Abstract
Background: Several studies suggest that cancer is reduced before and after a Parkinson’s disease (PD) diagnosis. However, determining relationships among diseases of ageing is challenging due to possible biases in ascertaining disease. This study evaluates the PD and cancer relationship, addressing potential biases.
Methods: Using Surveillance, Epidemiology, and End Results-Medicare linked data (1992–2005) of adults ≥ 65 years, we assessed PD risk after cancer comparing PD in 743 779 cancer patients with PD in a non-cancer group ( n = 419 432) in prospective cohort analyses. We also conducted a case-control study of 836 947 cancer cases and 142 869 controls to assess cancer following PD. We applied Cox proportional hazards models to estimate hazards ratios (HRs) for PD after cancer and unconditional logistic regression to estimate odds ratios (ORs) for PD preceding cancer, controlling for physician visits and other factors. To explore biases in ascertaining cancer, we examined relationships between cancer and automobile accident injuries, which we expected to be null.
Results: No association was observed between cancer and subsequent PD [HR = 0.97; 95% confidence interval (CI) = 0.92-1.01] nor between cancer and subsequent automobile injuries (HR = 1.03; 95% CI = 0.98-1.07). One site, lung cancer, was associated with subsequent reduced PD, which may reflect confounding by smoking. In the case-control analysis, PD was associated with reduced subsequent cancer, overall (OR = 0.77; 95% CI = 0.71-0.82) and for several cancer sites. However, the automobile injury/ subsequent cancer association was similar (OR = 0.83; 95% CI = 0.78-0.88), suggesting a cancer detection bias after serious health outcomes.
Conclusions: In totality, our data do not support a biological relationship between PD and cancer.
Keywords: : Cancer, Parkinson’s disease, Medicare, SEER Program
Key Messages
Several previous epidemiological studies reported reduced risks of cancer after Parkinson’s disease and vice versa.
Whether these inverse associations reflect biological processes rather than methodological limitations remains uncertain.
In this large study using Surveillance, Epidemiology, and End Results-Medicare linked data that comprise about one-quarter of the elderly US population, there was no association between cancer and a subsequent diagnosis of Parkinson’s disease.
The odds of a cancer diagnosis preceded by Parkinson’s disease was reduced by 23%, similar to the odds of cancer preceded by automobile accident injuries, although automobile injuries are unlikely to be biologically related to cancer.
These associations with subsequent cancer are consistent with a bias in ascertaining cancer after serious medical conditions, and provide little support for a biological relationship between cancer and Parkinson’s disease.
Introduction
Several studies suggest that cancer risk is lower after a Parkinson’s disease (PD) diagnosis 1–6 and that PD risk is lower after a cancer diagnosis, 3,7–9 although one study found most cancers increased after PD. 10 An inverse relationship is most evident for smoking-related cancers, 1,2,11,12 although studies with many inverse findings also observe higher risks for melanoma both occurring before 7 and after a PD diagnosis. 1,2,6 There is substantial interest in whether cancer and PD are biologically related or whether these associations reflect methodological biases or uncontrolled confounding.
Determining relationships among diseases of ageing, such as cancer and PD, is challenging. There may be ascertainment biases due to reduced cancer screening/testing after diagnosing PD, especially in the elderly, disabled or cognitively impaired. 13–15 Only one study 2 accounted for the intensity of medical surveillance after a PD diagnosis, which may influence ascertaining subsequent disease. In addition, disease order may matter, which highlights the importance of assessing risk in both temporal directions. Further, examining relationships for specific cancer sites requires a large population.
To evaluate PD/cancer relationships and address potential biases, we analysed data from Medicare patients residing within the population-based Surveillance, Epidemiology and End-Results (SEER) Program registry areas. We estimated hazard ratios (HRs) for incident PD after cancer using a prospective design, and estimated odds ratios (ORs) of cancer after prevalent PD using a case-control design.
Methods
This study used the SEER-Medicare linked database. The SEER registries cover about one-fourth of the US population. 16 Medicare is a federal health insurance programme for those aged ≥ 65 years. The SEER-Medicare dataset links demographic and clinical information on SEER cancer cases/patients with Medicare claims. 16 Also included are Medicare claims on a 5% random beneficiary sample in SEER areas; thus the database represents the Medicare population in SEER areas. 17
Study design and cancer case and control/non-cancer comparison group selection
The SEER-Medicare dataset was used for: (i) a cancer case-control study of the odds of PD preceding cancer; and (ii) a cohort study of cancer and subsequent PD risk.
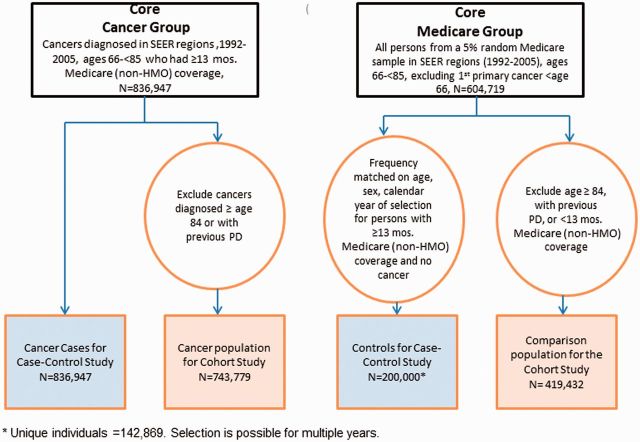
In the case-control study cancer cases were SEER patients with a first primary malignancy (1992–2005) in a SEER region. Cases were included if they had at least 13 months of Medicare coverage (Parts A and B and no health maintenance organization (HMO) participation) before cancer diagnosis, to ensure time to diagnose PD before the cancer diagnosis. HMO coverage was excluded because Medicare does not obtain HMO claims. 17 At diagnosis, cases were ages 66 through 84, because cancers may be under-ascertained in the oldest elderly. 15 Cases were also excluded if diagnosed only by autopsy or death certificate. Total cancer cases were N = 836 947 ( Figure 1 ).
Figure 1.
Flowchart of cancer and non-cancer groups.
