Abstract
Pulmonary arterial hypertension (PAH) is associated with metabolic derangements including insulin resistance, although their effects on the cardiopulmonary disease are unclear. We hypothesized that insulin resistance promotes pulmonary hypertension (PH) development and mutations in type 2 bone morphogenetic protein receptor (BMPR2) cause cellular insulin resistance. Using a BMPR2 transgenic murine model of PAH and two models of inducible diabetes mellitus, we explored the impact of hyperglycemia and/or hyperinsulinemia on development and severity of PH. We assessed insulin signaling and insulin-mediated glucose uptake in human endothelial cells with and without mutations in BMPR2. PH developed in control mice fed a Western diet and PH in BMPR2 mutant mice was increased by Western diet. Pulmonary artery pressure correlated strongly with fasting plasma insulin but not glucose. Reactive oxygen species were increased in lungs of insulin-resistant animals. BMPR2 mutation impaired insulin-mediated endothelial glucose uptake via reduced glucose transporter translocation despite intact insulin signaling. Experimental hyperinsulinemia is strongly associated with PH in both control and BMPR2-mutant mice, though to a greater degree in those with BMPR2 mutation. Human pulmonary endothelial cells with BMPR2 mutation have evidence of reduced glucose uptake due to impaired glucose transporter translocation. These experiments support a role for hyperinsulinemia in pulmonary vascular disease.
Keywords: insulin resistance, metabolic syndrome, BMPR2, pulmonary vascular disease
Pulmonary arterial hypertension (PAH) is characterized by progressive occlusion of small pulmonary arteries leading to increased right ventricular (RV) afterload with eventual RV failure and death. Mutations in the gene for type 2 bone morphogenetic protein receptor (BMPR2) are the most common genetic abnormality in heritable and idiopathic PAH.1 While clinically manifest as a pulmonary vascular disease (PVD), evidence suggests PAH is a multisystem disease with metabolic manifestations. Insulin-resistant PAH patients have increased mortality2–5 and a rodent model of BMPR2-mediated PAH develops metabolic alterations prior to PVD, suggesting a causative role of insulin resistance in promotion of PAH.6 How insulin resistance affects the pulmonary vasculature is poorly understood, but a mechanistic understanding of the interplay between insulin signaling and pulmonary endothelial function may identify new treatment targets in this deadly disease.
The endothelium plays a central role in the pathobiology of PAH, but is also an important mediator of insulin action in the systemic circuit.7–10 Insulin has two major kinase cascades – Akt and MAPK. Simplistically, the former causes immediate and delayed metabolic changes, while the latter promotes cellular growth and proliferation. While perturbations of insulin signaling have not previously been studied in PAH, uncontrolled endothelial and vascular smooth muscle cell proliferation are central features which result from altered BMP signaling in addition to other mechanisms.11 Cellular proliferation in PAH models is also associated with reactive oxygen species (ROS) generation which can be demonstrated in patient specimens.12 Oxidative injury is also associated with systemic endothelial dysfunction and diabetic complications.7,13
We sought to characterize the consequence of insulin resistance in a rodent model of PAH and to define alterations in insulin signaling in endothelial cells expressing mutant BMPR2 that may underlie the in vivo effects of insulin resistance in PAH. Because of the epidemiological associations between PAH and the metabolic syndrome and insulin’s pleiotropic effects including mitogenicity, we hypothesized that hyperinsulinemia, but not hyperglycemia alone, would promote PAH in BMPR2-mutant mice. We further hypothesized that BMPR2 mutation in endothelial cells would reduce insulin signaling and glucose transporter activation. Some of the results presented have been previously reported in the form of abstracts.14,15
Methods
Animal models
All animal procedures were approved by the Institutional Animal Care and Use Committee at Vanderbilt University, Nashville, TN, USA. We used male Rosa26-rtTA2*TetO7-Bmpr2R899X FVB/N inducible transgenic mice and transgene-negative male littermates as controls for all experiments, hereafter referred to as “BMPR2R899X” and “Control”, respectively.16 BMPR2R899X mice develop PAH with incomplete penetrance within four weeks of transgene activation.16 The animals were housed and managed by Vanderbilt University’s Division of Animal Care in standard social housing with no more than four other animals and ad libitum water access in ventilated, temperature-controlled, and humidity-controlled cages with 12-h light/dark cycle. Prior to the experiments, animals were fed Purina Rodent Diet 5L0D (Purina Lab Diet, St. Louis, MO, USA) supplied by the Department of Animal Care. Experimental diets were initiated at 10–14 weeks age and continued for six weeks until sacrifice. For experiments, mice were given either standard diet (SD, 16% kcal from fat), SD and streptozotocin (STZ), or a Western diet (WD, 60% kcal from fat). Diets (Bio-Serv, Flemington, NJ, USA) contained doxycycline (200 mg/kg) and were matched for sucrose and nutrient content other than fat. STZ (Sigma-Aldrich, St. Louis, MO, USA) was given by single intraperitoneal injection of 150 mg/kg at the time of diet initiation.17 Lung histology was used for assessment of vessel density and morphometry. Vessel measurements for calculating medial wall thickness were obtained in a previously described method.18 Additional details of animal models and experiments are available in the Online Supplement.
