Abstract
Anticoagulants are widely used in patients with pulmonary arterial hypertension (PAH) to prolong survival. However, there is a lack of robust evidence demonstrating the benefits of anticoagulants in PAH patients and very little is known about the complications of their use in this population. The objective of this study is to compare the safety of routine administration of oral anticoagulants between PAH patients who were and were not treated with oral anticoagulants. This observational, retrospective cohort study included consecutive patients with confirmed PAH from two centers: Brigham and Women’s Hospital in Boston and Hospital Universitario La Paz in Madrid from January 2009 to August 2015. The study group comprised patients who received therapeutic anticoagulation; patients who had never received anticoagulants were placed in the control group. Of the 201 included patients, 60.2% were treated with oral anticoagulants and 39.8% were not treated. The hazard ratio for major bleeding was 2.7 (95% confidence interval [CI] = 1.1–6.8; P = 0.036). The incidence rate for the anticoagulation group was 4.7 per 100 patient-years (95% CI = 2.5–8.0). The most frequent major hemorrhage was gastrointestinal bleeding with 24 cases (72.7%). Prior bleeding, poor anticoagulation, HAS-BLED score ≥3, diabetes, and number of medications were factors that increased the risk of major bleeding in patients using anticoagulants. The harmful effects of anticoagulants could outweigh the benefits in PAH patients. Therefore, anticoagulants should be prescribed on a case-by-case basis and should not be systematically recommended.
Keywords: anticoagulation therapy, major hemorrhage, pulmonary arterial hypertension, venous thromboembolism
Introduction
Pulmonary hypertension (PH) is a spectrum of diseases involving the pulmonary vasculature, defined as an elevation in pulmonary arterial pressures (mean pulmonary artery pressure [mPAP] ≥ 25 mmHg). Pulmonary arterial hypertension (PAH) is a relatively rare form of pulmonary hypertension that is characterized by symptoms of dyspnea, chest pain, and syncope. If left untreated, it leads to right-side heart failure and ultimately death.1 There is currently no cure for PAH, though pharmacologic specific treatment has been shown to improve morbidity and mortality. Anticoagulants are typically administered as adjuvants,2 because previous studies demonstrated a high prevalence of vascular thrombotic lesions in post-mortem evaluation of idiopathic PAH patients.3,4 Abnormalities in the coagulation cascade may contribute to a pro-thrombotic state in idiopathic PAH (IPAH).5,6 These physiological changes, along with concomitant conditions requiring anticoagulation such as cardiac valve replacement, atrial fibrillation, and risk factors for venous thromboembolism are the rationale for oral anticoagulants in PAH patients. However, the efficacy of anticoagulation therapy for prolonging survival has never been confirmed by randomized controlled trials and the results from recent PAH registries studies are controversial.7,8 With the exception of chronic thromboembolic PH, regulatory agencies have not authorized the use of anticoagulants in PH. The use of vitamin K antagonists (VKAs) requires special attention because of a narrow therapeutic window and significant variability among patients.9 Inadequate or excessive anticoagulation can lead to thrombotic or hemorrhagic complications, respectively.10 Certain etiologies and medications may increase the risk of bleeding in PAH patients, but only one study has been published on this matter.11
Therefore, we compared anticoagulation-related complications between PAH patients who were and were not treated with oral anticoagulants. We also analyzed several characteristics of PAH patients and their implications regarding these complications.
Methods
Study design
This retrospective observational cohort study included PAH patients from two centers: the Brigham and Women’s Hospital in Boston and the Hospital Universitario La Paz in Madrid. From January 2009 to August 2015, 201 consecutive patients were followed. We combined data from the hospitals, specialized anticoagulation clinics, and primary care institutions where patients were monitored. The study was approved by the Hospital La Paz Ethics Committee and underwent classification by the Spanish Medicines Agency. Patients who fulfilled the meeting protocol selection criteria were included. The Partners Healthcare institutional review board waived the need for informed consent.
Patients
The diagnosis of PAH was confirmed by right heart catheterization (RHC) based on a mPAP ≥ 25 mmHg and a mean pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg.
Patients who received therapeutic anticoagulation before or throughout the study period formed the anticoagulation group. Patients who did not receive therapeutic anticoagulation during the study period formed the control group. Patients from the anticoagulation group whose treatment was stopped during the study period were censored. Control patients that experienced a thrombotic event during the study period were started on anticoagulation therapy and switched to the anticoagulation group. They were considered part of the control cohort when assessing thrombotic-related events.
Most patients on anticoagulants were taking VKAs (warfarin in Boston, acenocoumarol in Madrid) on an individualized dosage regimen as per target international normalized ratio (INR) recommendations. Direct oral anticoagulants were also used, but to a lesser extent. Pulmonary vasodilators (prostacyclin analogues, endothelin-receptor antagonists, and phosphodiesterase-inhibitors) were prescribed according to PAH guidelines.
