Abstract
Right ventricular failure (RVF) is the most important prognostic factor for both morbidity and mortality in pulmonary arterial hypertension (PAH), but also occurs in numerous other common diseases and conditions, including left ventricle dysfunction. RVF remains understudied compared with left ventricular failure (LVF). However, right and left ventricles have many differences at the morphological level or the embryologic origin, and respond differently to pressure overload. Therefore, knowledge from the left ventricle cannot be extrapolated to the right ventricle. Few studies have focused on the right ventricle and have permitted to increase our knowledge on the right ventricular-specific mechanisms driving decompensation. Here we review basic principles such as mechanisms accounting for right ventricle hypertrophy, dysfunction, and transition toward failure, with a focus on epigenetics, inflammatory, and metabolic processes.
Keywords: right ventricle hypertrophy, cytokines, mitochondrial switch
Introduction
Pulmonary hypertension (PH) is a pathophysiological disease defined by a mean pulmonary artery (PA) pressure exceeding the upper limits of normal (i.e. 25 mmHg at rest).1 Considering the diverse causes and mechanisms contributing to its pathogenesis, PH has been classified by the World Health Organization (WHO) into five categories depending on common clinical parameters, potential etiological mechanisms, as well as pathological, pathophysiological, and therapeutic characteristics.2 One of these categories corresponds to pulmonary arterial hypertension (PAH), a proliferative vascular remodeling disease that leads to progressive obliteration of the pulmonary arterial lumen, a pressure overload-driven failure of the right ventricle (RV) and premature death. PAH is characterized by the proliferation and reduced apoptosis of pulmonary artery smooth muscle cells (PASMCs), inflammation, and vasoconstriction, which lead to an increased in pulmonary vascular resistance and ultimately to right heart failure. It is now known that the most important prognostic factor for both morbidity and mortality in PAH is not the degree of PA pressure elevation, but its consequences on the RV.1,3–5
The RV is a critical component of the cardiovascular system. Impairment of its functions occurs in numerous common diseases and conditions other than PAH, including heart failure with preserved6,7 or reduced8–11 ejection fraction,12–22 valvular heart disease,23–30 chronic respiratory disease such as pulmonary fibrosis31–36 and chronic obstructive pulmonary disease (COPD),37–41 and chronic thromboembolic PH (CTEPH), where right ventricular failure (RVF) is an important and independent predictor of patients’ outcomes. In PAH, RVF is the most important prognostic factor for both morbidity and mortality1,3,4 and is the cause of death of approximately 70% of PAH patients.42 Indeed, the increase in pulmonary resistance, which is the distinctive characteristic of PAH, poorly predicts patient outcomes. Thus, the survival in PH is determined by the condition of the RV rather than the degree of the afterload increase.42–44 Unfortunately, the RV is less able to adapt to pressure overload compared to the LV. A patient with systemic hypertension can develop adaptive LV hypertrophy and live asymptomatically for decades. Conversely, a patient with increases in afterload due to PAH generally lead to RVF and die within three years from the time of diagnosis when left untreated,1 although the RV compensatory response is highly variable among individuals.45,46
Thus, RV and LV responses to pressure overload are not similar, in addition to differences that appear at the level of the morphology or the embryologic origin.47 Therefore, knowledge from the LV cannot be extrapolated to the RV.3,4 Despite that, only few studies have specifically focused on the RV without applying concepts from the LV, and it is not surprising that the concept of RV-specific therapies remains embryonic.
Here we review the basic principles of mechanisms accounting for RV hypertrophy (RVH), dysfunction, and transition toward the failure as well as potential RV-specific therapies. However, it remains to be established what are the cellular, molecular, and metabolic insights that differ between LV and RV, and what makes the RV so susceptible to fail in response to pressure overload. Therefore, it is now critical to open new avenues of investigation, to enhance our understanding of RVF, our ability to predict it, and to develop RV-specific therapies to eventually be able to reverse RVF.