Hemodynamic and metabolic phenotyping
Two-dimensional echocardiography was performed the day prior to sacrifice using a Vevo 770 High-Resolution Image System (VisualSonics, Toronto, Canada) and the same technique that our group has previously described in audiovisual format.6,19 Afterwards, fasting blood samples were obtained for metabolic testing. On day of sacrifice, hemodynamics were obtained via open-chest RV puncture method under isoflurane anesthesia using a 1.4-F Mikro-Tip catheter (Millar Instruments, Houston, TX, USA).20 RV systolic pressure (RVSP) was recorded with ambient air mechanical ventilation, and again after 5 min of 10% hypoxia. In a subset, RVSP was measured after reinstitution of normoxia and tail vein injection of a synthetic thromboxane analogue, U46619 (1 mg/kg, Cayman Chemical, Ann Arbor, MI, USA). Immediately following invasive hemodynamic measurements, animals were euthanized by anesthesia overdose and exsanguination with subsequent harvest of tissues for analysis as further explained in the Online Supplement.
Human pulmonary microvascular endothelial cell culture
Human pulmonary microvascular endothelial cells (PMVECs) were grown in culture utilizing Endothelial Cell Growth Medium MV (PromoCell, Heidelberg, Germany) in standard culture conditions (humidified, 37℃, 5% CO2). PMVECs were transfected with BMPR2 mutation or empty vector and kept under G418S selection. Stable cell lines with BMPR2 mutation in the kinase domain (BMPR2R332X, referred to as Mutation1), or in the cytoplasmic tail domain (BMPR22580delT, referred to as Mutation2), or empty vector (referred to as No Mutation) were utilized for all in vitro experiments.21 PMVEC glucose update was assessed by two methods. First, flux was assessed by supernatant substrate concentration over time. Second, we measured insulin-mediated intracellular 2-deoxyglucose augmentation (Glucose Uptake Colorimetric Assay, BioVision Inc., Milpitas, CA, USA). Insulin signaling kinases were assessed by western blotting. We utilized polymerase chain reaction (PCR) to assess expression of insulin receptor splice isoforms IR-A and IR-B. These two splice isoforms differ in that IR-A excludes exon 11 while IR-B includes exon 11. PCR methods for messenger RNA (mRNA) quantitation of IR-A, IR-B, and the IR-A:IR-B ratio have been developed previously and were utilized in this experiment.22 Intracellular localization of GLUT4 and response to insulin were assessed by immunohistochemistry. Further detail of cell lines, methods of glucose uptake, PCR, western blotting, and immunohistochemistry are also available in the Online Supplement.
Statistical analysis
Figures and results are reported as mean ± standard deviation (SD). One-way ANOVA, two-way ANOVA, and t-tests were used to compare continuous variables; Chi-square was used to compare proportions between groups as appropriate (using Prism v5.0, GraphPad, San Diego, CA, USA). Statistical significance was taken as P < 0.05 using multiple comparison corrections.
Results
Western diet promotes pulmonary hypertension development in mice
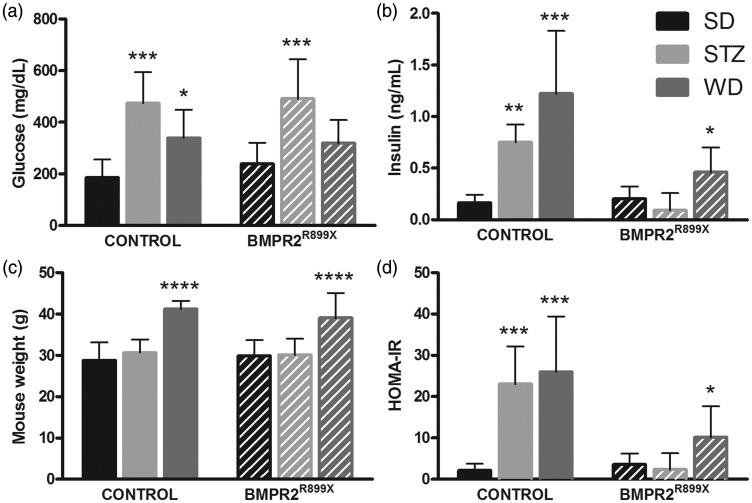
We first tested the hypothesis that hyperinsulinemia, but not hyperglycemia, is associated with PAH in control and BMPR2R899X mice. STZ resulted in fasting hyperglycemia (P < 0.001 versus SD for each genotype) without weight gain (Fig. 1) similar to human type 1 diabetes mellitus (DM). WD resulted in weight gain, hyperinsulinemia, and higher HOMA-IR (P < 0.05 versus SD for each genotype) consistent with human type 2 DM (Fig. 1).
Fig. 1.
Induced hyperglycemia and hyperinsulinemia models in control and BMPR2-mutant mice. (a, b) STZ and WD resulted in hyperglycemia with insulin levels being elevated most in animals fed WD. (c, d) Markers of insulin resistance were elevated by WD feeding. Comparison with SD-fed group of same genotype, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 7–10 per bar.