Data collection
The following was obtained from the patients’ medical records: demographics, clinical variables (PAH etiology, disease duration, functional capacity), hemodynamics, echocardiographic findings, specific PAH pharmacotherapy, PAH combined treatment, concomitant medications, morbidity, risk of bleeding, and death. To evaluate the safety of anticoagulants, we collected major and minor bleeding events: major hemorrhage (clinically overt and associated with a fall in hemoglobin of at least 20 g·L−1 [≥1.2 mmol·L−1]); required transfusion of at least two units of red cells; involved a critical site; or was fatal), minor hemorrhages, hematomas, and thrombotic events (related to the formation or presence of a thrombus). The anticoagulation follow-up and related events of Brigham and Women’s Hospital’s patients were collected from both DAWN AC® (anticoagulation management software used by the Anticoagulation Clinic) and patients’ medical records. A health intelligence platform incorporating the electronic medical record search engine QPID® (Queriable Patient Inference Dossier) was used to help identify events and ensure no information was missing. Among the key words used to identify possible anticoagulation events were: bled, bleeding, bruising, hematoma, hemorrhage, blood, bloody, bleed, hematuria, hemoptysis, epistaxis, melena, hemorrhagic and gastrointestinal bleeding. Events from Hospital La Paz’s patients were collected from the hospital electronic medical record. The primary care electronic medical record (HORUS®) was also consulted to avoid missing any anticoagulation related information or events that were not registered during a patient’s visit with their specialist.
Among the anticoagulation group, we recorded anticoagulant agents, target INRs, indications for anticoagulants, and time spent in therapeutic range (TTR). To identify risk factors for major bleeding, we analyzed age, sex, time from diagnosis of PAH, risk of bleeding, functional capacity, co-morbidities, PAH treatment, and related side effects.
Statistical analysis
Quantitative and ordinal variables were expressed as average and median (interquartile range [IQR]) values, respectively. Categorical variables were described through absolute and relative frequencies using percentages. For intergroup comparisons, we used the t-test for independent samples in parametric distributions and the Mann–Whitney U-test for non-parametric distributions. Categorical variables were compared using Pearson’s chi-square test. P values < 0.05 were significant.
To evaluate the association between the exposure (anticoagulants) and the outcome (anticoagulation-related event) we calculated the odds ratios (OR) with the associated 95% confidence intervals (CI) and P values. The hazard ratio (HR) from the time-to-event analysis was also obtained for major bleeding complications. The frequency of new anticoagulation-related events per population at risk was calculated using the incidence rate (IR) and first major bleeding IR (counting major bleeding events only once for each patient). The incidence rate ratio (IRR) was used to compare the IR between groups. Event-free survival (major bleeding/thrombotic events) time and cumulative incidence were analyzed with Kaplan–Meier survival curves.
These analyses were performed on all the anticoagulation patients and performed again after excluding patients with approved indications for anticoagulants other than PAH, to account for the effects of anticoagulants in patients with approved indications.
We performed a univariate analysis to discern the factors influencing the incidence of major bleeding in patients taking oral anticoagulants. This analysis included: a t-test for independent samples and analysis of variance for parametric distributions, and the Chi-square test and Mann–Whitney U-test for non-parametric distributions. Multivariate analysis was then performed with the Cox proportional hazards analysis of those factors that were either found to be significant, approached significance (P < 0.10), or had a clinical justification.
The following potential risk factors for major bleeding were evaluated: age, sex, six-minute walking test (6MWT) <300 m, World Health Organization functional class (WHO FC), three or more poor prognostic factors for PAH survival (the following determinants were considered: WHO FC IV, 6MWT <300 m, evidence of pericardial effusion, right atrial pressure >15 mmHg, and CI ≤2.0 L/min/m2), co-morbidities with increased risk of bleeding (atrial fibrillation, peripheral vascular disease, diabetes, and obesity), Charlson co-morbidity index, HAS-BLED score ≥3, history of bleeding or predisposition to bleed, combined PAH treatment, prostacyclin analogs, years since PAH diagnosis, number of medications, target INR >2.5, poor anticoagulation (TTR < 60%), and VKA interaction (moderate or major) with a medication from the patient’s medication list.
The statistical analysis utilized SPSS for Windows (version 15.0: SPSS Inc., Chicago, IL, USA) and MedCalc for Windows (version 16.4: MedCalc Software, Ostend, Belgium).
Results
Characteristics of participants
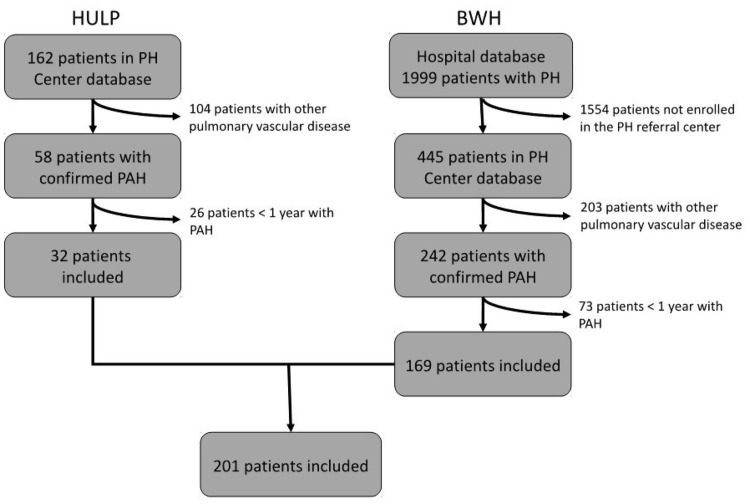
We included 201 patients in this study (Fig. 1): 100 (49.8%) diagnosed with PAH before the beginning of the follow-up (January 2009) and 101 diagnosed after (50.2%). Of these, 121 (60.2%) were treated with oral anticoagulants and 80 (39.8%) were not. The average age at diagnosis was 53 ± 17 years, the median WHO FC was III (IQR = II–III), and the average 6MWT was 353 ± 119 m (Table 1).
Fig. 1.
