamol exposure with asthma development might also reflect the severity of the condition leading to paracetamol use. It was not possible to further evaluate this using the information available.
We cannot exclude the possibility that the association of prenatal and infant paracetamol exposures with asthma development might be partly mediated by later paracetamol exposure.
The evaluation of maternal paracetamol use outside pregnancy and paternal paracetamol use was motivated by a negative controls approach. 12,13 Evaluation of these negative controls allowed an assessment of the likelihood of unmeasured confounding by characteristics that might influence the propensity for using paracetamol. Only one study has previously evaluated maternal paracetamol use after pregnancy and paternal paracetamol use. 25 In line with the findings from this previous study, we found no strong evidence for an association between maternal paracetamol use outside pregnancy or paternal paracetamol use with asthma in the offspring, supporting the conclusion that the results were not caused by underlying characteristics or health behaviour.
If the observed association between paracetamol exposure and asthma development reflects a true effect, proposed biological mechanisms include the ability of paracetamol to induce oxidative stress and enhance Th2 cell polarization and mediation of non-eosinophilic inflammatory responses. 26,27 Increased oxidative stress during pregnancy and infancy is hypothesized to increase the risk of asthma. 28 This hypothesis is also supported by studies reporting an inverse association between maternal antioxidant intake during pregnancy and asthma in the offspring. 29,30 Results from the Avon Longitudinal Study of Parents and Children also indicate that maternal antioxidant gene polymorphism may modify the association between prenatal paracetamol exposure and asthma development. 7
Ibuprofen is used for many of the same indications as paracetamol. According to Norwegian guidelines, ibuprofen is not recommended for pregnant women or infants who weigh less than 10 kg. 9 Based on our results, we could not conclude with certainty that prenatal ibuprofen exposure did not have a similar positive association with asthma development as observed for prenatal paracetamol exposure, since we likely had limited power to detect an association with our outcomes at 7 years. Ibuprofen is a COX-1 inhibitor, contributing to an up-regulation of leukotrienes, thought to play a role in asthma pathogenesis. 31
Strengths and limitations
Strengths of the current study include the size, detailed evaluation of different indications for prenatal paracetamol exposure and evaluation of prenatal ibuprofen exposure, in addition to the evaluation of maternal paracetamol use outside pregnancy and paternal paracetamol use as negative controls. This is the first study comparing the association between prenatal exposure with different indications, and use of paracetamol for different indications with development of asthma. It is also the first study examining the relative importance of prenatal vs infant paracetamol exposure in relation to asthma development, that was able to adjust for confounding by indication during both exposure periods.
The main limitation of the information available in MoBa was the ability to account for the amount of paracetamol used and severity of the underlying indications. However, by evaluating prenatal paracetamol exposure for more than one indication and during more than one time period of pregnancy, we evaluated two approaches to distinguish between amounts of exposure. The classification of prenatal and infant paracetamol exposures through questionnaires could have resulted in misclassification. Due to the prospective data collection, any misclassification of paracetamol exposure is unlikely to be differential by the child’s asthma status. Another limitation is that the information available for the child’s use of medications during the first 6 months of life did not include which specific condition the medications had been used for. By using maternal report of the child’s asthma status, there may also be misclassification of the outcome. However, maternal report that the child used asthma medications in the past year on the 7-years questionnaire in MoBa compared well with dispensed asthma medications in the prescription registry. 32 A comparison of MoBa participants against all Norwegian women who gave birth during the inclusion period indicated that several high-risk groups might be under-represented. 15 This might influence the generalizability of our results. 33–35 Our study further required information from a number of follow-up questionnaires. A comparison of eligible individuals with and without the necessary follow-up information indicated that mothers of children with the necessary follow-up information were older, were more likely to have higher education and were less likely to smoke ( Supplementary Table 1 ). We further evaluated bias due to loss to follow-up, by using dispensed asthma medications as an additional outcome which showed associations similar to those of the questionnaire-based outcome.
Conclusion
Our study is by far the largest study to provide evidence that prenatal and infant paracetamol exposures have independent positive associations with asthma development. Our findings suggest that the associations could not be fully explained by confounding by indication. Paracetamol is the most commonly used analgesic/antipyretic among pregnant women and infants, and uncovering potential adverse effects is of public health importance. Based on the inherent challenges in observational studies, evidence from a randomized controlled trial would be beneficial.