Disease spectrum
The first manifestation of RVH is mainly an adaptive response, also called compensated state, as the RV is able to balance the increase in pulmonary pressure. This compensatory phase is significantly shorter in the RV compared with the LV, and explains why the mortality in PAH occurs relatively rapidly after disease onset compared with patients with systemic hypertension. As the disease progresses, the adaptive response becomes maladaptive and unable to compensate for the rise in pressure, eventually resulting in RV dilatation and RVF.3
Adaptive hypertrophy
As described by Laplace in 1709, hypertrophy is an adaptive mechanism that follows the increase of intraluminal pressure to decrease the stress applied on the wall. Thickening of the wall and reduction of the internal radius of the chamber minimize the increase in wall tension required to withstand the increase in internal pressure, favoring RV compensation. In general, however, the RV adapts better to volume rather than pressure overload and to chronic rather than acute stressors.4 Cardiac hypertrophy in response to pressure overload has been attributed to an increase of the cross-sectional area of myocytes48–51 due to the parallel addition of sarcomeres in myofibrils,52 while their length is decreased. This differs from the response to volume overload where sarcomeres are added in series, resulting in an increase in the length without changes in the cross-sectional area of the cardiomyocytes.52–55
Mechanisms of mechano-transduction are strongly implicated in pressure overload-induced hypertrophy in the RV as in the LV. The mechanical forces created by an increase in afterload are sensed by cardiac cells and converted into biochemical or electrical signals that initiate structural and functional changes in cells and tissues. Integrins and focal adhesion complexes that are linked to both the extracellular matrix (ECM) and the cytoskeleton are specialized in the detection of extracellular stress and participate actively in signal mechano-transduction in ventricular hypertrophy56–58 by interacting with many signaling molecules, such as the focal adhesion kinase59 and integrin-linked kinase60 such as Src. This results in the activation of signaling pathways that are important for cardiomyocytes growth such as STAT3, ERK, and JNK.61 Cardiomyocytes growth needs to be paralleled by ECM synthesis. The matrix scaffold of the heart is predominantly composed of collagen and small amounts of fibronectin, laminin, and elastin.3 Pressure overload has been associated with the re-expression of fibronectin48 and increased levels of β1-integrin.62
Dysfunction in contractility
The α-myosin heavy chain (α-MHC) and the β-myosin heavy chain (β-MHC) isoforms are expressed in cardiomyocytes as critical components of the fundamental contractile unit, the sarcomere. As in LV hypertrophy (LVH), a proportional decrease in α-MHC and an increase in β-MHC have been observed in adult RVH.63 In the normal adult human RV, the α-MHC isoform makes up to approximately 23–34% of the total MHC, and the β-MHC the remainder. Although the β-MHC is already the predominant isoform in human, it has been suggested that even a small shift can significantly alter cardiomyocyte power output.64 The β-MHC is actually characterized by a lower adenosine triphosphatase activity and a lower filament sliding velocity compared to the α-MHC, two properties that contribute to cardiomyocyte contractile dysfunction.63 On the other hand, the β-MHC can generate cross-bridge forces with less consumption of energy65–67 and can be an adaptive response in order to preserve energy. In the LV, miRNA-208 has been identified as one of the key regulators of this contractile protein switch,68 but it remains to be established if this is the case in the RV as well. MHC gene expression is regulated in part by the thyroid hormone signaling. Interestingly, thyroid diseases are recognized as a predisposing condition for PH.69 The Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) demonstrated that approximately 20% of patients have thyroid disease,70,71 predominantly hypothyroidism. 3,3′,5-Triiodothyronine (T3) in myocytes regulates the expression of many other proteins in addition to α- and β-MHC, such as voltage-gated potassium channels and calcium transport/regulatory proteins: actually, all of them are implicated in the regulation of myocytes’ contractility.72–75 Thus, impaired T3 signaling may be a key factor in contractile dysfunctions observed in RVF. In keeping with this, a tissue-specific regulation of T3 signaling seems to occur in RV during hypertrophy processes. The type 3 deiodinase (D3) converts T3 in inactive metabolites and reverses T3 (rT3) and 3,3′-diiodothyronine (T2) via inner-ring deiodination. It has been demonstrated that D3 is specifically induced in the RV wall during RVH in a hypoxia-inducible factor-dependent manner, reducing locally T3 content and action.76
RV respond sub-optimally
Since they undergo the same type of stress (increase in afterload), common mechanisms must occur in both ventricles during the process of compensation, such as matrix remodeling, metabolic changes, and actin cytoskeletal alterations. Nonetheless, there are processes that diverge between the RV and LV in order to explain the RV suboptimal response. Some evidence can be found by carefully looking at the embryologic origin of the two chambers, which involve different pathways and expression of different proteins.