In the case-control analysis, controls were selected from the 5% Medicare sample in SEER areas (1992–2005) and restricted to those aged 66 through 84 years, N = 604 719. Controls were frequency-matched to cancer cases by sex, age (5-year categories) and calendar year of selection if they had ≥ 13 months of previous Part A/Part B/non-HMO Medicare coverage and no cancer at selection. Additional details are presented in Engels et al.17 A total of 200 000 controls (sampled with replacements) were frequency matched, resulting in N = 142 869 individual controls ( Figure 1 ).
In the cohort study (PD after cancer), cancer patients were the same as cancer cases described above, minus those diagnosed at age 84 or older (to allow follow-up until age 85); and minus those diagnosed with PD before cancer, for a total of N = 743 779 cancer patients ( Figure 1 ).
Subjects in the 5% random sample selected during the 1992–2005 period were eligible to be part of the non-cancer comparison group in the cohort study if at selection they were ages 66-<84 years, had ≥ 13 months of Medicare coverage (Parts A/B/no HMO), with no cancer or PD: N = 419 432 ( Figure 1 ).
In both study designs, cancer sites were classified using the SEER ‘site recode with Kaposi sarcoma and mesothelioma’ variable according to International Classification of Diseases for Oncology (third edition, ICD-O-3). Cancers were also grouped into smoking-related and other cancers because of a strong inverse PD relationship with smoking-related cancers, 1,2,4,7 which included cancers of the oral cavity/pharynx, lip, pancreas, lung/bronchus, larynx, cervix, kidney/ renal pelvis, bladder, oesophagus and stomach. 18
Ascertainment of PD
PD was based on ICD-9 code 332.0 in Medicare claims. A person was considered diagnosed with PD if there was one hospital or two physician/outpatient PD claims at least 30 days apart (because hospital claims are more thoroughly audited), 17 a method for ascertaining disease similar to that used for other SEER-Medicare studies. 17,19
Statistical analyses
In the cohort analysis, we compared PD incidence in cancer patients with PD incidence in individuals without cancer. Follow-up began at cancer diagnosis or selection age (for the comparison group) and ended at the earliest age of PD diagnosis, discontinued Part B coverage, HMO transfer, death, cancer diagnosis (for the comparison group), attaining age 85 or 31 December 2005. We used Cox proportional hazards models with age as the time scale to estimate HRs and 95% confidence intervals (CIs) of PD associated with cancer.
In the case-control analyses, we compared the PD prevalence in cancer cases and controls using unconditional logistic regression models to estimate ORs and 95% CIs. We accounted for the control selection at multiple times and the use of controls who became cases in the variance calculation. 17
Common elements to both designs
We assessed associations stratified by sex, race/ethnicity and age at time of selection (66–< 70; 70–< 79; 80–< 85 years), and separately for specific cancer sites and smoking-related and other cancers. Associations by age strata for selected cancer sites are also presented. We limited cancer-site specific analyses to sites with at least 20 PD cases.
All models were adjusted for: sex, race/ethnicity, age (as the time metric in cohort models; in 5-year groups in the case-control models), cancer registry (due to differences in background incidences) and frequency of physician visits. For cohort models, the baseline hazard was also stratified on birth year (to account for secular trends in, e.g., diagnostic practices), and case-control analyses were adjusted for year of selection (1992–94, 1995–98, 1999–2005).
The first PD claim date was treated as the diagnosis date. PD risks were analysed across time intervals: < 1 year; 1–< 5 years; 5–< 10 years; and 0–< 10 years after cancer (prospective cohort study); and < 1 year; 1–< 5 years; and 0–< 5 years before cancer (case-control study) because some claims were limited to 5 years. 17 Also, because patients with serious disease often receive heightened medical surveillance, we adjusted for physician visit frequency. In the cohort analyses, physician visits were counted during 6-month intervals between the selection and censor dates and, in the case-control analyses, we adjusted for the average number of visits across all intervals (both analyses excluding the first and last interval.) Claims by physicians with limited responsibility for direct patient care (i.e. radiologists, anaesthesiologists, pathologists) were excluded.
Studies of cancer associated with automobile accident injuries
To evaluate ascertainment bias, we examined the relationship between cancer and automobile accident injuries (ICD-9 E810-819) occurring both before and after cancer. We expected no associations because we were unable to suggest a plausible hypothesis biologically relating automobile injuries to cancer. We also examined the risk of prostate and breast cancer after automobile accidents, two cancers on which potential confounding by smoking or alcohol would have little impact. As an acute injury, automobile injury claims were based on one medical visit. In prospective cohort analyses of cancer followed by automobile injuries, cancer patients were excluded if they had previous automobile injuries. In other respects, the analyses followed the models for cancer and PD.
We applied the Bonferroni correction to account for multiple comparisons when interpreting results of all the sub-groups and site-specific cancers for the 0–10 year follow-up period in the cohort analysis and thus used a corrected P -value of P < 0.0019 as a threshold for associations. P -values were based on two-sided tests. All analyses used SAS (Version 9.2, SAS Institute, Inc., Cary, NC). This study was exempted by the National Institutes of Health (NIH) Office of Human Subjects Research from institutional board approval.
Results
Prospective cohort study of PD after cancer
The characteristics of the 743 779 cancer patients and 419 432 persons in the comparison group in the cohort analysis are presented in Table 1a . The cancer patients were more likely to be older, male and selected later, but the racial/ethnicity distributions were similar. There were 2.1 million person-years of follow-up (average 2.8 years) in the cancer patients and 2.4 million (average 5.8 years), in the comparison group.
Table 1a.