Funding
The data collection in the Norwegian Mother and Child Cohort Study is supported by National Institutes of Health [National Institute of Environmental Health Sciences contract number N01-ES-75558, National Institute of Neurological Disorders and Stroke grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1] and the Norwegian Research Council/FUGE [grant number 151918/S10]. This work was supported by the Norwegian Research Council [grant number 221919] and the Norwegian Extra-Foundation for Health and Rehabilitation [grant number 2011.2.0218 to M.C.M.]. The funding sources had no role in the study design; the collection, analysis and interpretation of data; or the writing of the article and the decision to submit it for publication. This work was supported by the UK Medical Research Council Integrative Epidemiology Unit and the University of Bristol (MC_UU_12013_1).
Supplementary Material
Acknowledgements
The authors are grateful to all families participating in the Norwegian Mother and Child Cohort Study.
Author contributions
The study was initiated by M.C.M and W.N., and designed by M.C.M., W.N. and S.E.H., and P.N.and M.C.M. performed the statistical analysis and wrote the initial draft with supervision from W.N., S.E.H. and P.N. Ø.K. and G.D.S. contributed with invaluable support for data analyses, interpretation of findings and critical revision of the manuscript. W.N. and M.C.M. obtained the financial support. All authors had full access to data and reviewed and approved the final version of the article submitted for publication. M.C.M. will act as the guarantor of the manuscript. The references have been checked for accuracy and completeness by M.C.M.
Conflict of interest: All authors report no conflict of interest.
References
- 1. Akinbami L. The state of childhood asthma, United States, 1980-2005 . Adv Data 2006. : 1 – 24 . [PubMed] [Google Scholar]
- 2. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) . Thorax 2009. ; 64 : 476 – 83 . [DOI] [PubMed] [Google Scholar]
- 3. Cheelo M, Lodge CJ, Dharmage SC. et al. . Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis . Arch Dis Child 2014. ; 100:81 – 89 . [DOI] [PubMed] [Google Scholar]
- 4. Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis . Chest 2009. ; 136 : 1316 – 23 . [DOI] [PubMed] [Google Scholar]
- 5. Eyers S, Weatherall M, Jefferies S, Beasley R. Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis . Clin Exp Allergy 2011. ; 41 : 482 – 89 . [DOI] [PubMed] [Google Scholar]
- 6. Bakkeheim E, Mowinckel P, Carlsen KH, Haland G, Carlsen KC. Paracetamol in early infancy: the risk of childhood allergy and asthma . Acta Paediatr 2011. ; 100 : 90 – 96 . [DOI] [PubMed] [Google Scholar]
- 7. Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma . J Allergy Clin Immunol 2010. ; 126 : 1141 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sordillo JE, Scirica CV, Rifas-Shiman SL. et al. . Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children . J Allergy Clin Immunol 2015. ; 135 : 441 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norwegian Medicines Agency . Use of Prescription Free Pain Killers . 2015. . http://www.legemiddelverket.no/Bruk_og_raad/raadtilforbruker/Smertestillende/Sider/default.aspx (25 February 2015, date last accessed).