Evidence found in the embryologic origin
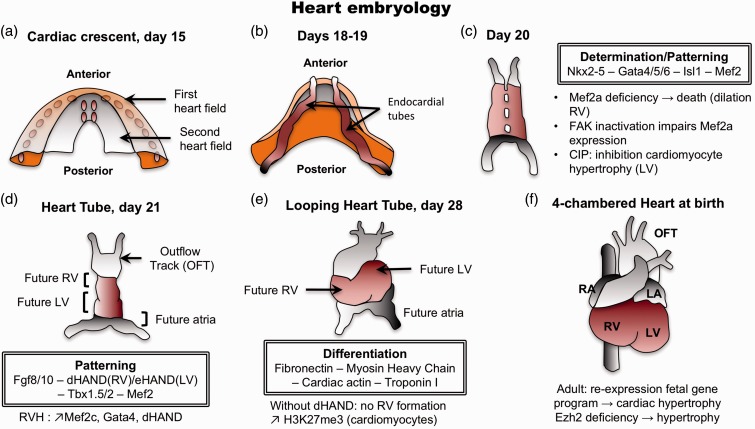
One of the featured theories enounced to answer the question of what are the mechanisms driving heart hypertrophy and failure is the possible re-expression of a fetal gene program. Contrary to the initial thoughts, the RV and LV do not originate from the same region of the primary heart field (or cardiac crescent) (Fig. 1a).77 In fact, the two ventricles are already separated in the early stages of heart development, suggesting that if a fetal program is re-expressed in the stressed RV, it should not be the same as the one stimulated in the stressed LV. Cell tracing experiments have demonstrated that RV progenitors (as well as outflow tract and septum progenitors) are located in the widest region of the cardiac crescent called the anterior heart field,78 while LV progenitors are present in the narrower region called the posterior mesoderm.79 Those progenitors migrate to generate the primitive heart tube that is patterned to form along the anterior–posterior axis, the aortic sac, the outflow tract, RV, LV, and atria. At this stage of development, the identity of heart chambers is evident morphologically (Fig. 1d), but also with the presence of different contractile properties and the expression of specific genes.80–82 For example, expression of the LIM homeodomain transcription factor islet-1 (Isl-1) is required in progenitors of the anterior heart field to give rise to the outflow tract and a majority of cells of the RV and the atria, and some cells within the LV. At the opposite, progenitors that will give rise to the majority of cells of the LV and some atrial cells do not express Isl-1.83 The role of Isl-1 in RVH/RVF has not yet been studied and may offer some important clues. The cardiac Isl-1-interacting protein (CIP) has been recently identified and characterized as a novel cardiac-specific nuclear protein that, as suggested by its nomenclature, interacts with Isl-1 and serves as a cofactor of Isl-1 to regulate its transcriptional activity. CIP has been described as repressed in LVH secondary to transverse aortic constriction in mice and, most importantly, had an inhibitory effect on cardiomyocytes hypertrophy.84 It has also been described that the MADS domain transcription factor Mef2c is required for the proper formation of the cardiac outflow tract and RV, and that Mef2c is dependent on GATA and Isl-1 direct binding.85 Interestingly, the predominant member of the MEF2 family of protein expressed in postnatal cardiac muscle is MEF2a. It has been shown that mice expressing a MEF2a transgenic deficiency had a susceptibility to die suddenly within the first week of life with pronounced dilation of the RV, myofibrillar fragmentation, mitochondrial disorganization, and activation of a fetal cardiac gene program.86 Recently, a study established that MEF2a could be regulated by FAK (focal adhesion kinase) and conditional inactivation of FAK in the costameres of the embryonic heart compromised MEF2a expression. This impaired MEF2a expression in FAK knock-out (KO) hearts led again to death in the embryonic stage for the majority of mice, and the small fraction of mice able to survive to adulthood developed spontaneous eccentric RVF.87 This suggests that FAK/MEF2a expression may be critical for the transition from RVH to RVF. Finally, Mef2 proteins appeared to be recruited by GATA4 for a synergic activation of atrial natriuretic peptide, as well as cardiac actin and MHC expressions,88 which can also contribute to the contractile dysfunction seen in RVH/RVF.