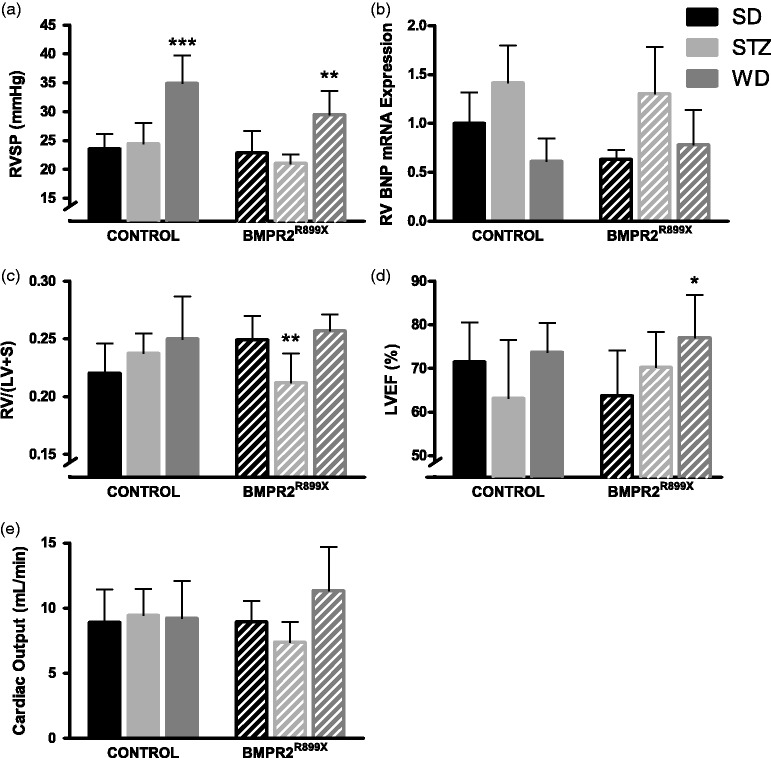
Both genotypes of mice developed elevated RVSP when fed WD (P < 0.01; Fig. 2a) but STZ had no effect. The magnitude of RVSP elevation was generally mild as has been reported previously.23 Fulton index (RV/LV+S) was no different between animals on SD and WD, but lower in BMPR2R899X animals given STZ (P < 0.01 compared with SD or WD; Fig. 2c). Left ventricular ejection fraction was not reduced by either STZ or WD (Fig. 2d). Cardiac output did not vary between groups (Fig. 2e). Expression of BNP was assessed in RV specimens by quantitative PCR (qPCR) for BNP mRNA. By two-way ANOVA, there was an effect of diabetogenic exposure on RV BNP expression (Fig. 2) but genotype had no effect on RV BNP expression. Small increases in RVSP were seen with acute hypoxia and larger increases with U46619. In SD-treated mice, those with BMPR2 mutation had less vasoreactivity to U46619 (P < 0.01; Fig. S1). The effect of diet did not have an effect on vasoreactivity in BMPR2R899X mice, but WD prevented vasoconstriction due to U46619 in control mice (P < 0.01)
Fig. 2.
Western diet raises RVSP in both control animals and a transgenic mouse model of human PAH. (a) RVSP was elevated by WD regardless of genotype. Treatment with STZ had no effect on RVSP. (b) RV BNP expression was affected by diabetogenic exposure (two-way ANOVA P < 0.05), without genotype effect. (c) Fulton index was no different across groups. (d) Reduced LVEF did not explain WD-induced PH. (e) Cardiac output did not vary by genotype or diet. Symbols are same as in Fig. 1. n = 3–4 per bar for BNP expression, otherwise 5–12 per bar.
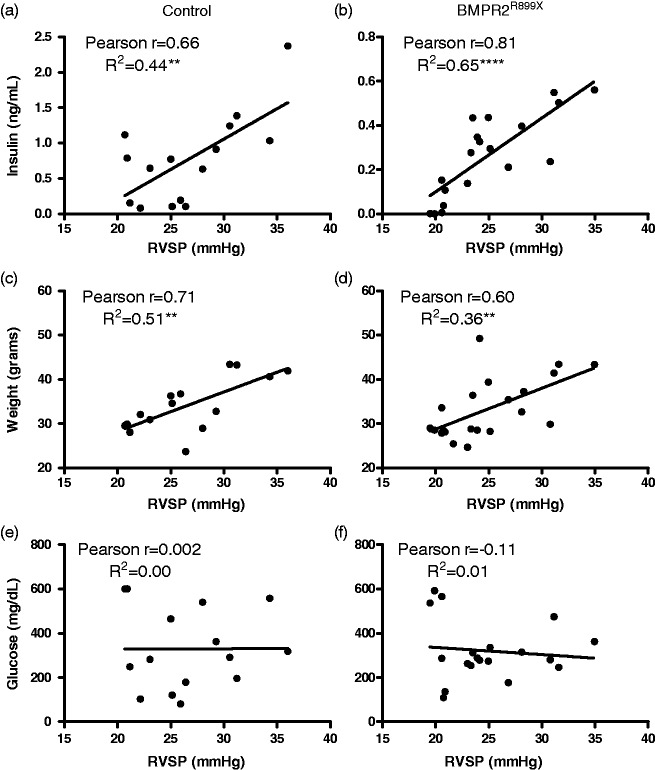
There was a strong correlation between fasting insulin and RVSP and weight and RVSP but no correlation between fasting glucose and RVSP (Fig. 3). Correlations were similar irrespective of BMPR2 mutation and when all animals were analyzed in aggregate (data not shown). These data confirm that hyperinsulinemia, but not hyperglycemia, associates with RVSP in control animals and a transgenic mouse model of human PAH.
Fig. 3.