- 10. Shaheen SO, Newson RB, Henderson AJ. et al. . Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood . Clin Exp Allergy 2005. ; 35 : 18 – 25 . [DOI] [PubMed] [Google Scholar]
- 11. Rebordosa C, Kogevinas M, Sorensen HT, Olsen J. Pre-natal exposure to paracetamol and risk of wheezing and asthma in children: a birth cohort study . Int J Epidemiol 2008. ; 37 : 583 – 90 . [DOI] [PubMed] [Google Scholar]
- 12. Richmond RC, Al-Amin A, Davey Smith G, Relton CL. Approaches for drawing causal inferences from epidemiological birth cohorts: a review . Early Hum Dev 2014. ; 90 : 769 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol 2008. ; 102 : 245 – 56 . [DOI] [PubMed] [Google Scholar]
- 14. Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) . Int J Epidemiol 2006. ; 35 : 1146 – 50 . [DOI] [PubMed] [Google Scholar]
- 15. Nilsen RM, Vollset SE, Gjessing HK. et al. . Self-selection and bias in a large prospective pregnancy cohort in Norway . Paediatr Perinat Epidemiol 2009. ; 23 : 597 – 608 . [DOI] [PubMed] [Google Scholar]
- 16. Kreiner-Moller E, Sevelsted A, Vissing NH, Schoos AM, Bisgaard H. Infant acetaminophen use associates with early asthmatic symptoms independently of respiratory tract infections: the Copenhagen Prospective Study on Asthma in Childhood 2000 (COPSAC(2000)) cohort . J Allergy Clin Immunol 2012. ; 130 : 1434 – 36 . [DOI] [PubMed] [Google Scholar]
- 17. Lowe AJ, Carlin JB, Bennett CM. et al. . Paracetamol use in early life and asthma: prospective birth cohort study . BMJ 2010. ; 341 : c4616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnabel E, Heinrich J. Respiratory tract infections and not paracetamol medication during infancy are associated with asthma development in childhood . J Allergy Clin Immunol 2010. ; 126 : 1071 – 73 . [DOI] [PubMed] [Google Scholar]
- 19. Wickens K, Beasley R, Town I. et al. . The effects of early and late paracetamol exposure on asthma and atopy: a birth cohort . Clin Exp Allergy 2011. ; 41 : 399 – 406 . [DOI] [PubMed] [Google Scholar]
- 20. Simon RA, Dazy KM, Waldram JD. Aspirin-exacerbated respiratory disease: characteristics and management strategies . Expert Rev Clin Immunol 2015. ; 11 : 805 – 17 . [DOI] [PubMed] [Google Scholar]
- 21. Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children . J Allergy Clin Immunol 2009. ; 123 : 847 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang F, Hoglund CO, Arck P. et al. . Maternal bereavement and childhood asthma - analyses in two large samples of Swedish children . PLoS One 2011. ; 6 : e27202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozyrskyj AL, Mai XM, McGrath P, Hayglass KT, Becker AB, Macneil B. Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma . Am J Respir Crit Care Med 2008. ; 177 : 142 – 47 . [DOI] [PubMed] [Google Scholar]
- 24. Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study . Am J Respir Crit Care Med 2002. ; 165 : 358 – 65 . [DOI] [PubMed] [Google Scholar]
- 25. Shaheen SO, Newson RB, Davey Smith G, Henderson AJ. Prenatal paracetamol exposure and asthma: further evidence against confounding . Int J Epidemiol 2010. ; 39:790 – 94 . [DOI] [PubMed] [Google Scholar]
- 26. Grainge CL, Davies DE. Epithelial injury and repair in airways diseases . Chest 2013. ; 144 : 1906 – 12 . [DOI] [PubMed] [Google Scholar]
- 27. Nassini R, Materazzi S, Andre E. et al. . Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents . FASEB J 2010. ; 24 : 4904 – 16 . [DOI] [PubMed] [Google Scholar]
- 28. Patelarou E, Giourgouli G, Lykeridou A. et al. . Association between biomarker-quantified antioxidant status during pregnancy and infancy and allergic disease during early childhood: a systematic review . Nutr Rev 2011. ; 69 : 627 – 41 . [DOI] [PubMed] [Google Scholar]
- 29. Devereux G, Turner SW, Craig LC. et al. . Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children . Am J Respir Crit Care Med 2006. ; 174 : 499 – 507 . [DOI] [PubMed] [Google Scholar]
- 30. Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life . Am J Respir Crit Care Med 2005. ; 171 : 121 – 28 . [DOI] [PubMed] [Google Scholar]
- 31. Levy S, Volans G. The use of analgesics in patients with asthma . Drug Saf 2001. ; 24 : 829 – 41 . [DOI] [PubMed] [Google Scholar]
- 32. Furu K, Karlstad O, Skurtveit S. et al. . High validity of mother-reported use of antiasthmatics among children: a comparison with a population-based prescription database . J Clin Epidemiol 2011. ; 64 : 878 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebrahim S, Davey Smith G. Commentary: Should we always deliberately be non-representative? Int J Epidemiol 2013. ; 42 : 1022 – 26 . [DOI] [PubMed] [Google Scholar]
- 34. Richiardi L, Pizzi C, Pearce N. Commentary: Representativeness is usually not necessary and often should be avoided . Int J Epidemiol 2013. ; 42 : 1018 – 22 . [DOI] [PubMed] [Google Scholar]
- 35. Rothman K, Hatch E, Gallacher J. Representativeness is not helpful in studying heterogeneity of effects across subgroups . Int J Epidemiol 2014. ; 43 : 633 – 34 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.