Creation of the study population. The present study was a retrospective observational cohort study of patients with PAH from two centers. Diagnosis of PAH confirmed by right heart catheterization based on a mPAP ≥ 25 mmHg, and a mean PAWP ≤ 15 mmHg. BWH, Brigham and Women’s Hospital; HULP, Hospital Universitario La Paz; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension.
Table 1.
Patients’ characteristics at the time of PAH diagnosis.
| Entire population (n = 201) (average ± SD/ frequency, %) | Anticoagulation group (n = 121) (average ± SD/ frequency, %) | Control group (n = 80) (average ± SD/ frequency, %) | P | |
|---|---|---|---|---|
| Gender | 1.00 | |||
| Male | 51 (25.4) | 31 (25.6) | 20 (25.0) | |
| Female | 150 (74.6) | 90 (74.4) | 60 (75.0) | |
| Age (years) | 0.745 | |||
| <50 | 76 (38.8) | 49 (40.8) | 27 (35.5) | |
| 50–70 | 92 (46.9) | 54 (45.0) | 38 (50.0) | |
| >70 | 28 (14.3) | 17 (14.2) | 11 (14.5) | |
| Race | 0.412 | |||
| White | 170 (84.6) | 100 (82.7) | 70 (87.5) | |
| Black | 14 (7.0) | 11 (9.1) | 3 (3.8) | |
| Hispanic | 16 (8) | 9 (7.4) | 7 (8.8) | |
| Asian | 1 (0.5) | 1 (0.8) | 0 | |
| Center | 0.276 | |||
| BWH | 169 (84.1) | 105 (86.8) | 64 (80.0) | |
| HULP | 32 (15.9) | 16 (13.2) | 16 (20.0) | |
| Weight | 0.023 | |||
| Pounds | 170.6 ± 48.6 | 176.7 ± 49.4 | 161.5 ± 41.3 | |
| Kilograms | 77.4 ± 22.1 | 80.2 ± 22.4 | 73.3 ± 18.7 | |
| Height | 0.089 | |||
| Inches | 64.3 ± 4.0 | 64.7 ± 4.1 | 63.7 ± 3.8 | |
| Centimeters | 163.3 ± 10.2 | 164.3 ± 10.4 | 161.8 ± 9.7 | |
| BMI | 29.0 ± 7.6 | 29.6 ± 7.8 | 28.1 ± 7.2 | |
| PAH etiologies | 0.164 | |||
| Idiopathic | 86 (43) | 58 (48) | 28 (35) | |
| CTD | 49 (25) | 28 (23) | 21 (26) | |
| CHD | 31 (16) | 19 (16) | 12 (15) | |
| PoPH | 12 (6) | 4 (3) | 8 (10) | |
| HPAH | 8 (4) | 4 (3) | 4 (5) | |
| HIV | 8 (4) | 3 (2) | 5 (6) | |
| Others | 4 (2) | 3 (3) | 1 (1) | |
| Hemodynamics | ||||
| mPAP (mmHg) (n = 179) | 48.4 ± 17.0 | 49.0 ± 16.6 | 47.3 ± 17.6 | 0.516 |
| PAWP (mmHg) (n = 177) | 10.1 ± 4.9 | 10.4 ± 4.9 | 9.8 ± 5.0 | 0.359 |
| Cardiac Index (L/min/m2) | 2.57 ± 0.80 | 2.52 ± 0.77 | 2.64 ± 0.84 | 0.333 |
| PVR (dyn*s*cm−5) | 750 ± 466 | 748 ± 451 | 753 ± 490 | 0.944 |
| Vasoreactive patients (%) | 30 (16.8) | 20 (30.3) | 10 (9.2) | 0.614 |
| Echocardiography | ||||
| Estimated systolic PAP (n = 171) | 67.9 ± 24.0 | 68.3 ± 25.4 | 67.4 ± 21.8 | 0.811 |
| RV dysfunction (n = 197) | 107 (54.3) | 70 (58.8) | 37 (47.4) | 0.117 |
| WHO FC | ||||
| Median (IQR) | III (II–III) | III (II–III) | III (II–III) | 0.759 |
| 6MWT (m) | 353 ± 119 | 360 ± 119 | 341 ± 120 | 0.318 |
6MWT, six-minute walking test; BMI, body mass index; BWH, Brigham and Women’s Hospital; CHD, congenital heart disease; CTD, connective-tissue disease; HAPA, heritable pulmonary arterial hypertension; HIV, human immunodeficiency virus; HULP, Hospital Universitario La Paz; mPAP, mean pulmonary arterial pressure; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PoPH, portal pulmonary hypertension; RV, right ventricle; SD, standard deviation; WHO, World Health Organization.
The median number of prescribed medications per patient was 10 (IQR = 7–13). PAH-specific therapy was administered to 192 (95.5%) patients. The most prevalent co-morbidities were high blood pressure, obesity, and hyperlipidemia with 89 (44.3%), 76 (37.8%), and 59 (29.4%) patients, respectively. The median Charlson index score at diagnosis was 4.0 (IQR = 2–6). Thirty patients (14.9%) died during the follow-up, of which 16 belonged to the anticoagulation group (13.2%) and 14 to the non-anticoagulation group (17.5%), a non-significant difference. The overall median HAS-BLED score was 2 (IQR = 1–3) at the beginning of the follow-up, with 39% patients scoring ≥3.
In addition to PAH, patients had other conditions with indication for anticoagulation. Sixty-four patients (52.9%) were on anticoagulants to treat PAH, 28 patients presented with PAH and atrial fibrillation (23.1%), 27 patients had PAH along with venous thromboembolism (22.3%), and one patient had all three conditions (0.8%). Furthermore, 16 patients (13.2%) were on anticoagulation due to central venous catheter drug administration system.
