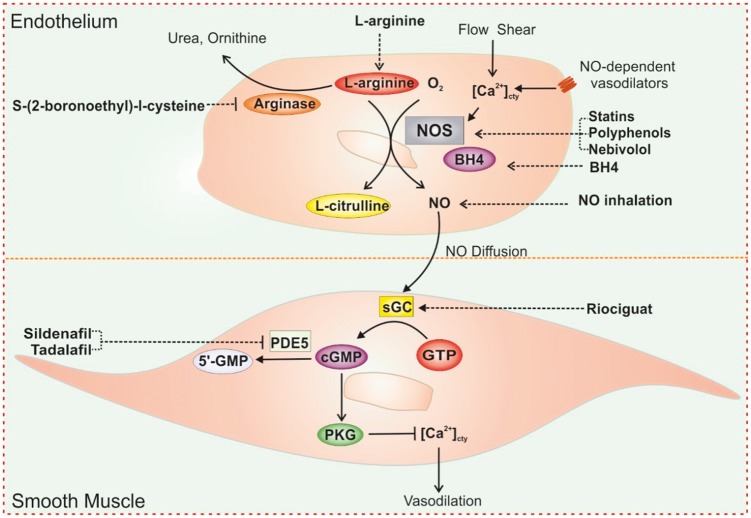
Idiopathic pulmonary arterial hypertension (IPAH) remains a devastating and deadly disease with a poor long-term prognosis. The median survival for untreated patients is only 2.8 years, with pitiful three-year, five-year, and seven-year survival rates of 68%, 57%, and 49%, respectively.1 The goal of a breakthrough discovery leading to a meaningfully life-extending treatment protocol has not yet been achieved, as the exact pathogenic mechanisms of pulmonary arterial hypertension (PAH) are too complex for current understanding. Research has, on the bright side, yielded a promising, increasingly detailed map of disease processes. Endothelium-derived nitric oxide (NO) is a major vasodilator, while its downstream effectors, cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG), have been demonstrated to cause vasodilative, anti-proliferative, anti-coagulant, and anti-inflammatory effects on pulmonary vasculature. Therefore, the L-arginine-NO-cGMP-PKG signaling cascade is an important pathway for developing therapies for PAH (Fig. 1).
Fig. 1.
Strategies for targeting L-arginine-nitric oxide signaling pathways in PH treatment. The increase of [Ca2+]cty in response to receptor-mediated agonists or increased shear stress results in activation of NOS in ECs. Activated NOS converts L-arginine in the presence of oxygen to NO and L-citrulline. These processes also require BH4. NO released from ECs diffuses into PASMCs and binds to its intracellular receptor sGC, which produces cGMP from GTP. The increase in intracellular cGMP concentration results in activation of PKG. Activation of PKG leads to the decrease of [Ca2+]cty and relaxation of smooth muscle via several mechanisms. cGMP are also determined by activity of type PDE5. There are several therapeutic strategies for targeting NO signaling in PAH including increasing NO formation or direct inhalation of NO, stimulating sGC activation, and preventing the breakdown of cGMP. Compounds, such as L-arinine, S-(2-boronoethly)-l-cysteine, L-citrulline, statins, polyphenols, Nebivolol, and BH4 can increase substrates or NOS activation leading to increase of NO formation. NO inhalation can also enhance NO signaling. Therapeutics also takes advantage of enhancing NO downstream pathways including stimulators or activators of sGC such as Riociguat and PED5 inhibitors, such as Sildenafil and Tadalafil.
L-arginine is a semi-essential basic amino acid that contains four nitrogen molecules and serves as a substrate for NO synthase (NOS). Activated NOS produces NO by converting L-arginine to L-citrulline in the presence of O2, Ca2+, heat shock protein 90 (Hsp 90), and tetrahydrobiopterin (BH4). There are three known NOS isoforms: neuronal constitutive NOS (nNOS or NOS1); inducible NOS (iNOS or NOS2); and endothelial constitutive NOS (eNOS or NOS3). NOS1/nNOS and NOS3/eNOS are constitutive NOS (cNOS). Under normal conditions, continual NO production by cNOS is Ca2+/calmodulin-dependent. An increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in endothelial cells (ECs), induced by shear force (flow-dependent NO formation) or EC membrane receptors (receptor-stimulated NO formation), can activate cNOS, increase NO synthesis and release, and result in vasodilation. The activity of iNOS, which is also expressed in ECs, is very low under normal and basal conditions, and its activation is independent of Ca2+. During inflammation, iNOS can be activated by bacterial endotoxins (e.g. lipopolysaccharide) and cytokines (e.g. tumor necrosis factor) and produce NO at about 1000-fold greater levels than that produced by cNOS. NO is a potent vasodilator and a modulator of pulmonary hemodynamics. NO rapidly diffuses from the endothelium to the vascular smooth muscle cells (SMCs). Once in the SMC, NO activates the soluble guanylate cyclase (sGC) by binding to the heme-NO/O2-binding domain on the b1 subunit of sGC. Activated sGC catalyzes guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) which is then catalyzed by phosphodiesterase 5 (PDE5) to become 5-GMP (Fig. 1). cGMP is an important intracellular second messenger that activates PKG, cGMP-gated, or cGMP/PKG-sensitive ion channels and Ca2+-activated K+ (BK) channels in SMCs. Increased cytoplasmic cGMP and activated PKG exert relaxant and anti-proliferative effects on pulmonary arterial smooth muscle cells (PASMCs) through activation of cGMP-gated K+ channels and BK channels, blockade of Ca2+-permeable channels (e.g. VDCC and TRP channels), and inhibition of specific intracellular signaling cascades that are related to cell proliferation, growth, and migration.