Characteristics of cancer patients and non-cancer comparison group in prospective cohort analysis of Parkinson’s disease following cancer
| Cancer patients ( N = 743779) | Comparison group ( N = 419432) | |
|---|---|---|
| Age a at baseline in years, (%) | ||
| 66–< 70 | 23.1% | 64.7% |
| 70–< 80 | 59.8% | 29.6% |
| 80–< 85 | 17.1% | 5.7% |
| Median age | 74 | 67 |
| Sex, n (%) | ||
| Male | 55.1% | 41.5% |
| Female | 44.9% | 58.5% |
| Selection year, n (%) | ||
| 1992–94 | 17.7% | 57.9% |
| 1995–98 | 20.2% | 14.9% |
| 1999–2005 | 62.1% | 27.2% |
| Race/ethnicity, n (%) | ||
| White | 86.0% | 83.6% |
| Non-White | 14.0% | 16.4% |
| Black | 7.8% | 7.7% |
| Asian | 2.5% | 3.5% |
| Hispanic | 1.4% | 2.4% |
| Native American Indian | 0.2% | 0.3% |
| Other/unknown | 2.0% | 2.6% |
| Person-years | 2109511 | 2435783 |
Overall there was no association with PD within 10 years after cancer diagnosis (HR = 0.97; 95% CI = 0.92-1.01); Table 2 ; Supplementary Tables 1 and 2 , for unadjusted HRs, and age-specific incidence rates in cancer patients and the comparison groups, respectively, available as Supplementary data at IJE online). There were slight inverse associations between cancer and subsequent PD in men and in those aged between 70 and 84 years at baseline; however, these associations did not withstand multiple testing corrections. The associations for selected cancers (i.e. breast and prostate cancer, and melanoma) were similar across age groups ( Supplementary Table 3a , available as Supplementary data at IJE online).
Table 2.
Hazard ratio (HR) for Parkinson’s disease (PD) after first primary cancer diagnosis, 1992–2005. aP -values are not adjusted for multiple testing in the table
|
< 1-year follow-up
|
1-< 5-year follow-up
|
5–< 10-year follow-up
|
0–< 10-year follow-up
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | Comparison group | Cancer patients | HR | 95% CI | |
| PD cases ( n ) b | PD cases ( n ) b | |||||||||
| Overall | 1.15 | 0.96-1.37 | 0.83 | 0.77-0.91 | 0.93 | 0.85-1.03 | 5105 | 5893 | 0.97 | 0.92-1.01 |
| Sex | ||||||||||
| Men | 0.97 | 0.77-1.22 | 0.83 | 0.74-0.92. | 0.92 | 0.81-1.04 | 2506 | 3878 | 0.93 | 0.87-0.99 j |
| Women | 1.44 | 1.09-1.90 | 0.84 | 0.73-0.97 | 0.94 | 0.80-1.09 | 2599 | 2015 | 1.01 | 0.94-1.09 |
| Race | ||||||||||
| White | 1.19 | 0.98-1.45 | 0.84 | 0.77-0.92 | 0.92 | 0.83-1.01 | 4451 | 5263 | 0.97 | 0.92-1.02 |
| Non-White | 0.93 | 0.59-1.44 | 0.82 | 0.65-1.03 | 1.15 | 0.88-1.50 | 654 | 630 | 0.95 | 0.84-1.09 |
| Age at cancer diagnosis, (years) | ||||||||||
| 66–< 70 | 1.54 | 1.14-2.07 | 0.82 | 0.72-0.96 | 0.95 | 0.82-1.10 | 2390 | 1079 | 0.98 | 0.90-1.07 |
| 70–< 80 | 1.04 | 0.84-1.28 | 0.81 | 0.73-0.89 | 0.86 | 0.77-0.96 | 4438 | 3978 | 0.94 | 0.89-0.99 j |
| 80–< 84 | 1.01 | 0.77-1.33 | 0.82 | 0.73-0.92 | − c | − c | 1807 | 836 | 0.90 | 0.82-0.99 |
| Smoking-related cancers d | 1.14 | 0.88-1.48 | 0.76 | 0.66-0.88 | 0.95 | 0.80-1.15 | 5105 | 1117 | 0.91 | 0.85-0.9 j |
| Non smoking-related cancers e | 1.14 | 0.94-1.38 | 0.84 | 0.77-0.92 | 0.92 | 0.84-1.02 | 5105 | 4776 | 0.97 | 0.92-1.02 |
| Individual cancer sites | ||||||||||
| Oral cavity f | 0.86 | 0.14-1.69 | 0.77 | 0.50-1.19 | 1.14 | 0.63-2.06 | 5105 | 55 | 0.86 | 0.66-1.13 |
| Oesophageal | 1.02 | 0.49-2.16 | 0.87 | 0.46-1.64 | 0.89 | 0.21-3.74 | 5105 | 30 | 1.11 | 077-1.60 |
| Stomach | 1.57 | 0.90-2.73 | 0.60 | 0.36-0.98 | 1.0 | 0.48-2.05 | 5105 | 58 | 0.93 | 0.71-1.22 |
| Colon | 1.26 | 0.89-1.77 | 0.84 | 0.71-1.01 | 0.91 | 0.71-1.15 | 5105 | 556 | 0.94 | 0.85-1.04 |
| Rectum g | 1.02 | 0.63-1.66 | 0.77 | 0.60-1.00 | 0.92 | 0.63-1.33 | 5105 | 190 | 0.88 | 0.75-1.03 |
| Pancreas | 0.97 | 0.53-1.80 | 0.33 | 0.12-0.91 | – | – | 5105 | 24 | 0.76 | 0.51-1.15 |
| Larynx | 0.68 | 0.30-1.51 | 0.65 | 0.41-1.02 | 0.50 | 0.22-1.13 | 5105 | 42 | 0.68 | 0.49-0.93 j |
| Lung and bronchus | 0.96 | 0.69-1.35 | 0.63 | 0.50-0.79 | 1.20 | 0.88-1.64 | 5105 | 330 | 0.81 | 0.72-0.92 j |
| Melanoma h | 1.29 | 0.74-2.24 | 1.07 | 0.81-1.42 | 1.09 | 0.75-1.57 | 4451 | 189 | 1.12 | 0.95-1.31 |
| Breast (female) | 1.25 | 0.84-1.86 | 0.91 | 0.76-1.09 | 0.85 | 0.69-1.05 | 2599 | 801 | 1.01 | 0.91-1.11 |
| Cervix | 2.94 | 1.06-8.11 | 1.36 | 0.66-2.80 | 1.91 | 0.78-4.69 | 2599 | 23 | 1.66 | 1.09-2.52 j |
| Uterus i | 2.33 | 1.38-3.92 | 0.93 | 0.67-1.28 | 0.89 | 0.60-1.31 | 2599 | 152 | 1.04 | 0.87-1.24 |
| Ovary | 1.52 | 0.70-3.32 | 0.46 | 0.26-0.84 | 0.32 | 0.08-1.28 | 2599 | 34 | 0.65 | 0.46-0.93 j |
| Prostate | 0.85 | 0.64-1.14 | 0.82 | 0.73-0.93 | 0.93 | 0.81-1.07 | 2506 | 2146 | 0.92 | 0.86-0.99 j |
| Urinary bladder | 1.25 | 0.86-1.84 | 0.83 | 0.68-1.02 | 0.83 | 0.63-1.09 | 5105 | 404 | 0.95 | 0.85-1.07 |
| Kidney/renal pelvis | 1.15 | 0.68-1.97 | 0.87 | 0.64-1.18 | 1.11 | 0.74-1.67 | 5105 | 134 | 1.01 | 0.84-1.21 |