The median (IQR) time on anticoagulants was 6.1 years (IQR = 2.6–10.5) with 92 patients (76.0%) receiving anticoagulants for the entire period. The TTR was 57%.
Safety analysis
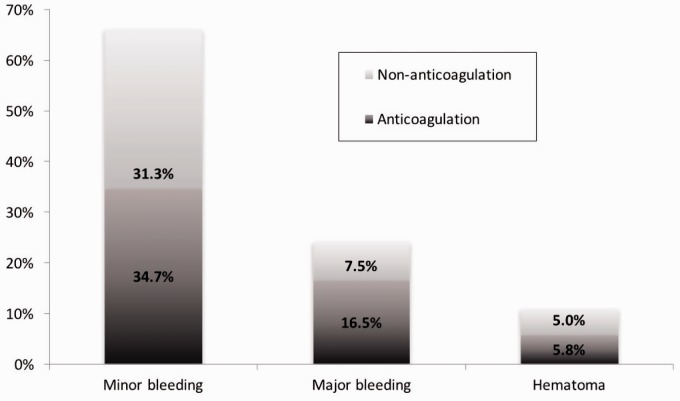
A total of 155 events were documented in 93 patients (46.3%) during the study period. The event OR for the anticoagulation versus the non-anticoagulation group was 2.0 (95% CI = 1.1–3.5; P = 0.021). Of those 155 events, 141 were non-thrombotic events (91%) and occurred in 85 patients (42.3% of the entire population; Fig. 2). Table 2 shows the list of non-thrombotic events.
Fig. 2.
Non-thrombotic events in patients with PAH. We recorded 141 non-thrombotic events, which occurred in 85 patients (42.3% of the entire population).
Table 2.
Frequency of non-thrombotic events.
| Anticoagulation-related complication | Frequency (%) | |
|---|---|---|
| Minor* n = 97) | Major (n = 33) | |
| Epistaxis | 29 (30) | 4 (12) |
| GI bleeding | 21 (22) | 24 (73) |
| Hemoptysis | 14 (14) | − |
| Hematuria | 8 (8) | − |
| Vaginal bleeding | 6 (6) | 2 (6) |
| Ophthalmic bleeding | 5 (5) | − |
| Mouth bleeding | 4 (4) | − |
| Skin tear bleeding | 4 (4) | − |
| Bleeding after procedure | 3 (3) | − |
| Not specified | 3 (3) | − |
| Subdural hemorrhage | − | 2 (6) |
| Brain hemorrhage | − | 1 (3) |
| Hematoma | 11 | |
Minor bleeding was the most frequent type of event with 97 documented episodes (68.8%).
GI, gastrointestinal.
A total of 26 patients (12.9%) suffered 33 major bleeding events. Of these, 20 belonged to the treated group (16.5%) and six to the non-treated group (7.5%). In patients taking oral anticoagulants exclusively for PAH (n = 65), the OR for major bleeding in the anticoagulation versus non-anticoagulation group was 2.8 (95% CI = 1.0–7.9; P = 0.047). The most frequent major hemorrhage was gastrointestinal bleeding, with 24 cases (72.7%). The gastrointestinal bleeding OR was 3.9 (95% CI = 1.2–12.8; P = 0.019). There were two cases of subdural hemorrhage (6.1%) and one case of brain hemorrhage (3.0%). The median (IQR) time to first major bleeding was 2.2 years (IQR = 1.5–3.8), with a median FC of II (IQR = II–III) and 6MWT of 339 m (IQR = 242–401). Patients who experienced major bleeding had a group TTR of 55.0% and time above range of 14.5% at the time of the event. The INRs at the event were out of range in 14 cases (11 above and three below), with two within range and four with no documented INRs.
The IR for the treatment group was 4.7 per 100 patient-years (95% CI = 2.5–8.0), compared to 2.6 per 100 patient-years (95% CI = 1.1–5.1) in the controls. The IRR was 1.8 (95% CI = 0.8–4.3; P = 0.110). When accounting for only the first major hemorrhage, the IRs were 3.9 (95% CI = 2.4–6.0) and 1.9 (95% CI = 0.7–4.2) events per 100 patient-years in the treated and non-treated groups, respectively.
The IR of gastrointestinal bleeding in the anticoagulation group was 4.0 per 100 patient-years (95% CI = 2.0–7.1), compared to 1.3 per 100 patient-years (95% CI = 0.4–3.3) in the controls, with an IRR of 3.1 (95% CI = 0.9–13.4; P = 0.041). We found no significant difference between the groups regarding the association between gastrointestinal side effects from PAH-specific treatment and major gastrointestinal bleeding.
Among the different PAH etiologies, portal PAH had the highest major hemorrhage IR, with 7.0 events per 100 patient-years (95% CI = 0.2–38.7) in the anticoagulation group, followed by connective tissue disease-associated PAH with 6.3 (95% CI = 2.3–13.7), IPAH with 5.0 (95% CI = 2.6–8.5), and congenital heart disease-associated PAH with 2.1 events per 100 patient-years (95% CI = 0.3–7.5).
Anticoagulation patients with only PAH as an indication (n = 65) had a higher six-year cumulative major bleeding IR than the control patients, although not significantly so (log rank = 3.05; P = 0.081). The cumulative six-year gastrointestinal bleeding IR was also higher in this subgroup, compared with the controls (log rank = 4.49; P = 0.034).