Upregulation and maintenance of the L-arginine-NO-cGMP signaling pathway is an attractive therapeutic strategy in PAH because of the extensive evidence that altered NO levels play a pivotal role in the pathogenesis of sustained vasoconstriction and excessive vascular remodeling. Intermittent administration of inhaled NO effectively lowers PA pressure in patients with PAH and a patient’s vasoreactive response to NO is a strong predictor of long-term survival.2,3 Augmenting the activity of sGC, a downstream target of NO signaling, also provides a reasonable strategy to ameliorate the development of pulmonary hypertension (PH). Riociguat, a sGC stimulator, is the recent drug approved by Food and Drug Administration (FDA) for the treatment of PAH and chronic thromboembolic pulmonary hypertension (CTEPH).4 Two of PDE5 inhibitors, sildenafil and tadalafil, which inhibit cGMP hydrolysis, have also been approved for the treatment of PAH.5 Another therapeutic strategy is to improve and restore the activity of endothelial NOS by increasing the availability of the substrates (L-arginine) or stabilizing NOS to improve NO production. Administration of L-arginine has been shown to restore vascular endothelial NO production and reduce pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) in animal models with experimental PH and patients with PAH.6 NOS activity is regulated by the cofactor BH4 which increases the stability of the activated NOS dimer. Oral administration of BH4 has been shown to inhibit acute hypoxic pulmonary vasoconstriction and attenuate the development of chronic hypoxia-induced PH.7
In the article published in this issue of Pulmonary Circulation, Schreiber et al. hypothesized that the combined oral administration of L-arginine and BH4 would increase enzymatic turnover of eNOS and thereby lower PAP more effectively than either substance alone.8 The results of their study showed that, while the administration of L-arginine or BH4 did have a beneficial effect on the pulmonary vasculature, there was no synergistic effect when the two substances were combined. Previous studies have taken a similar approach by combining the administration of L-arginine and Naringenin to improve NO availability and protect against monocrotalin (MCT)-induced PH in rats.9 The authors of the current study were investigating the ability of combined L-arginine and BH4 administration in the reversal of MCT-induced PH by waiting to administer the drugs until the manifestation of advanced pulmonary vascular disease. This approach better reflects the practical conditions under which PAH patients are commonly treated. Clinical symptoms develop rather late in the course of PAH, when the PVR is already markedly increased and medical therapies should be able to exert their effects despite a severely altered pulmonary endothelium. Although both L-arginine and BH4 significantly lowered PAP, the results in this study do not encourage a combined administration of L-arginine and BH4 for PAH treatment.
Overall, there is tremendous promise targeting the L-arginine-NO-cGMP-PKG pathway as well as associated molecules and processes. Many efforts have been made aiming to discover new drug and substantial progress, yet none of the current battery of drugs available in the market have provide a significant improvement in long-term patient outcomes. Therefore, there a definite need exists to expand the understanding of molecular mechanisms and signaling pathways involved in the development of PH. Collaborative efforts involving physicians, clinical investigators, pathophysiologists, and epidemiologists are also necessary to realize this promise and develop new treatment strategies.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL125208 and HL135807).
References
- 1.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Channick R, Ivy D, et al. Clinical perspectives with long-term pulsed inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Pulm Circ 2012; 2: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra R, Hess D, Lewis GD, et al. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ 2011; 1: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halank M, Hoeper MM, Ghofrani H-A, et al. Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Results from a phase II long-term extension study. Respir Med 2017; 128: 50–56. [DOI] [PubMed] [Google Scholar]
- 5.Sofer A, Ryan MJ, Tedford RJ, et al. A systematic review of transition studies of pulmonary arterial hypertension specific medications. Pulm Circ 2017; 7: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta S, Stewart DJ, Langleben D, et al. Short-term pulmonary vasodilation with l-arginine in pulmonary hypertension. Circulation 1995; 92: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 7.Francis BN, Hale A, Channon KM, et al. Effects of tetrahydrobiopterin oral treatment in hypoxia-induced pulmonary hypertension in rat. Pulm Circ 2014; 4: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber C, Eilenberg MS, Panzenboeck A, et al. Combined oral administration of L-arginine and tetrahydrobiopterin in a rat model of pulmonary arterial hypertension. Pulm Circ 2017; 7: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed LA, Obaid AAZ, Zaki HF, et al. Naringenin adds to the protective effect of l-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation and nitric oxide. Eur J Pharm Sci 2014; 62: 161–170. [DOI] [PubMed] [Google Scholar]