| Thyroid | 0.86 | 0.32-2.32 | 0.62 | 0.34-1.11 | 0.88 | 0.39-1.98 | 5105 | 29 | 0.83 | 0.57-1.21 |
| Leukaemia | 1.07 | 0.61-1.88 | 0.80 | 0.58-1.11 | 0.81 | 0.46-1.43 | 5105 | 109 | 0.96 | 0.78-1.17 |
a Models have been adjusted for race, sex and number of doctors’ visits, stratified on birth year and cancer registry area, except that sex was not adjusted for in the subpopulation based on sex, nor race in the subpopulation defined by race. There were a total of 743 779 cancer patients and 419 432 persons in the comparison population. Data source is SEER-Medicare. Cancers were classified by using the ‘SEER site recode with Kaposi sarcoma and mesothelioma’. Refer to [ http://seer.cancer.gov ] and for details, see site recode ICD-O-3.
b Number of PD diagnoses in cancer patients/comparison group.
c For this age group, follow-up was less than 5 years.
d Smoking-related cancers include oral cavity, phyarynx, lip, pancreas, lung and bronchus, larynx, cervix, kidney and renal pelvis, bladder, oesophagus and stomach.
e Non smoking-related cancers include all cancers other than smoking-related cancers.
f Includes tongue, floor of mouth, gum and mouth, tonsil, oropharynx, hypopharynx.
g Includes rectum and rectosigmoid junction.
h Only Whites.
i Includes corpus uteri and uterus, not otherwise specified.
j P -values for the associations between the overall cancer groups and specific cancer sites with PD (for 0–< 10 years) (before multiple comparison corrections) varied between P = 0.01 and 0.03, except for lung and bronchus cancer, for which the P -value was 0.0009. After correcting for multiple comparisons [ n = 27 comparisons, based on all associations for 0–< 10 years, other than the overall population (e.g. men, Whites, oral cavity cancer)], none of the associations withstood multiple comparisons except for lung and bronchus cancer ( P -value = 0.024).
In the first year after a cancer diagnosis, risk of PD diagnosis was elevated for total cancer and all subgroups other than men and non-Whites ( Table 2 ). In years 1 through 5 after cancer diagnosis, HRs for PD were lower, but after 5 years, HRs were typically closer to one ( Table 2 ).
Of the 18 specific cancer sites examined for subsequent PD risk, associations were null for 13 sites and for the group of non-smoking related cancers. Sites with inverse associations within 10 years of the cancer diagnosis included two key smoking-related cancer sites: lung and bronchus (HR = 0.81; 95% CI = 0.72-0.92), and larynx (HR = 0.68; 95% CI = 0.49-0.93), as well as the group of smoking-related cancer sites (HR = 0.91; 95% CI = 0.85-0.99). Of these, only the association with lung and bronchus cancer withstood multiple comparisons correction ( Table 2 ).
In addition, there were nominal inverse associations with PD after prostate (HR = 0.92; 95% CI = 0.86-0.99) and ovarian cancer (HR = 0.65; 95% CI = 0.46-0.93) and a positive association with cervical cancer (HR = 1.66; 95% CI = 1.09-2.52), none of which withstood multiple comparisons correction. PD was not related to melanoma (HR = 1.12; 95% CI = 0.95-1.31). There was also no relationship between cancer and subsequent automobile injuries (the negative control; HR = 1.03; 95% CI 0.98-1.07; Table 3 ; Supplementary Table 1 for unadjusted HRs).
Table 3.
Relationship between cancer before and after injuries due to automobile accidents a
| HRs of injuries due to automobile accidents after cancer | ||||||||||
| < 1-year follow-up | 1–< 5-years follow-up | 5–< 10-years follow-up | 0–< 10-years follow-up | |||||||
| Automobile accident injury cases | ||||||||||
|
| ||||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | Comparison group c | Cancer patients c | HR | 95% CI | |
|
| ||||||||||
| Overall | 1.08 | 0.91-1.27 | 0.96 | 0.89-1.04 | 0.97 | 0.88-1.08 | 6746 | 6236 | 1.03 | 0.98-1.07 |
| ORs of previous injuries due to automobile accidents in individuals diagnosed with cancer b | ||||||||||
|
| ||||||||||
| < 1 year prior to cancer | 1–< 5 years prior to cancer | 0–< 5 years prior to cancer | ||||||||
|
| ||||||||||
| OR | 95% CI | OR | 95% CI | Controls c | Cancer cases c | OR | 95% CI | |||
|
| ||||||||||
| Overall | 0.86 | 0.76-0.96 | 0.81 | 0.76-0.87 | 1519 | 8403 | 0.83 | 0.78-0.88 | ||
a Models have been adjusted for race, sex and number of doctors’ visits, stratified on birth year and cancer registry area. The study populations of the cancer cohort and comparison cohort both excluded subjects with claims prior to baseline for auto accidents based on Medicare claims. Data source is SEER-Medicare.
b Models have been adjusted for age, race, sex, number of doctors’ visits, cancer registry area and selection years. Data source is SEER-Medicare.
c Number of cancer cases/controls with automobile accident injuries.