After adjusting for follow-up time and prior bleeding/predisposition to bleed, the Cox proportional hazards analysis showed that the major bleeding HR for patients taking anticoagulants was 2.7 (95% CI = 1.1–6.8; P = 0.036).
Regarding direct oral anticoagulants, there were seven patients (5.8%) treated with theses agents (three dabigatran, three rivaroxaban, and one apixaban). A total of six non-thrombotic events were registered in five patients (two dabigatran, two rivaroxaban, and one apixaban). None were categorized as major bleeding. Dabigatran registered three episodes (rectal, vaginal, and non-specified minor bleeding), rivaroxaban two (nose bleeding and hemoptysis), and apixaban one (headache).
There were 14 thrombotic events in 14 patients, six from the anticoagulation group (5.3%) and eight from the non-anticoagulation group (9.2%). The thrombotic event OR in anticoagulation patients with PAH as the only indication (n = 58) was 0.7 (95% CI = 0.2–2.6; P = 0.624). There were two central line occlusions, one internal jugular vein thrombosis and one pulmonary embolism. Of the two patients with PAH and atrial fibrillation who had a thrombotic event, one experienced myocardial infarction, the other cardioembolic stroke. In the control group, there were four cases of pulmonary embolism, two of deep venous thrombosis, one of atherothrombotic stroke, and one of vena-cava syndrome. The venous thromboembolism OR in patients with only PAH as an indication for the anticoagulation versus non-anticoagulation group was 0.2 (95% CI = 0.03–2.02; P = 0.188).
The IR of thrombotic events was 1.4 (95% CI = 0.4–3.7) and 2.4 (95% CI = 1.0–4.8) per 100 patient-years for the anticoagulation and non-anticoagulation groups, respectively. The thrombotic event IRR was 0.6 (95% CI = 0.1–2.2; P = 0.362). The IR of venous thromboembolism in the treated group was 2.0 (95% CI = 0.1–11.2) events per 1000 patient-years and 18.4 (95% CI = 6.8–40.6) events per 1000 patient-years in the non-treated group. The IRR was 0.11 (95% CI = 0.002–0.891; P = 0.012).
The Kaplan–Meier analysis showed that non-anticoagulant users had worse thrombosis-free survival (log rank = 1.65; P = 0.199) and venous thromboembolism-free survival (log rank = 5.82; P = 0.016) than patients treated with anticoagulants.
Analysis of major bleeding risk factors
Table 3 lists the risk factors for major bleeding within the anticoagulation population that either showed or approached statistical significance. Table 4 lists the major bleeding HRs, according to the Cox proportional hazards analysis, adjusted by follow-up time and prior bleeding/predisposition to bleed.
Table 3.
Potential factors that increased the risk of major bleeding.
| Potential modifying factor | OR | 95% CI | P |
|---|---|---|---|
| 6MWT <300 m | 2.80 | 0.94–8.32 | 0.057 |
| HAS-BLED score ≥3 | 11.17 | 3.06–40.77 | <0.001 |
| Prior bleeding/predisposition to bleed | 27.36 | 5.93–126.25 | <0.001 |
| Poor anticoagulation | 2.72 | 1.00–7.40 | 0.045 |
| Diabetes | 2.66 | 0.93–7.65 | 0.063 |
| Target INR >2.5 | 3.87 | 0.97–15.35 | 0.064 |
6MWT, six-minute walking test; CI, confidence interval; INR, international normalized ratio; OR, odds ratio.
Table 4.
COX regression analysis with hazard ratios for specific variables of potential influence on major bleeding.
| Variables | HR | 95% CI | Sig. |
|---|---|---|---|
| Diabetes | 4.36 | 1.69–11.24 | 0.002 |
| 6MWT < 300 m at diagnosis | 1.98 | 0.72–5.41 | 0.085 |
| Poor anticoagulation (HAS-BLED item 5) | 2.38 | 0.94–6.01 | 0.066 |
| Number of medications | 1.11 | 1.00–1.24 | 0.049 |
6MWT, six-minute walking test; CI, confidence interval; HR, hazard ratio; INR, international normalized ratio.
Discussion
PAH patients on anticoagulants had a threefold greater risk of major bleeding than control patients. PAH patients had a greater incidence of major bleeding when compared with other cases where anticoagulation was clearly indicated. Gastrointestinal bleeding was the most frequent form of major bleeding. While patients not receiving anticoagulants had a greater risk of venous thromboembolism, the difference was not significant. Portal PAH, connective tissue disease-associated PAH, and IPAH were the group 1 etiologies (WHO clinical classification system) with the highest rates of major bleeding.
Thus far, no study has provided robust evidence of the efficacy and safety of oral anticoagulants in patients with PAH. Because a randomized clinical trial is unlikely to be viable, the present study offers new insights into the debate. The strength of the current study is that it included a substantial sample of a low-prevalence disease across two different centers, in the United States and Europe.
Characteristics of participants
In the anticoagulation group, approximately half of the patients were prescribed an anticoagulant because of PAH alone. The remainder had co-morbidities that were indications for anticoagulation such as atrial fibrillation and venous thromboembolism in most instances. The number of patients with dual indications for anticoagulation in our study may have been elevated because of the presence of right heart dysfunction and failure, and co-morbid atrial fibrillation or flutter. In other cases, atrial fibrillation or venous thromboembolism may have developed along with PAH due to the patients’ advanced age, and the presence of pro-thrombotic risk factors such as immobility, obesity, chronic heart failure, antiphospholipid syndrome, chronic obstructive pulmonary disease, steroid therapy, and use of central lines.