Fig. 1.
Human heart development. The determination and differentiation patterns are mentioned at each concerned stage. (a) Formation of the cardiac crescent at day 15, showing the first and second heart field (also known as anterior and posterior heart field, respectively). (b) At days 18–19, endocardial tubes appear. RV progenitors are located in the anterior heart field, whereas LV progenitors are positioned in the posterior mesoderm. (c) Fusion to form the heart tube. (d) At day 21, identification of heart future regions is morphologically possible. (e) Reorientation of heart’s anterior portion (looping) along the left/right axis of the embryo. (f) The four-chamber heart is now entirely formed. LA, left atrium; LV, left ventricle; OFT, outflow tract; RA, right atrium; RV, right ventricle.
To align the presumptive structures and form the primitive heart that presages the definitive morphology, the heart tube undergoes looping to reorient its anterior portion along the left–right axis of the embryo at day 28 (Fig. 1e). The basic helix–loop–helix transcription factors dHAND and eHAND are expressed in the right and left ventricles, respectively, in a chamber-specific manner. dHAND-null mice have been shown to fail in the formation of the RV despite a correct looping, indicating that HAND genes are involved in the development of the chambers rather than controlling the looping.89 Mef-2c, GATA-4, and dHAND expressions have been measured in the PA banding (PAB) model and found unregulated at the protein level.90 These transcription factors with a specific role in the regulation of the RV development may also have a specific role in the regulation of RV hypertrophy and failure.
Evidence found in evolution
The emergence of an air-breathing living witnessed the need to improve circulatory systems. Thus birds, mammals, and crocodilians evolved to a four-chamber heart, with complete septation into the left and right sides, allowing the separation between pulmonary and systemic circulatory systems. However, fish, amphibian, lizard, and turtle species conserved a three-chamber heart with a single ventricle. Because the LV clearly demonstrates outstanding performances such as inflow and outflow valves that are superior to those of the RV, it has been believed that the RV has been added in order to handle the pulmonary circulation only. In the last decade, the discovery of the transcription factor Isl-1 has opened a new chapter: the morphological RV as the ancient heart. Indeed, as stated below, Isl-1 in mammals is required for the fashioning of the outflow tract and RV. Intriguingly, analysis of Isl-1 homologs expression in Xenopus laevis and Drosophila melanogaster revealed the presence of a cardiac Isl-1 progenitor pool in the developing heart despite the lack of an anatomically separated RV in adults.91,92 In keeping with these observations, it has been previously described that dHAND is the unique HAND gene expressed in the zebrafish Danio renio and driving the development of a single ventricle.93 This suggests that lower vertebrates may be helpful in the study of RV hypertrophy and failure. But above all, as the RV is defined as the Isl-1-positive chamber proximal to the arterial trunk, it seems that the RV corresponds to the ancient heart, at least morphologically, and that the LV is the new evolutionary ventricle. Following this, and considering that the RV may have undergone several millions of years of refinement, it is maybe inappropriate to associate its poor performance during disease to its design.