Hyperinsulinemia, but not hyperglycemia, is associated with RVSP in control and BMPR2 mutant mice. Pearson correlations are shown for fasting insulin (a, b), body weight (c, d), and fasting glucose (e, f). There was strong correlation of RVSP with both weight and insulin. n = 15–21 for each correlation. Symbols denoting statistical significance of the R2 value are the same as given in Fig. 1.
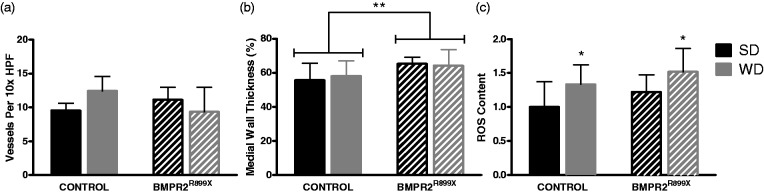
The number and morphometry of small pulmonary vessels were assessed. In fluorescence-labeled alpha smooth muscle actin stained lung sections, small vessels were counted manually per high power microscope field. Pulmonary vessel density was unchanged by genotype or diabetogenic exposure (Fig. 4). Medial wall thickness was also assessed. By two-way ANOVA, there was an increase in medial wall thickness by BMPR2 mutation, but no effect of diabetogenic exposure (Fig. 4). Representative histology is given in supplemental figures Figs. S2 and S3.
Fig. 4.
BMPR2 mutation and WD have varied results on small pulmonary vessels. (a) Small pulmonary vessel density was not altered by diet or genotype. Mean vessels per 10× high power field is given (averaged over ten fields, n = 3–5 mice per bar). (b) Pulmonary vessels in mice with BMPR2 mutation showed increased medial wall thickness, but this did not vary based on diet exposure (two-way ANOVA P < 0.01 for effect of genotype, P = NS for effect of diabetogenic exposure). The mean medial wall thickness from all vessels on 15 high powered fields is given for n = 4 mice. (c) WD and mutation each significantly increased lung ROS (P < 0.01 for WD, P < 0.05 for BMPR2 mutation, n = 10–12 per bar). ROS given as fold change compared with control genotype, SD group. Comparison with SD-fed group of same genotype, *P < 0.05.
Increased lung oxidizing capacity in control and BMPR2R899X mice fed Western diet
Given that no histologic pulmonary vascular changes were identified to be related to WD-induced PAH, we assessed ROS as a principal mediator of endothelial dysfunction by measuring total oxidant content in lung lysates with a dichlorofluorescin assay, comparing animals on WD with SD controls. ROS was increased independently by WD and BMPR2 mutation compared with respective controls (P < 0.01 for WD, P < 0.05 for BMPR2 mutation; Fig. 4) when analyzed with two-way ANOVA. It is known that ROS results in both endothelial dysfunction of the systemic circulation and insulin resistance25 and this, coupled with pulmonary vessel remodeling, may partially explain our finding of reduced pulmonary vasoreactivity in animals with BMPR2 mutation.
Effect of Western diet on insulin-mediated intracellular signaling in whole lung
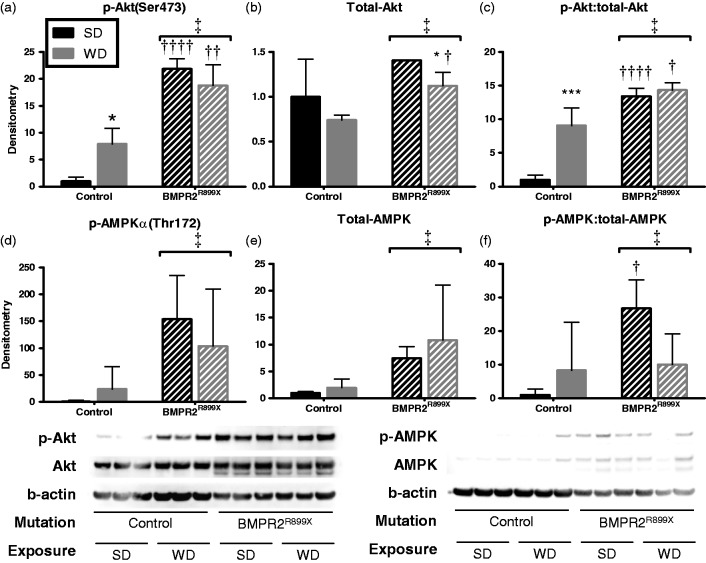
Because insulin resistance is characterized by downregulation of Akt and its targets; we used lung lysates to assess the tissue-specific effect on these signaling pathways in our animals. We found that compared with control, BMPR2R899X mice had higher total and phospho-Akt (1.4-fold and 22-fold versus control, respectively) and total and phospho-AMPKα (7.4-fold and 154-fold, respectively) (Fig. 5) when both were fed SD. In control mice, WD feeding resulted in a 7.9-fold increase in the amount of phospho-Akt(Ser473) and a 24-fold increase in phospho-AMPKα(Thr172) versus SD. However, in BMPR2R899X mice, these phosphorylated kinases were not further increased by WD versus SD, suggesting a ceiling effect.
Fig. 5.
BMPR2 mutation increases insulin signaling protein expression in lung. Total and phosphorylated Akt (a–c) and AMPK (d–f) were increased by the presence of BMPR2 mutation compared to control. WD increased phosphorylation of Akt and AMPK in control mice, but in BMPR2 mutant animals no further increase was seen compared with SD fed animals. Comparison with SD-fed group of same genotype, *P < 0.05, ***P < 0.001. BMPR2R899X animals compared with littermate control, †P < 0.05, ††P < 0.01, †††P < 0.001, ††††P < 0.0001. ‡Effect of mutation P < 0.05 by two-way ANOVA. n = 3 mice per bar run in technical triplicate.