Complications due to supratherapeutic anticoagulation
Nearly half the study patients suffered an event of some kind. The anticoagulation group had considerably more events than the non-treatment group.
The vast majority of the events were clinically mild, including minor bleeding and hematoma. However, under-reporting of minor bleeding events is common in patients taking oral anticoagulants. The most frequent minor bleeding reported was epistaxis, followed by gastrointestinal bleeding, and hemoptysis. The latter is a severe uncommon complication in PAH, likely due to bronchial artery hypertrophy and/or rupture. The incidence of hemoptysis in PAH patients is unknown and is likely under-reported in scientific literature (12). All hemoptysis episodes in this study were minor and without further complications. Because anticoagulation may aggravate hemoptysis, patients on anticoagulants should be carefully monitored for this symptom.
One-fourth of non-thrombotic events involved major hemorrhage. The significantly higher odds and hazard of major bleeding in the anticoagulation group underscores the risk of major bleeding in such patients. The median PAH functional parameters of patients who experienced any event were consistent with moderate disease stage (FC II, 6MWT > 300 m). Prior to a major bleeding event, the group TTR for anticoagulation patients was low, with approximately half of the time spent in therapeutic range, reflecting a poor control of anticoagulation. Poor anticoagulation/low TTRs in atrial fibrillation patients has been linked to an increased risk of thrombosis and/or bleeding.13,14 Moreover, most of the INRs at the time of the event were above therapeutic range.
The incidence of major bleeding in the anticoagulation group was almost twice as high as that of the control group, although the difference was not significant. The first major hemorrhage IR in our study (3.9 events per 100 patient-years) was lower than that of a Netherlands cohort with 8.1 events (5.4 for idiopathic PAH and 19.0 for connective tissue disease-associated PAH) per 100 patient-years,11 which might be due to their overall higher target INR. The Netherlands cohort had 79% IPAH patients and 91% connective tissue disease-associated PAH patients with target ranges over 2.0–3.0. These ranges were rarely seen in our study. Conversely, our major bleeding rate was higher than the rates reported in recent direct oral anticoagulants clinical trials (Table 5), where the major bleeding rate on warfarin was consistently 3.4 events per 100 patient-years. The IR obtained in our study was also higher than that from retrospective community registries (two events per 100 patient-years),15 i.e. the two PAH works (Netherlands and our study) had the highest major bleeding rates per year, suggesting that PAH patients may be at a higher risk of major bleeding than populations with other indications for anticoagulants.
Table 5.
Summary of major bleeding rates in recent clinical trials of oral anticoagulants.
| Author | Type of study | Year | Indication | INR | Major bleeding IR (%-year) | GI IR (%-year) |
|---|---|---|---|---|---|---|
| Current study | Retrospective (n = 121) | 2015 | PAH | 94% ≤ 2.0–3.0 6% > 2.0–3.0 | 4.8/3.9* | 4.0 |
| Hokusai-VTE et al.16 | RCT (n = 4122) | 2013 | VTE | 2.0–3.0 | 1.6 | NA |
| Giugliano et al. (ENGAGE)17 | RCT (n = 7036) | 2013 | AF | 2.0–3.0 | 3.4 | 1.2 |
| Graner et al. (ARISTOTLE)18 | RCT (n = 9081) | 2011 | AF | 2.0–3.0 | 3.1 | 0.9 |
| Patel et al. (ROCKET)19 | RCT (n = 7125) | 2011 | AF | 2.0–3.0 | 3.4 | NA |
| Connolly et al. (RE-LY)20 | RCT (n = 6022) | 2009 | AF | 2.0–3.0 | 3.4 | 1.0 |
| Henkes et al.11 | Retrospective (n = 138) | 2013 | IHAP CTD-PAH | 2.0–3.0 2.5–3.5 3.0–4.0 | 8.1* | NA |
| Wieloch et al.21 | Retrospective registry (n = 18,391) | 2011 | AF | 2.0–3.0 | 2.6 | NA |
| Friberg et al.22 | Retrospective registry (n = 68,306) | 2012 | AF | NA | 1.9 | NA |
| Abraham et al.23 | Retrospective (n = 7749) (n = 732) | 2015 | AF Non-AF | NA | – | 3.1 1.6 |
| Kearon et al.24 | RCT (n = 369) | 2003 | VTE | 2.0–3.0 | 0.9 | NA |
First major bleeding incidence.
AF, atrial fibrillation; CTD-PAH, connective tissue disease-associated PAH; GI, gastrointestinal; INR, international normalized ratio; IPAH, idiopathic PAH; IR, incidence ratio; NA, not available; PAH, pulmonary arterial hypertension; RCT, randomized clinical trial; VTE, venous thromboembolism.