Epigenetic mechanisms involved in RVF
Epigenetic processes are defined as changes in gene expression secondary to factors unrelated to changes in DNA sequence.94,95 Here we detail three epigenetic mechanisms that occur in RVF secondary to PH: DNA methylation; modification of histone proteins; and the role of specific non-coding RNAs, microRNAs (miRNAs).
DNA methylation
DNA methylation is defined as the addition of a methyl group to the carbon 5′ position of the nucleotide cytosine ring. Cytosine methylation in mammals is most commonly found in the context of the sequence 5′-CG-3′, which is also referred to as a CpG dinucleotide. The methylation occurs via DNA methyltransferases (DNMTs) and usually in silenced genes:96 DNMTs can both repress and activate DNA transcription.97,98 In the mammalian genome, it is estimated that 70% of all CpGs are methylated.99 Unmethylated CpG, on the other hand, are largely grouped in clusters called “CpG islands” in the 5′ regulatory region of many genes. CpG islands mark 70% of annotated mammalian promoters.100 Also, CpG methylation is essential for proper gene expression, development, and genome stability.101 Notably, differential methylation of CpG islands is part of the epigenetic variation found in humans.99,102
DNA methylation is highly dynamic during cardiomyocyte development and postnatal maturation: it is important for the perinatal switch in sarcomere protein isoforms and postnatal cardiomyocyte maturation and adaptation. A substantial number of CpGs are differentially methylated between newborn and adult cardiomyocytes.103 For example, two prototypical cardiomyocyte genes, α- and β-MHC (Myh6 and Myh7), show a CpG demethylation in cardiomyocytes purified from newborns compared with adults. On the other hand, genes that are hypermethylated in cardiomyocytes after birth are involved in myocyte contraction, cardiac morphogenesis, cell differentiation, and other processes. Postnatal de novo DNA methylation is then required for the expression of several sarcomere components and is dependent on the DNA methyltransferases 3A/B.103 Demethylated regions of adult cardiomyocytes were shown to be significantly enriched for motifs of known cardiac transcription factors, including MEF2C, GATA1-4, and others. Postnatal life is therefore accompanied by de novo methylation of fetal genes and demethylation of adult isoforms.103
Recent studies have investigated DNA methylation in human cardiac tissue biopsies from patients with chronic heart failure of different etiologies.104,105 Both studies described distinct signatures of DNA methylation in failing versus non-failing hearts. Disease-associated differentially methylated regions were adjacent to genes involved in cardiomyocyte development, cardiac morphogenesis, and energy metabolism, indicating adaptation of DNA methylation in disease-relevant regions.103 Therefore, it will be important to uncover the role of DNA methylation during RVH and RVF in the different cardiac cell types (e.g. fibroblasts, endothelial cells, immune cells) to determine their epigenetic contribution.