Endothelial cells with BMPR2 mutation have reduced glucose uptake in response to insulin
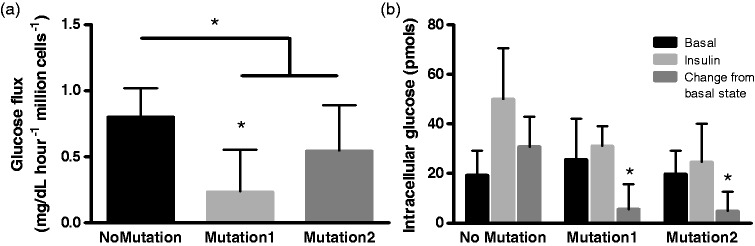
We next evaluated the effects of BMPR2 mutation and insulin on PMVECs in vitro. By two independent methods, insulin-stimulated glucose flux was demonstrated to be reduced in BMPR2 Mutant1 and Mutant2 PMVECs without a difference between the two mutations studied (Fig. 6)
Fig. 6.
Insulin-induced glucose flux is reduced by BPMR2 mutation in isolated endothelial cells. (a) The rate of utilization of glucose from supernatant media was reduced by BMPR2 mutation with Mutation1 having a more profound effect than Mutation2. (b) There was increased intracellular glucose analogue in PMVECs under insulin stimulation, an effect significantly reduced by the presence of BMRP2 mutation. *P < 0.05 compared with cells without BMPR2 mutation. Six technical replicates used for each experiment.
Insulin receptor isoform expression in cultured endothelial cells
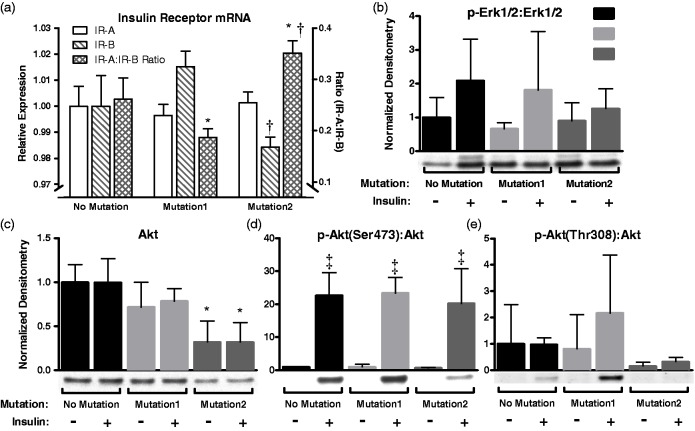
We next sought to define the molecular basis of our finding of reduced insulin sensitivity in BMPR2-mutant PMVECs as well as our findings in control and BMPR2R899X mice. Increased expression of IR-A, with an increase in the ratio of IR-A to IR-B has been demonstrated to relate to proliferative index in malignant tissues, thought due to pro-proliferative IR-A signaling. IR isoform A is known to signal similarly to insulin like growth factor receptor and has minimal to no expression in mature tissues. We found that wild-type PMVECs in culture conditions express mRNA of both isoforms and that BMPR2 mutation had no effect on expression of IR-A. The ratio of IR-A:IR-B varied by BMPR2 mutation type (one increased, one decreased). This seemed driven by expression of IR-B as IR-A expression was the same for all cell lines (Fig. 7).
Fig. 7.
Expression of insulin receptor isoforms and downstream insulin signaling molecules in cultured endothelial cells with and without BMPR2 mutation. (a) Insulin receptor isoform A was not differentially expressed according to mutation status. Isoform B expression varied by mutation status, but the ratio was not skewed significantly towards increased presence of IR-A. (b) The MAPK signaling molecule Erk1/2 had no statistically significant change in phosphorylation by mutation or insulin status. (c–e) Akt, however, had reduced expression with one of two mutations in BMPR2 but relative phosphorylation of the Ser473 site remained intact. *P < 0.05 compared with cells without mutation (two-way ANOVA); †P < 0.05 compared with Mutation1 (two-way ANOVA); ‡P < 0.05 compared with no insulin (two-way ANOVA).
Insulin signaling in cultured endothelial cells
Total Akt in PMVECs was reduced by BMPR2 mutation (mutation effect 69% of variance, P < 0.001; Fig. 7). Two different Akt phosphorylation sites were evaluated – phospho-Akt(Ser473) and phospho-Akt(Thr308) – as the critical activation site in endothelial cells is uncertain. Phospho-Akt(Ser473) was reduced by mutation (similar to total Akt) and increased by insulin (mutation effect 13% of variation, P < 0.05; insulin effect 64% of variation, P < 0.0001; Fig. S1). With normalization for total-Akt, there was no effect of mutation on phospho-Akt(Ser473) (insulin effect, P < 0.0001; mutation effect, P = 0.85; Fig. 6d). No differences in phospho-Akt(Thr308) based on experimental conditions were observed (Fig. 7 and Fig. S4). Similar effects of BMPR2 mutation was observed for the downstream target of Akt, GSK-3β with less activation by insulin treatment (Fig. S4). Total- and phospho-P70-S6K and phospho-Erk1/2 did not vary by condition (Fig. S4 and Fig. 7, respectively).