Case-control study of PD before cancer
Table 1b presents the characteristics of the 836 947 cancer cases and 142 869 frequency-matched controls. There were no substantial differences between the cancer cases and controls for the matching variables (age, sex, selection year), nor for race/ethnicity.
Table 1b.
Characteristics of cancer cases and non-cancer control group in retrospective case-control analysis of Parkinson’s disease (PD) before cancer
| Cancer cases ( N = 836947) | Control group ( N = 142869 ) | |
|---|---|---|
| Age a in years, n (%) | ||
| 66–< 70 | 27.0% | 27.9% |
| 70–< 80 | 55.2% | 54.7% |
| 80–< 85 | 17.9% | 17.4% |
| Median age | 74 | 74 |
| Sex, n (%) | ||
| Male | 54.5% | 54.8% |
| Female | 45.5% | 45.2% |
| Selection year, n (%) | ||
| 1992–94 | 17.4% | 17.2% |
| 1995–98 | 20.2% | 20.0% |
| 1999–2005 | 62.4% | 62.8% |
| Race/ethnicity, n (%) | ||
| White | 86.1% | 84.3% |
| Non-white | 13.9% | 15.7% |
| Black | 7.8% | 6.7% |
| Asian | 2.5% | 3.9% |
| Hispanic | 1.4% | 2.4% |
| Native American Indian | 0.2% | 0.4% |
| Other/unknown | 2.0% | 2.3% |
a For cancer patients/cases, age is based on age at cancer diagnosis; for the comparison group/controls, age is based on age at selection as a control.
Overall there was an inverse relationship between cancer and PD (OR = 0.77; 95% CI = 0.71-0.82; Table 4 ; Supplementary Table 1 for unadjusted HRs). The inverse relationship was observed in men, women and Whites, but not in non-Whites. Inverse associations were seen in all age groups and there was no heterogeneity in associations by age strata (data not shown). Also, associations were not heterogeneous by age strata for breast cancer, prostate cancer and melanoma ( Supplementary Table 3b ).
Table 4.
Odds ratio (OR) of a previous Parkinson’s disease (PD) diagnosis in cancer cases compared with non-cancer controls, 1992–2005 a
|
< 1 year prior to cancer |
1–< 5 years prior to cancer
|
0–< 5 years prior to cancer
|
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | Controls | Cancer cases | OR | 95% CI | |
| ( n ) b | ( n ) b | |||||||
| Overall | 0.84 | 0.74-0.94 | 0.74 | 0.68-0.81 | 1165 | 5829 | 0.77 | 0.71-0.82 |
| Sex | ||||||||
| Men | 0.73 | 0.63-0.85 | 0.70 | 0.63-0.78 | 721 | 3442 | 0.70 | 0.64-0.77 |
| Women | 1.08 | 0.87-1.33 | 0.80 | 0.71-0.92 | 444 | 2387 | 0.87 | 0.78-0.98 |
| Race | ||||||||
| White | 0.81 | 0.71-0.92 | 0.72 | 0.66-0.78 | 1036 | 5151 | 0.74 | 0.69-0.80 |
| Non-White | 1.02 | 0.73-1.42 | 1.00 | 0.76-1.31 | 129 | 678 | 0.99 | 0.79-1.24 |
| Age at cancer diagnosis (years) | ||||||||
| 66–< 70 | 0.82 | 0.57-1.18 | 0.55 | 0.42-0.73 | 112 | 475 | 0.64 | 0.51-0.81 |
| 70–< 80 | 0.83 | 0.70-0.97 | 0.75 | 0.68-0.84 | 644 | 3328 | 0.77 | 0.70-0.85 |
| 80–< 85 | 0.85 | 0.69-1.05 | 0.77 | 0.67-0.88 | 409 | 2026 | 0.79 | 0.70-0.89 |
| Smoking-related cancers c | 0.82 | 0.71-0.95 | 0.71 | 0.64-0.78 | 1165 | 1827 | 0.74 | 0.68-0.81 |
| Nonsmoking-related cancers d | 0.83 | 0.74-0.94 | 0.75 | 0.69-0.82 | 1165 | 4002 | 0.77 | 0.72-0.83 |
| Individual cancer sites | ||||||||
| Oral cavity e | 0.55 | 0.33-0.93 | 0.84 | 0.63-1.13 | 1165 | 66 | 0.77 | 0.59-0.99 |
| Oesophageal | 0.72 | 0.45-1.15 | 0.68 | 0.49-0.94 | 1165 | 55 | 0.67 | 0.51-0.89 |
| Stomach | 1.07 | 0.79-1.45 | 0.75 | 0.59-0.95 | 1165 | 129 | 0.84 | 0.70-1.02 |
| Colon | 1.10 | 0.93-1.31 | 0.83 | 0.73-0.94 | 1165 | 677 | 0.91 | 0.82-1.01 |
| Rectum f | 0.60 | 0.43-0.82 | 0.63 | 0.51-0.77 | 1165 | 149 | 0.63 | 0.53-0.75 |
| Pancreas | 0.95 | 0.72-1.25 | 0.80 | 0.66-0.98 | 1165 | 191 | 0.84 | 0.71-1.00 |
| Larynx | 0.50 | 0.26-0.97 | 0.45 | 0.28-0.72 | 1165 | 28 | 0.48 | 0.32-0.70 |
| Lung and bronchus | 0.76 | 0.64-0.90 | 0.66 | 0.59-0.74 | 1165 | 836 | 0.69 | 0.62-0.76 |
| Melanoma g | 0.95 | 0.72-1.25 | 1.10 | 0.91-1.31 | 1036 | 205 | 1.03 | 0.88-1.21 |
| Breast (female) | 0.88 | 0.68-1.14 | 0.83 | 0.71-0.96 | 444 | 561 | 0.84 | 0.73-0.96 |
| Cervix | 1.06 | 0.43-2.60 | 1.02 | 0.60-1.73 | 444 | 20 | 1.05 | 0.67-1.66 |
| Uterus h | 0.91 | 0.60-1.36 | 0.73 | 0.57-0.95 | 444 | 102 | 0.75 | 0.59-0.94 |
| Ovary | 0.98 | 0.62-1.53 | 0.53 | 0.37-0.77 | 444 | 56 | 0.67 | 0.50-0.89 |
| Prostate | 0.56 | 0.47-0.67 | 0.55 | 0.48-0.62 | 721 | 951 | 0.55 | 0.49-0.61 |
| Urinary bladder | 0.67 | 0.53-0.86 | 0.69 | 0.59-0.81 | 1165 | 334 | 0.68 | 0.59-0.78 |
| Kidney/renal pelvis | 0.98 | 0.73-1.33 | 0.69 | 0.54-0.87 | 1165 | 146 | 0.79 | 0.66-0.96 |
| Thyroid | 0.71 | 0.35-1.44 | 0.50 | 0.29-0.87 | 1165 | 20 | 0.53 | 0.33-0.83 |
| Leukaemia | 0.89 | 0.66-1.19 | 0.77 | 0.62-0.95 | 1165 | 174 | 0.80 | 0.68-0.96 |
a Models have been adjusted for age, race, sex, number of doctors’ visits, cancer registry area and selection years, except that sex was not adjusted for in the subpopulation based on sex, nor race in the subpopulation defined by race. There were a total of 836 947 cancer patients and 142 869 persons in the comparison population. Data source is SEER-Medicare. Cancers were classified by using the ‘SEER site recode with Kaposi sarcoma and mesothelioma’. Refer to [ http://seer.cancer.gov ] and for details, see site recode ICD-O-3.