Modification of histone proteins
Histones are the building blocks of the nucleosomes and, as such, alter the exposure of the DNA to the transcription machinery. In addition to DNA methylation, there are histone modifications that alter the structure of chromatin and thus DNA transcription.4 The histone acetylation and deacetylation are forms of post-translational modifications that lead to increased and decreased transcription of genes, respectively. The acetylation occurs via histone acetyltransferases (HATs), whereas deacetylation is accomplished through histone deacetylases (HDACs).95,96
Several reports have addressed the effects of HDAC inhibitors in models of RV remodeling with discordant results. The valproic acid has been shown to block RV cardiac hypertrophy in response to PAB and monocrotaline (MCT)-induced PH,106 and to reduce established hypoxia-induced PH in another animal model.107 As valproic acid has many additional effects, including regulation of ion channels, glycogen synthase kinase-3β, and mitogen-activated protein (MAP) kinase, it is still unclear if the observed effects are only due to HDAC inhibition. In contrast, another study showed that trichostatin A—a potent pan-HDAC inhibitor—and valproic acid did not prevent the development of RV hypertrophy and was associated with RV dysfunction, capillary rarefaction, fibrosis, and increased cardiomyocytes death in the PAB model.108 Other HDAC inhibitors, specifically focused on class I HDACs, can modulate the hypoxia-induced cardiopulmonary remodeling via anti-proliferative mechanisms.109 The role of HDACs in RV remodeling still needs to be elucidated as previous studies primarily focused on LV remodeling.110
Ezh2 is a histone methyltransferase (HMT) that trimethylates the histone H3 at lysine 27 (H3K27me3). Ezh2 is expressed in cardiomyocytes and H3K27me3 is increased in cardiac progenitor cells during differentiation to cardiomyocytes. Mice with specific inactivation of Ezh2 by Cre-mediated recombination in the anterior heart field developed normally structured hearts that became enlarged after birth in an RV-restricted manner.111 This suggests that the presence of Ezh2 suppresses the expression of genes that promote cardiac hypertrophy. Indeed, Ezh2 has been shown to prevent cardiac hypertrophy by repressing the homeodomain transcription factor gene Six1, as genetically reduced Six1 levels rescued the pathology of Ezh2-deficient hearts. These results indicate that epigenetic mechanisms are critical for cardiac hypertrophy, but more importantly they show that embryonic epigenetic dysregulation can predispose to adult disease and maladaptive stress response. Because RV and LV have different embryonic origins, it is possible that RV possesses an embryonic epigenetic-associated predisposition that can explain its suboptimal respond to stress-induced hypertrophy.
Non-coding RNAs: microRNAs
miRNAs are short non-coding RNAs (∼22 nt) that are involved in post-transcriptional regulation of gene expression in multicellular organisms by affecting both the stability and translation of messenger RNAs (mRNAs). miRNAs are the most studied class of non-coding RNAs and control gene expression by binding to the 39-untranslated region of mRNA, which leads to either mRNA degradation or inhibition of protein translation.94,112
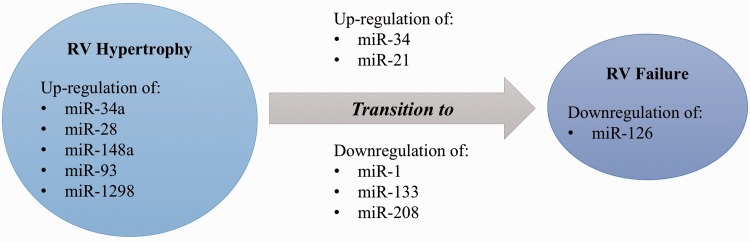
In order to define the molecular insights characterizing the transition from RVH to RVF, miRNA profile of expression has been studied in the PAB model112 in different stages, including at day 2, during the RVH stage (day 4), and when the RV failed (RVF at day 10). First of all, it has been shown that most miRNAs are similarly expressed in the RVH/RVF compared with LVH/left ventricular failure (LVF), but some miRNAs such as miR-34 a, miR-28, miR-148 a, and miR-93112 are upregulated in RVH/RVF while they have been reported downregulated in LVH/LVF.113–115 Moreover, the transition from RVH to RVF might be characterized by the expression of miR-34 (upregulated at day 10 only), miR-21 (upregulated at day 4 and to a lesser extent at day 10), and miR-1 (mostly downregulated at day 4).112 They also assessed the miRNA profile in the RVH hypoxia model compared with the RVF Sugen/Hypoxia (Su/Hx) model where they observed that miR-133 was downregulated in the Su/Hx RVF (Fig. 2).116 These two studies defined a molecular RVF program in which there is a loss of genes promoting cell growth and cell cycle (insulin-like growth factor-1, Akt, Kuppler-like factor-5, Ccne1, cdc25A), impairment of angiogenesis (vascular endothelial growth factor [VEGF] and Akt), and expression of genes encoding glycolytic enzymes (hexokinase-1, phosphofructokinase, uncoupling protein 2)112,116 (Table 1).