Glucose transporter translocation
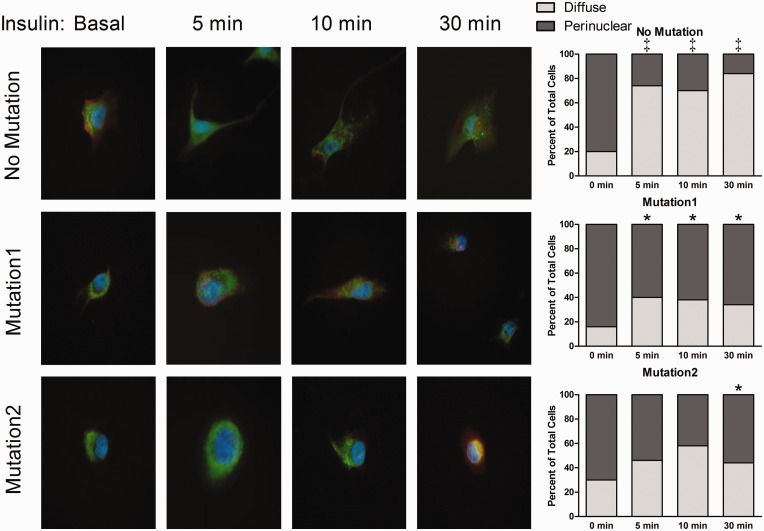
Insulin-augmented glucose uptake requires GLUT4 translocation to the cell surface, a process dependent on Akt and intact cytoskeleton.26,27 Having demonstrated intact insulin signaling, we explored whether cytoskeletal abnormalities may underlie our and others’ findings in mice and humans with PAH.4,16,28 GLUT4 localization was no different between cells with and without BMPR2 mutation at basal conditions (Fig. 8). Upon insulin stimulation, No Mutation PMVECs had rapid redistribution of GLUT4 from a perinuclear location to the cell periphery (P < 0.05). Cells expressing Mutation1 or Mutation2 lacked an insulin-mediated GLUT4 redistribution (P = NS for comparison between basal state and insulin time points), a finding significantly different from PMVECs with No Mutation (P < 0.05 at all time points for Mutation1; P < 0.05 at 30 min for Mutation2). This finding of impaired GLUT4 trafficking is in keeping with prior studies demonstrating BMPR2 mutation causes impaired nuclear trafficking of glucocorticoid receptors.16
Fig. 8.
Insulin-induced translocation of glucose transporter type 4 is reduced by BMPR2 mutation. GLUT4 immunolocalization (green) was demonstrated at various time points of insulin exposure in endothelial cells co-stained with DAPI (blue) and actin (red). The proportion of cells with GLUT4 predominantly located in a perinuclear region versus diffusely throughout the cytoplasm was represented graphically. *P < 0.05 compared to basal condition (zero minutes); ‡P < 0.05 compared to cells without mutation. Statistical comparison is by chi-square test.
Discussion
While the relationships among insulin resistance, endothelial dysfunction, and complications of systemic vascular disease have been long studied, the effects on the pulmonary vasculature is only recently beginning to be explored. Based on recent observations of an association of insulin-resistant states with PAH and BMPR2-mediated metabolic dysregulation, we hypothesized that hyperinsulinemia due to insulin resistance would be injurious specifically to the pulmonary vasculature and promote PAH development. Indeed, these experiments demonstrate that hyperinsulinemia, but not hyperglycemia, associates with mild PAH development demonstrated by a strong correlation between RVSP and fasting insulin not explained by changes in LV structure or function (Figs. 2 and 3). These data suggest that insulin resistance induced by WD or exposure to hyperinsulinemia may have a direct pathogenic effect on the pulmonary vascular bed. While both type 1 DM and the metabolic syndrome are complicated by hyperglycemia and the development of vascular disease, only the latter is known to be associated with PAH. Here we have replicated this observation in both control animals and a transgenic model of PAH. In our animal model of type 2 DM (WD feeding), we observed more pronounced hyperinsulinemia (with similar hyperglycemia and body weight) in control animals compared with those with BMPR2 mutation, a finding that was not statistically significant. Mathematically, this also resulted in higher HOMA-IR in control animals (also not statistically different between control and BMPR2R899X). Increased variation of fasting insulin and HOMA-IR was observed in control animals compared to BMPR2R899X.
Acknowledging the poorly understood differences of vascular and endothelial biology between the systemic and pulmonary systems, we explored the presence of ROS and correlates of known effects of insulin resistance and ROS on systemic endothelial dysfunction.25,29 Indeed, we observed significantly higher ROS in the lungs of animals fed WD and those with BMPR2 mutation with an additive effect (Fig. 4). Our hypothesis was that WD would result in pulmonary vascular injury and rarefaction in PAH-prone animals, though this was not demonstrated in these short-duration experiments. Likewise, we did not observe major structural alterations in small pulmonary vessel morphometry with the diabetes models used in this experiment. Universal expression of BMPR2 mutation did result in a small increase in pulmonary vessel medial wall thickness. As a marker of pulmonary vasoreactivity, we utilized hypoxia- and non-hypoxia-induced vasoconstriction and found reduced vasoreactivity due to BMPR2 mutation, but diet had no effect. Taken together, these data are consistent with prior reports of the complex interplay of insulin action and the endothelium. Others have shown transgenic animals with disrupted insulin signaling (IRS-1−/−, IRS-2−/−, or IR+/−) have systemic vascular effects, but depending on the model, a second stressor is needed to demonstrate pathologic endothelial dysfunction.30–33 Moreover, endothelial-specific insulin resistance has been shown to increase ROS, further supporting the pathogenic role ROS may play in endothelial dysfunction and underscoring the importance of WD- and BMPR2-induced increased ROS demonstrated in our experiments. We hypothesize that the oxidant injury induced by a Western diet, in concert with vascular remodeling occurring in context of BMPR2 mutation, together limit vasoreactivity.