b Number of PD cases in cancer cases/controls.
c Smoking-related cancers include oral cavity and pharynx, lip, pancreas, lung and bronchus, larynx, cervix, kidney and renal pelvis, bladder, oesophagus, and stomach.
d Nonsmoking-related cancers include all cancers other than smoking-related cancers.
e Includes tongue, floor of mouth, gum and mouth, tonsil, oropharynx, hypopharynx.
f Includes rectum and rectosigmoid junction.
g Only Whites.
h Includes corpus uteri and uterus, not otherwise specified.
An inverse association with a previous PD diagnosis was observed for most specific cancer sites ( Table 4 ). Among the lowest ORs were PD before cancers of the larynx (OR = 0.48; 95% CI = 0.32-0.70), thyroid (OR = 0.53; 95% CI = 0.33-0.83), prostate (OR = 0.55; 95% CI = 0.49-0.61) and ovary (OR = 0.67; 95% CI = 0.50-0.89). Having had a previous PD diagnosis was associated with a lower OR for both smoking-related and non-smoking-related cancers to a similar extent (OR = 0.74 vs 0.77, respectively). There were no associations with cervical cancer or melanoma.
The above results should be interpreted with care, as there was also an inverse association between automobile injuries and subsequent overall cancer risk (OR = 0.83; 95% CI = 0.78-0.88; Table 3 ; Supplementary Table 1 for unadjusted ORs), as well as inverse associations between automobile accidents and prostate cancer (OR = 0.81; 95% CI = 0.72-0.91) and breast cancer (0.86; 95% CI = 0.75-0.97).
Discussion
We undertook two population-based SEER-Medicare studies: a prospective cohort study of the relationship between cancer and subsequent PD risk, and a case-control study of cancer compared with non-cancer controls and previous PD. We also analysed the associations between automobile accident injuries and cancer, because car accidents are unlikely to affect cancer risk biologically in either direction. Overall cancer was unrelated to subsequent PD diagnosis. That cancer was unrelated to subsequent automobile injuries supports the validity of our prospective analysis. In analysing PD risk after cancer at specific sites, we did observe an inverse association for PD after lung/bronchus cancer, but not for other sites (after correcting for multiple comparisons). In addition, when we examined associations with overall and specific cancers, we saw similar null associations across age strata, further supporting the lack of association between cancers and PD.
In contrast, in the case-control analyses, PD was inversely related to subsequent overall cancer, cancer in most demographic groups, as well as to both smoking and non-smoking-related cancers. Notably, however, automobile injuries, the negative comparison control, were also reduced before cancer, nearly to the same degree. The use of a negative comparison control such as automobile accidents is important, as screening or diagnostic tests may be scheduled less frequently for persons disabled from PD (or accidents). This may especially apply to persons in nursing homes. 20 That other studies of PD and cancer did not include negative comparison controls limits interpreting the associations observed, both the inverse findings 1–4,8,9,11,12 and a recent study 10 which found adverse associations between PD and cancers in Taiwan, as health care systems may vary widely in the aggressiveness with which cancer screening and medical work-ups are pursued in those already confronting a medically debilitating condition. Thus, our findings of reduced risk of cancer after both automobile accidents and PD are consistent with under-ascertainment of cancer after at least some unrelated health outcomes.
Unmeasured confounders, e.g. smoking and alcohol, could potentially account for the reduced cancer risk after automobile accidents that we observed. Yet, as noted, we see inverse relationships between automobile accidents and both prostate and breast cancer, two cancer sites not strongly associated with smoking and alcohol. The only hypothetical confounders that would contribute to a lower cancer risk after automobile accident injuries would be unmeasured confounders that were positively related to cancer and negatively related to automobile accidents or vice versa. We cannot think of a factor that meets this constellation of conditions. Therefore, although we observed several inverse associations of PD with cancer, as did most previous studies, 1–4,8,9,11,12 under-ascertainment of cancer after PD and after automobile accidents is a plausible explanation for the inverse associations observed.
The same ascertainment bias is less likely to occur for studies of PD risk or automobile injuries following cancer diagnosis. Automobile injuries often require immediate medical attention, regardless of cancer history. PD, although a chronic disease, is diagnosed based on motor abnormalities (i.e. rigidity, tremor, balance problems) that are visible to clinicians 21,22 without requiring burdensome or invasive tests.