Fig. 2.
Differential miRNA expression between right ventricular hypertrophy and right ventricular failure.
Table 1.
Genes characterizing right ventricular failure.
| Promotion of cell growth and cell cycle | |
| Insulin-like growth factor 1 | Downregulated compared with RVH |
| Akt | Downregulated compared with RVH |
| Kuppler-like factor-5 | Downregulated compared with RVH |
| Ccne1 | Downregulated compared with RVH |
| cdc25A | Downregulated compared with RVH |
| Impairment of angiogenesis | |
| Vascular endothelial growth factor (VEGF) | Downregulated compared with RVH |
| Enhancement of glycolytic enzymes | |
| Hexokinase-1 (HK1) | Upregulated compared with RVH |
| Phosphofructokinase (PFKM) | Upregulated compared with RVH |
| Uncoupling protein 2 (UCP2) | Upregulated compared with RVH |
Later, the miRNA expression profile between RVH and RVF has been studied in the MCT model. In this model, RVF was defined as a state of ongoing RVH in which the RV systolic pressure decreases because of contractile failure (while the PA pressure remains elevated): the RV end-diastolic pressure increases; the cardiac output decreases; clinical evidence of heart failure (ascites, decreased appetite, and activity, > 20% weight loss) develops; and death occurs. In this model, Mef2c protein expression was sharply increased in late RVH stages, contributing to the adaptive nature of this stage as for the physiological RVH of the fetal circulation in utero, but was lost again at the RVF stage.117 In contrast to what is known in LVH, levels of miR-208 (a myocardium-specific miRNA) were continuously decreasing as RVH progressed toward RVF117 (Fig. 3). The subunit MED13 of the complex mediator of transcription has been identified as the miR-208 direct target gene in the heart.68 The decreased expression of miR-208 was therefore associated with an increase in MED13 expression as well as an increased activity of its partner NCoR1, resulting in hypoacetylation of histones and a chromatin structure resistant to the transcription, allowing the repression of Mef2117 and the exit from a fetal-like compensatory phase in RVF. In this model, as in human tissues, it has also been shown that the transition from RVH to RVF is associated with a decreased in miR-126 expression. MiR-126 downregulation was associated with increased SPRED-1, leading to decreased activation of RAF (P-RAF/RAF) and MAP kinase (P-MAP/MAP), thus inhibiting the VEGF pathway and capillary density.118 During RVF, miR-126 has been specified to contribute to a decrease in the RV vascular density, promoting the progression towards RVF.
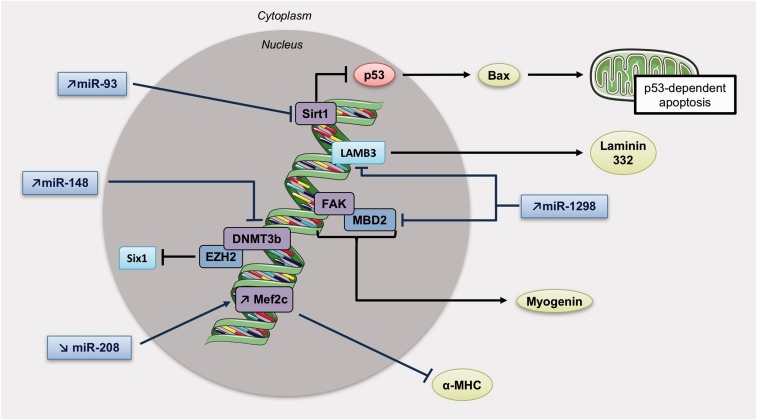
Fig. 3.