In skeletal muscle from obese humans and animal models of insulin resistance, insulin-mediated Akt activation is reduced.8,9,34 The impact of BMPR2 mutation on insulin signaling was previously unexplored. In lung tissue from animals expressing mutant BMPR2, we observed higher expression and activation of Akt compared to control animals. WD feeding resulted in increased phospho-Akt, a finding restricted to control mice, possibly due to a ceiling reached in BMPR2R899X animals. Regardless, insulin-mediated signaling within lung tissue appears intact which may promote downstream metabolic and non-metabolic effects. Because we used tissue specimens, we were unable to determine if individual cell types within the lung are more or less affected by circulating hyperinsulinemia and therefore drive the physiologic responses seen. For this reason, we turned to in vitro studies of PMVECs with and without BMPR2 mutation.
Using PMVECs, we demonstrated a cellular phenotype of reduced insulin-mediated glucose uptake as a result of BMPR2 mutation, possibly mediated by reduced GLUT4 translocation to the cell membrane. On the surface, this finding may contrast with previously described increases in 18FDG uptake in lung and RV in humans with and animal models of PAH.35–37 However, those studies are unable to identify cell types responsible for glucose uptake and do not assess responsiveness to insulin. Rather, they demonstrate increased glucose uptake by insulin-independent means – presumed via constitutively-expressed GLUT1 as demonstrated in an animal model.37 In the study by Zhao et al., primary lung fibrocytes from subjects with idiopathic PAH were shown to have increased basal 18FDG uptake (compared with donor controls). Our study of the response to insulin stimulation on PMVECs expands on those findings by investigating a different cell type and exploring the effect of insulin. In exploring mechanisms for reduced insulin-stimulated glucose uptake, we identified impaired GLUT4 trafficking within cells expressing BMPR2 mutation. Known cytoskeletal defects caused by BMPR2 mutation likely explain this finding as GLUT4 is dependent on actin cytoskeleton for its function.16,27,38 Our new finding of failure of insulin-augmented glucose uptake in PMVECs with BMPR2 mutation may have further significance in tissues such as cardiomyocytes and is an interest of future research.
Our study has several limitations. Our model of universal BMPR2 mutation expression is known to have variable penetrance of PH,6,39,40 and the penetrance was lower in these data. The RVSP given in Fig. 2 are mean values, so some animals had substantially higher RVSP values. Overall, the current data suggest development of mild PAH. In other PAH animal models, RV hypertrophy is a measurable effect. We showed no difference in Fulton index with BMPR2 mutation, consistent with our prior work showing lack of RV hypertrophy despite PH development with universal expression of BMPR2.41 We believe this also explains the lack of higher BNP expression in animals with BMPR2 mutation. We used common inducible models of diabetes in this experiment. STZ is an alkylating agent with pancreatic beta cell specificity via cellular uptake through GLUT2 and while it has long been used as a type 1 DM model, its effect is known to vary by gender, age, strain, species, and other factors.42–44 Our animals differed only in the presence of BMPR2 mutation (male littermates, matched for age), but BMPR2R899X animals demonstrated increased STZ sensitivity. There are no data on the impact of BMPR2 mutation on pancreatic beta cells or GLUT2 expression. There is scant data that BMPR2 mutation may impair DNA repair mechanisms, which would be consistent with our observations.45 While fasting insulin was found to be highly correlated with RVSP, this does not clearly demonstrate causation. Animal weight also positively correlated to RVSP (though not as strongly) and it could be hypothesized that other circulating adipokines promote PAH separate from or in concert with hyperinsulinemia. Our in vitro studies focused on PMVECs. We were unable to assess other cell types such as vascular smooth muscle cells, skeletal myocytes, or adipocytes which may provide additional insight into the increased presence of insulin resistance at an organism level, as demonstrated in PAH.3,4
In conclusion, we have demonstrated that hyperinsulinemia as the result of Western diet exposure is associated with PAH development not only in PAH-susceptible BMPR2R899X mice, but also in control mice. Western diet increases lung ROS generation but has no large effects on vascular density. In vitro, BMPR2 mutation results in reduced endothelial cell glucose transporter activation and insulin-mediated augmentation of glucose flux while insulin signaling kinase activation remains intact. Further study of the implications of insulin resistance in patients is needed, including whether treatment of insulin resistance improves outcomes of PAH and whether patients with insulin resistance have increased development of PVD.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was supported by funding from the National Institutes of Health - 1P01HL108800. KDN, JPF, JHN, JDW, and ARH have each received research funding from the NIH. JPF has received research funding from the Parker B. Francis Foundation, Actelion, and Gilead Sciences. ARH has received research funding from United Therapeutics and speakers’ fees from United Therapeutics, Actelion, Bayer, and Pfizer.