Henkes et al. (Netherlands cohort) concluded that the major bleeding risk during VKA therapy differed among PAH etiologies.11 They observed higher rates in connective tissue disease-associated PAH than in IPAH. Three groups of PAH patients have been found to have an increased risk of bleeding:15 patients with connective tissue diseases such as systemic sclerosis, who often experience microvasculature lesions;25 those with low platelet counts and varices, who may experience gastrointestinal bleeding in portal PAH; and PAH patients with congenital heart disease, who have an increased risk of hemoptysis.15 In our cohort, however, the major bleeding IR for congenital heart disease was lower than that of the other etiologies. The PAH etiologies with the highest IR were portal PAH and connective tissue disease-associated PAH. Portal PAH also had the highest IR of major bleeding in non-anticoagulation patients (three events per 100 patient-years) compared with IPAH (one event per 100 patient-years) and congenital heart/connective tissue disease-associated PAH (none), similar to other publications.15,26
The most frequent major hemorrhage was gastrointestinal bleeding with an incidence of 13% in the anticoagulation group, similar to the 15% in the study by Henkes et al. Oral anticoagulants increased the odds of major gastrointestinal bleeding by nearly four times. PAH patients on anticoagulants had three times the IR of gastrointestinal bleeding than the control group. The IR of major gastrointestinal bleeding in studies comparing warfarin to direct oral anticoagulants was consistently around one event per 100 patient-years (Table 5). Data from a large population-based cohort study showed slightly higher rates,23 which may be more representative of a clinical setting without inclusion/exclusion criteria. In both situations, the IR for gastrointestinal bleeding was lower than that found in our study (4.0 events per 100 patient-years), indicating that the PAH cohort might have a higher risk of gastrointestinal bleeding than other groups. As mentioned above, there are two PAH etiologies with an increased risk of gastrointestinal bleeding, connective tissue disease-associated PAH and portal PAH, likely due to the presence of angiodysplasia and varices, respectively. Twenty-five percent of the patients with a major gastrointestinal bleeding event had CTD or portal PAH, which may have caused the higher gastrointestinal bleeding rate. However, other factors might also contribute to the overall risk of gastrointestinal bleeding. We assessed gastrointestinal-related adverse events from the use of PAH-specific pharmacotherapy as a possible factor for major gastrointestinal bleeding. However, those patients who experienced gastrointestinal-related adverse events did not suffer further major gastrointestinal bleeding.
In line with the findings of Gabriel et al., there were only a few patients using direct oral anticoagulants in our study. This was anticipated given that direct oral anticoagulants are not approved for use in PAH patients. Furthermore, recent findings from the aforementioned study, showed that PAH patients are at a higher risk of bioaccumulating these newer agents, therefore therapy should be individualized.27 While none of the seven patients taking direct oral anticoagulants had a major bleeding episode, because of the small number of patients, we cannot make any conclusions as to the safety of these medications over any other oral anticoagulant.
Complications due to infratherapeutic anticoagulation
The goal of anticoagulants in PAH patients is prolonging survival by preventing in-situ micro thrombosis in pulmonary arteries, which was observed in several autopsies of deceased IPAH patients.28 In our study, we detected a relatively high number of thrombotic events, especially in the non-anticoagulation group (9% incidence). Most of the thrombotic events reported in the anticoagulation cohort were related to underlying causes. In patients with PAH as the only anticoagulation indication using intravenous prostanoids, the thrombotic events were related to central catheter administration (line occlusion and jugular thrombosis), and in patients with atrial fibrillation the two episodes were related to atrial thrombus formation and co-existing atherosclerotic risk factors. In the control group, there were six venous thromboembolisms (mostly pulmonary embolism), an atherothrombotic stroke, and vena-cava syndrome in a patient using a central system administration device.
Venous thromboembolism was the most common thrombotic complication in the study. The annual IR of venous thromboembolism was 18 per 1000 patients for the non-anticoagulation group. Other studies reported an incidence of venous thromboembolism 1–2 per 1000 people in the US29 and 0.9 per 1000 person-years in Canada.30 These are similar to the venous thromboembolism IR for the anticoagulation group, but notably lower than that of the control group in our study. Therefore, PAH may predispose to venous thromboembolism, but age, congestive heart failure, high blood pressure, inactivity, and obesity might also play a part.31
Risk factors for bleeding
There were several factors found to increase the risk of major bleeding in the anticoagulation group.
HAS-BLED score
This risk stratification tool helps distinguish patients with a low/high risk of bleeding in atrial fibrillation.32 In our study, patients who experienced any hemorrhage had a significantly higher median risk score (3; high risk) compared with those who did not (2; moderate risk). The HAS-BLED system recommends alternatives to anticoagulation in patients with a risk score ≥3, although it has not been validated for patients with PAH. A large percentage of patients whose HAS-BLED scores reflected prior bleeding/predisposition to bleed experienced actual bleeding/hematoma during the observation period. Numerous studies have demonstrated the highly predictive association between prior bleeding (especially in the gastrointestinal tract) and future major bleeding.32–35 Therefore, we recommend that PAH patients with a history of bleeding avoid anticoagulation therapy. If therapy must be initiated, a low-intensity regimen (target INR = 1.5–2.5 or 2.0–2.5) could be suitable for patients with a high risk of bleeding. Poor anticoagulation at the beginning of the follow-up also showed a significant association with major bleeding, a finding that has been supported by several studies.13,14,36 This association was almost significant in the multivariate analysis.
Number of medications
Patients with PAH have many home medications. The median was ten, with 90% of the participants using more than five (polypharmacy). These results are in accordance with those presented in Gabriel et al.’s study with a mean of nine different drugs per patient.27 The risk of having a major bleeding event increased by 10% per medication. Increasing therapy complexity in multimorbid patients raises the risk of drug-related problems (interactions, poor adherence, etc.). High-risk conditions for drug-related problems include polypharmacy, significant changes in drug therapy or existing diseases, insufficient responses to therapy, suspected lack of therapy, side effects, and hospital discharges.37 These circumstances are common in PAH patients. To mitigate this, patients’ medication profiles should be thoroughly reviewed to optimize the treatment, find possible interactions, predict potential non-compliance, and provide patient counseling.