The relationship of prostate cancer and PD risk illustrates the asymmetry of the ascertainment bias. Screening tests [i.e. prostate-specific antigen (PSA)], have contributed to prostate cancer being among the most common solid cancers diagnosed and treated. 23 Ascertainment bias could lead to reduced prostate cancer screening/testing in PD and accident patients compared with those without these conditions. A study of older adults with and without probable disability found the latter group less likely to report receiving prostate cancer screening. 24 In fact, the ORs of diagnosing prostate cancer after PD was low (OR = 0.55) but the PD risk after prostate cancer was nearly null (HR = 0.92). The same disparate PD/prostate cancer relationships, depending on disease order, are found in other studies that reported prostate cancer risk bi-directionally: 0.80 vs 1.01 in a California Kaiser Permanente study; 25 0.7 vs 0.9 in a British National Health Service Study; 3 0.74 vs 0.99 in Danish registry studies; 1,7 and 0.77 vs 1.12 (cancer up to 1 year before PD) in a Swedish registry study, 11 for PD first vs second, respectively.
Previous findings of cancer before PD could also reflect such biases when using prevalent PD cases, such as those drawn from hospital records. 26 Some cancers that could potentially have been diagnosed before a prevalent PD case was selected may have been missed if medical work-ups were reduced in the immediate period before selecting the prevalent PD case. Results from studies that relied on hospital records to identify inpatient PD cases, 3,7 may reflect these limitations.
A recent study by Akushevich et al ., 27 which also used SEER-Medicare data to look some cancer sites and PD, found primarily reduced PD before cancer, but null relationships when PD was second, like our study. The specific associations differed somewhat from ours, and there were design differences between the studies. Akushevich et al. suggest that ascertainment bias may not fully account for inverse associations between another neurodegenerative disease, Alzheimer’s, and cancer because other diseases (e.g. myocardial infarction, renal disease and ulcer) were not lower after Alzheimer’s. However, such diseases may be more evident to clinicians, and are thus more likely to be ascertained than cancer.
Although we observed little evidence of an overall relationship between PD and total cancer, PD was linked to some smoking-related cancers. Although smoking is adversely related to many cancer sites, 28 there is substantial evidence that smoking decreases PD risk 21,29,30 and that PD patients smoke less. 21 We observed inverse associations of smoking-related cancers after PD in the case-control analyses, and the inverse relationship with PD after lung cancer in the prospective cohort analyses withstood adjustment for multiple comparisons. These inverse observations were consistent with other studies, 1,2,9,12 and the possibility that confounding from unmeasured smoking may have contributed to inverse associations warrants careful evaluation.
We found nominal inverse associations between PD and ovarian cancer in both directions (> 30%). Only a few studies 1,3,11 were large enough to assess associations with PD and some found lower ovarian cancer risk following a PD diagnosis. 1,3 Explanations may involve confounding from female reproductive factors, including oral contraceptive use. 31–35
Melanoma is one of the few cancer sites that have been positively related to PD. 36 A recent meta-analysis of PD and melanoma found a greater than 3-fold risk for melanoma after PD, but no risk before PD. 36 In our study, melanoma was unrelated to PD in both directions, and thus did not confirm previous associations.
We saw no relationship between PD after breast cancer in the cohort analysis and a nominal inverse association for breast cancer after PD in the case-control analysis. In contrast, two studies observed elevated risks of breast cancer after PD, 1,5 whereas other studies have been null. 2,3,11,25 Thus, the weight of the evidence does not support an adverse relationship between the diseases.
This study has several major strengths. First, it is very large, with several-fold more cases than most other studies, which allowed assessing associations over time by gender, age and race and by cancer sites. Moreover, we used a bidirectional design (PD before and after cancer). Other strengths include: the availability of incident PD cases identified by physician visits (to identify PD cases earlier in severity) as well as hospital stays; comprehensive and unbiased ascertainment of SEER cancers; data on frequency of physician visits to control for surveillance intensity; adjustment for multiple comparisons; nationwide claims data, which reduced loss to out-migration; and inclusion of automobile injuries as a negative comparison control.
Limitations include the lack of information on covariates, such as smoking history. Further, Medicare claims do not constitute clinical data, yet were the basis for ascertaining PD outcomes. 37 As noted, to reduce misclassification, we categorized subjects as having PD only if they had either an inpatient diagnosis or multiple outpatient claims. We also assumed that deaths from causes other than PD are independent of PD risks separately for cancer patients and the comparison groups. However, this is a standard assumption (conditionally independent censoring) made in survival analysis.
In summary, we found little evidence of a biological relationship between PD and total cancer. There was an inverse association between one smoking-related site, lung cancer, and PD, for PD before and after lung cancer. The associations with individual sites may reflect confounding by environmental factors, such as smoking and sex hormones, as well as possible bias in cancer ascertainment after PD.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute and the National Institute of Environmental Health Sciences, National Institutes of Health and the US Public Health Service.
Supplementary Material
Acknowledgements
We thank Drs Barry Graubard and Joan Warren for their thoughtful contributions to this study and Ms Winnie Ricker of Information Management Services, Inc., for biomedical computer assistance. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of: the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services, Information Managements Services, Inc.; and the SEER Program registries in the creation of the database.
Conflict of interest : None declared.