References
- 1.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res 2014; 115(1): 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136(1): 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J. Heart Lung Transplant 2011; 30(8): 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33(2): 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson L, Brittain EL, Pugh ME, et al. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ 2014; 4(2): 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West J, Niswender KD, Johnson JA, et al. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 2013; 41(4): 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab 2009; 297(3): E568–E577. [DOI] [PubMed] [Google Scholar]
- 8.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012; 148(5): 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 2013; 14(1): 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota T, Kubota N, Kadowaki T. The role of endothelial insulin signaling in the regulation of glucose metabolism. Rev Endocr Metab Disord 2013; 14(2): 207–216. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2012; 122(12): 4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 2012; 52(9): 1970–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan LJ. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. Journal of Diabetes Research 2014; 2014: 137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trammell A, Talati M, Brittain E, et al. Bmpr2 mutation results in impaired glut4 trafficking. Am J Respir Crit Care Med 2013; 187: A1735. [Google Scholar]
- 15.Trammell AW, Penner N, Fessel JP, et al. Hyperinsulinemia promotes pulmonary hypertension development in mice. Am J Respir Crit Care Med 2014; 189: A2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JA, Hemnes AR, Perrien DS, et al. Cytoskeletal defects in bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2012; 302(5): L474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosius F. Diabetic complications consortium. Animal Models of Diabetic Complications Consortium: High-Dose Streptozotocin Induction Protocol (Mouse). Available at: http://diacomp.org (accessed 15 December 2014).
- 18.Chazova I, Loyd JE, Zhdanov VS, et al. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 1995; 146(2): 389–397. [PMC free article] [PubMed] [Google Scholar]
- 19.Brittain E, Penner NL, West J, et al. Echocardiographic assessment of the right heart in mice. J Vis Exp 2013; 81: 50912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JA, West J, Maynard KB, et al. Ace2 improves right ventricular function in a pressure overload model. PLoS One 2011; 6(6): e20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krump-Konvalinkova V, Bittinger F, Unger RE, et al. Generation of human pulmonary microvascular endothelial cell lines. Lab Invest 2001; 81(12): 1717–1727. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier R, Hamel F, Beaulieu D, et al. Absence of a differentiation defect in muscle satellite cells from dm2 patients. Neurobiol Dis 2009; 36(1): 181–190. [DOI] [PubMed] [Google Scholar]
- 23.West J, Harral J, Lane K, et al. Mice expressing bmpr2r899x transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 2008; 295(5): L744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long L, MacLean MR, Jeffery TK, et al. Serotonin increases susceptibility to pulmonary hypertension in bmpr2-deficient mice. Circ Res 2006; 98(6): 818–827. [DOI] [PubMed] [Google Scholar]
- 25.Youn JY, Siu KL, Lob HE, et al. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 2014; 63(7): 2344–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sylow L, Jensen TE, Kleinert M, et al. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 2013; 62(6): 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/glut4 circuitry. Traffic (Copenhagen, Denmark) 2011; 12(6): 672–681. [DOI] [PubMed] [Google Scholar]
- 28.Pugh ME, Hemnes AR. Metabolic and hormonal derangements in pulmonary hypertension: From mouse to man. Int J Clin Pract Suppl 2010; 64(168): 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414(6865): 813–820. [DOI] [PubMed] [Google Scholar]
- 30.Wheatcroft SB, Shah AM, Li JM, et al. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Diabetes 2004; 53(10): 2645–2652. [DOI] [PubMed] [Google Scholar]
- 31.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes 2008; 57(12): 3307–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubota T, Kubota N, Moroi M, et al. Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation 2003; 107(24): 3073–3080. [DOI] [PubMed] [Google Scholar]
- 33.Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 2003; 111(9): 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zdychova J, Komers R. Emerging role of akt kinase/protein kinase b signaling in pathophysiology of diabetes and its complications. Physiol Res 2005; 54(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 35.Oikawa M, Kagaya Y, Otani H, et al. Increased [18f]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005; 45(11): 1849–1855. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Ashek A, Wang L, et al. Heterogeneity in lung (18)fdg uptake in pulmonary arterial hypertension: Potential of dynamic (18)fdg positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 2013; 128(11): 1214–1224. [DOI] [PubMed] [Google Scholar]
- 37.Marsboom G, Wietholt C, Haney CR, et al. Lung (1)(8)f-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185(6): 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foletta VC, Lim MA, Soosairajah J, et al. Direct signaling by the bmp type ii receptor via the cytoskeletal regulator limk1. J Cell Biol 2003; 162(6): 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beppu H, Ichinose F, Kawai N, et al. Bmpr-ii heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 2004; 287(6): L1241–1247. [DOI] [PubMed] [Google Scholar]
- 40.Soon E, Crosby A, Southwood M, et al. Bone morphogenetic protein receptor type ii deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192(7): 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemnes AR, Brittain EL, Trammell AW, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J. Respir Crit Care Med 2014; 189(3): 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008; 51(2): 216–226. [DOI] [PubMed] [Google Scholar]
- 43.Deeds MC, Anderson JM, Armstrong AS, et al. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab Anim 2011; 45(3): 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham ML, Janecek JL, Kittredge JA, et al. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp Med 2011; 61(4): 356–360. [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Vattulainen S, Aho J, et al. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2014; 50(6): 1118–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.