Limitations
The retrospective design and lack of randomization between the study and control groups might have resulted in selection biases. Despite the small sample size, the US and Spanish groups are representative of the general populations of interest. The exposure to the anticoagulant and subsequent event might have occurred before the start of the study for some patients; the initial anticoagulant effects (when INRs are more unstable) might not have been accounted for these patients. Lastly, under-reporting of minor bleeding events is common, but this is unlikely to affect major bleeding events since any such episode is likely to be reported.
Conclusions
PAH patients who were treated with anticoagulants had a higher risk of major bleeding compared to the control group and had a higher IR than populations requiring anticoagulants for other conditions. The number of venous thromboembolism episodes observed in our control cohort suggests that patients with a risk of thrombosis could benefit from these agents, but the association between PAH and venous thromboembolism is unclear. Our findings suggest that the harmful effects of anticoagulation therapy outweigh the benefits in PAH patients with a non-validated indication for anticoagulation. Therefore, anticoagulants should only be prescribed on a case-by-case basis and not systematically recommended, especially in patients with diabetes, a HAS-BLED score ≥3, prior history of bleeding, poor anticoagulation control, or polypharmacy.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by a grant from the Alfonso Martin Escudero Foundation, Madrid, Spain.
References
- 1.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Storstein O, Efskind L, Müller C, et al. Primary pulmonary hypertension with emphasis on its etiology and treatment. Acta Med Scand 1966; 179: 197–212. [DOI] [PubMed] [Google Scholar]
- 4.Fuster F, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984; 70: 580–587. [DOI] [PubMed] [Google Scholar]
- 5.Berger G, Azzam ZS, Hoffman R, et al. Coagulation and anticoagulation in pulmonary arterial hypertension. Isr Med Assoc J 2009; 11: 376–379. [PubMed] [Google Scholar]
- 6.Welsh CH, Hassell KL, Badesch DB, et al. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 1996; 110: 710–717. [DOI] [PubMed] [Google Scholar]
- 7.Preston IE, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the registry to evaluate early and long-term PAH disease management (REVEAL). Circulation 2015; 132: 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 9.Ageno W, Gallus AS, Wittkowsky A, et al. Oral Anticoagulant Therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oake N, Fergusson DA, Forster AJ, et al. Frequency of adverse events in patients with poor anticoagulation: a meta-analysis. CMAJ 2007; 176: 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henkes IR, Hazenoot T, Boonstra A, et al. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J 2013; 41: 872–878. [DOI] [PubMed] [Google Scholar]
- 12.Cantu J, Wang D, Safdar Z. Clinical implications of hemoptysis in patients with pulmonary arterial hypertension. Int J Clin Pract Suppl 2012; 177: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones M, McEwan P, Morgan CL, et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart 2005; 91: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167: 239–245. [DOI] [PubMed] [Google Scholar]
- 15.Optiz CF, Kirch W, Mueller EA, et al. Bleeding events in pulmonary arterial hypertension. Eur J Clin Invest 2009; 39: 68–73. [DOI] [PubMed] [Google Scholar]
- 16.The Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 19.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 21.Wieloch M, Själander A, Frykman V, et al. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J 2011; 32: 2282–2289. [DOI] [PubMed] [Google Scholar]
- 22.Friberg L, Rosenqvist M, Lip G. Net clinical befefit of warfarin in patients with atrial fibrillation. Circulation 2012; 125: 2298–2307. [DOI] [PubMed] [Google Scholar]
- 23.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015; 350: h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349: 631–639. [DOI] [PubMed] [Google Scholar]
- 25.Duchini A, Sessoms SL. Gastrointestinal hemorrhage in patients with sytemic sclerosis and CREST syndrome. Am J Gastroenterol 1998; 93: 1453–1456. [DOI] [PubMed] [Google Scholar]
- 26.Badesch DB, Abman SH, Ahearn GS, et al. Medical therapy for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004; 126: 35S–62S. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel L, Delavenne X, Bedouch P, et al. Risk of direct oral anticoagulant bioaccumulation in patients with pulmonary hypertension. Respiration 2016; 91: 307–315. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SR, Granton JT, Mehta S. Thrombotic arteriopathy and anticoagulation in pulmonary hypertension. Chest 2006; 130: 445–552. [DOI] [PubMed] [Google Scholar]
- 29.Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med 2010; 38: S495–501. [DOI] [PubMed] [Google Scholar]
- 30.Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE study cohort. Am J Med 2013; 126: 832.e13–21. [DOI] [PubMed] [Google Scholar]
- 31.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2017; 388(10063): 3060–3073. [DOI] [PubMed] [Google Scholar]
- 32.Pisters R, Lane DA, Niewlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 33.Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis 2013; 35: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearon C, Akl EA, Omelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149: 315–352. [DOI] [PubMed] [Google Scholar]
- 35.Veeger NJ, Piersma-Wichers M, Meijer K, et al. Minor bleeds alert for subsequent major bleeding in patients using vitamin K antagonists. BJH 2011; 153: 598–514. [DOI] [PubMed] [Google Scholar]
- 36.Veeger NJ, Piersma-Wichers M, Hillege HL, et al. Early detection of patients with a poor response to vitamin K antagonists: the clinical impact of individual time within target range in patients with heart disease. J Thromb Haemost 2006; 4: 1625–1627. [DOI] [PubMed] [Google Scholar]
- 37.Messerli M, Blozik E, Vriends N, et al. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy - a prospective randomised controlled trial. BMC Health Serv Res 2016; 16: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]