References
- 1. Rugbjerg K, Friis S, Lassen CF, Ritz B, Olsen JH. Malignant melanoma, breast cancer and other cancers in patients with Parkinson's disease . Int J Cancer 2012. ; 131:1904 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Cancer risk in association with Parkinson disease: a population-based study . Parkinsonism Relat Disord 2010. ; 16:186 – 90 . [DOI] [PubMed] [Google Scholar]
- 3. Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson's disease: record linkage studies . J Neurol Neurosurg Psychiatry 2010. ; 81:215 – 21 . [DOI] [PubMed] [Google Scholar]
- 4. Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease . Cancer Epidemiol Biomarkers Prev 2007. ; 16:1260 – 65 . [DOI] [PubMed] [Google Scholar]
- 5. Minami Y, Yamamoto R, Nishikouri M, Fukao A, Hisamichi S. Mortality and cancer incidence in patients with Parkinson's disease . J Neurol 2000. ; 247:429 – 34 . [DOI] [PubMed] [Google Scholar]
- 6. Ong EL, Goldacre R, Goldacre M. Differential risks of cancer types in people with Parkinson's disease: A national record-linkage study . Eur J Cancer 2014. ; 50:2456 – 62 . [DOI] [PubMed] [Google Scholar]
- 7. Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease . Epidemiology 2006. ; 17:582 – 87 . [DOI] [PubMed] [Google Scholar]
- 8. D'Amelio M, Ragonese P, Morgante L. et al. . Tumor diagnosis preceding Parkinson's disease: a case-control study . Mov Disord 2004. ; 19:807 – 11 . [DOI] [PubMed] [Google Scholar]
- 9. Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case-control study of nonfatal cancer preceding the diagnosis of Parkinson's disease . Cancer Causes Control 2007. ; 18:705 – 11 . [DOI] [PubMed] [Google Scholar]
- 10. Lin PY, Chang SN, Hsiao TH, Huang BT, Lin CH, Yang PC. Association Between Parkinson Disease and Risk of Cancer in Taiwan . JAMA Oncol 2015. ; 1:633 – 40 . [DOI] [PubMed] [Google Scholar]
- 11. Wirdefeldt K, Weibull CE, Chen H. et al. . Parkinson's disease and cancer: A register-based family study . Am J Epidemiol 2014. ; 179:85 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bajaj A, Driver JA, Schernhammer ES. Parkinson's disease and cancer risk: a systematic review and meta-analysis . Cancer Causes Control 2010. ; 21:697 – 707 . [DOI] [PubMed] [Google Scholar]
- 13. Iezzoni LI, McCarthy EP, Davis RB, Siebens H. Mobility impairments and use of screening and preventive services . Am J Public Health 2000. ; 90:955 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mettlin C, Bonfiglio J, Berg RL. et al. . Cancer control and the older person. Prevention and detection in older persons . Cancer 1991. ; 68(Suppl 11):2530 – 33 . [DOI] [PubMed] [Google Scholar]
- 15. Driver JA, Djousse L, Logroscino G, Gaziano JM, Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study . BMJ 2008. ; 337:a2467 .. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population . Med Care 2002. ; 40(Suppl 8):IV–3 – 18 . [DOI] [PubMed] [Google Scholar]
- 17. Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly . Am J Epidemiol 2011. ; 174 : 860 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. U.S. Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General . Rockville, MD: : U.S. Department of Health and Human Services; , 2004. . [Google Scholar]
- 19. Chang CM, Warren JL, Engels EA. Chronic fatigue syndrome and subsequent risk of cancer among elderly US adults . Cancer 2012. ; 118:5929 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bassett SD, Smyer T. Health screening practices in rural long-term care facilities . J Gerontol Nurs 2003. ; 29:42 – 49 . [DOI] [PubMed] [Google Scholar]
- 21. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence . Eur J Epidemiol 2011. ; 26(Suppl 1):S1 – 58 . [DOI] [PubMed] [Google Scholar]
- 22. D'Amelio M, Ragonese P, Sconzo G, Aridon P, Savettieri G. Parkinson's disease and cancer: insights for pathogenesis from epidemiology . Ann N Y Acad Sci 2009. ; 1155:324 – 34 . [DOI] [PubMed] [Google Scholar]
- 23. Jani AB, Johnstone PA, Liauw SL, Master VA, Brawley OW. Age and grade trends in prostate cancer (1974-2003): a Surveillance, Epidemiology, and End Results Registry analysis . Am J Clin Oncol 2008. ; 31:375 – 78 . [DOI] [PubMed] [Google Scholar]
- 24. Ramirez A, Farmer GC, Grant D, Papachristou T. Disability and preventive cancer screening: results from the 2001 California Health Interview Survey . Am J Public Health 2005. ; 95:2057 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo RY, Tanner CM, Van Den Eeden SK, Albers KB, Leimpeter AD, Nelson LM. Comorbid cancer in Parkinson's disease . Mov Disord 2010. ; 25:1809 – 17 . [DOI] [PubMed] [Google Scholar]
- 26. Chou KL, Zamudio J, Schmidt P. et al. . Hospitalization in Parkinson disease: a survey of National Parkinson Foundation Centers . Parkinsonism Relat Disord 2011. ; 17:440 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Kulminski A, Yashin AI. Morbidity risks among older adults with pre-existing age-related diseases . Exp Gerontol 2013. ; 48:1395 – 401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shopland DR. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking . Environ Health Perspect 1995. ; 103(Suppl 8) : 131 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanner CM, Goldman SM, Aston DA. et al. . Smoking and Parkinson's disease in twins . Neurology 2002. ; 58:581 – 88 . [DOI] [PubMed] [Google Scholar]
- 30. Chen H, Huang X, Guo X. et al. . Smoking duration, intensity, and risk of Parkinson disease . Neurology 2010. ; 74:878 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls . Lancet 2008. ; 371:303 – 14 . [DOI] [PubMed] [Google Scholar]
- 32. Rugbjerg K, Christensen J, Tjonneland A, Olsen JH. Exposure to estrogen and women's risk for Parkinson's disease: a prospective cohort study in Denmark . Parkinsonism Relat Disord 2013. ; 19:457 – 60 . [DOI] [PubMed] [Google Scholar]
- 33. Nicoletti A, Nicoletti G, Arabia G. et al. . Reproductive factors and Parkinson's disease: a multicenter case-control study . Mov Disord 2011. ; 26:2563 – 66 . [DOI] [PubMed] [Google Scholar]
- 34. Simon KC, Chen H, Gao X, Schwarzschild MA, Ascherio A. Reproductive factors, exogenous estrogen use, and risk of Parkinson's disease . Mov Disord 2009. ; 24:1359 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu R, Baird D, Park Y. et al. . Female reproductive factors, menopausal hormone use, and Parkinson's disease . Mov Disord 2014. ; 29:889 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma . Neurology 2011. ; 76:2002 – 09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noyes K, Liu H, Holloway R, Dick AW. Accuracy of Medicare claims data in identifying Parkinsonism cases: comparison with the Medicare current beneficiary survey . Mov Disord 2007. ; 22:509 – 14 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.