MiRNAs and methylation patterns involved in right ventricular hypertrophy. Increase in miR-93 inhibits Sirt1 activity, which usually repress p53 known to induce apoptosis through the mitochondria. Mir-148 upregulation obstructs DNMT3b activity, and miR-1298 can both inhibit FAK and LAMB3 (Laminin subunit beta 3). Also, a diminution of miR-208 is responsible of Mef2c upregulation and inhibition of α-MHC expression. DNMT3b, DNA Methyltransferase 3 Beta; LAMB3, Laminin subunit beta 3; Sirt1, Sirtuin 1.
Finally, a recent study revealed that miR-1298 is increased in RVH but not in LVH. miR-1298 inhibits connexin-43 expression,112 a gap junction protein required for maintenance of the normal cardiac rhythm, regulation of vascular tone, and endothelial function,119 suggesting that miR-1298-mediated decrease of connexin-43 could be one of the causes of arrhythmia and impaired coronary artery function in the right heart of PH rats.120
Inflammation in RVF
Inflammation is a complex set of interactions among soluble factors and cells that can arise in any tissue in response to traumatic, infectious, post-ischemic, toxic, or auto-immune injury. Inflammation can lead to persistent tissue damage by leucocytes, lymphocytes, or collagen.1 Moreover, inflammation plays a significant role in the pathogenesis of PH. However, its role in RVH and RVF is still not well understood. RVF is less prevalent and occurs later in those with congenital heart disease (i.e. Eisenmenger syndrome)121 and idiopathic PAH122,123 versus in those with scleroderma-associated PAH.121–124 suggesting that RVF is predominant in patients having an inflammatory burden. Overbeek et al.125 confirmed that RV from scleroderma-associated PAH (n = 5) had more neutrophilic granulocytes, macrophages, and lymphocytes than in idiopathic PAH (n = 9) or controls (n = 4), whereas RV interstitial fibrosis was similar in all groups. Nonetheless, the participation of inflammation to RVF is also suspected in all types of PAH. Cardiomyocytes are believed to produce a trigger for autocrine, paracrine, and neuroendocrine signaling pathways leading to a vicious circle of RV inflammation and ischemia, leading to cardiomyocytes apoptosis and RVF.3
Role of cytokines
Activation of cytokines may play an important role in patients with RVF. In patients with selected forms of congenital heart disease and RVF, elevated levels of tumor necrosis factor (TNF) and endotoxin were associated with more symptomatic disease.126 Levels of pro-inflammatory cytokines like interleukin (IL)-6 and IL-1β, which production is mediated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB),127 are linked to the severity of heart failure.128,129 In the pressure-overloaded RV, several chemokines are upregulated (CXCL10, CXCL6, CX3CL1, CCL5, CXCL16, and CCL2130) and stimulate the expression of small leucine rich-proteoglycans (SLRP), which are recognized to act as signaling molecules and directly regulate inflammatory processes.131,132
Tumor necrosis factor-alpha
Tumor necrosis factor-alpha (TNF-α) is a polypeptide hormone produced by activated monocytes/macrophages and an effector molecule in various inflammatory processes. Growing evidence has implicated TNF-α in the pathogenesis of heart failure: the healthy heart does not produce TNF-α, but the insufficient myocardium does.133 Experimentally, it has been demonstrated that transgenic mice that chronically overexpress myocardial TNF-α develop cardiac hypertrophy, fibrosis, both left and right dilated myocardiopathy, and premature death.134 Also, TNF-α depletion in a banding pressure-overload model improves cardiac hypertrophy and remodeling.135 TNF-α involvement in the pathogenesis of heart failure has been also suggested by demonstrating a correlation between patients’functional class and circulating TNF-α levels.136 TNF-α binds to TNF receptors type 1 (TNF-R1) and 2 (TNF-R2) to exert its biological functions. These receptors are significantly increased in relation to the severity of heart failure,137 as observed in 1200 patients randomized in the VEST study, in which the levels of TNF-α-R1s and TNF-α-R2s correlated with functional classes and were significant independent predictors of mortality.138
Similarly, a strong correlation has been established between the level of TNF-α mRNA or protein in the donor heart and the development of RVF after transplantation, which is an important problem after heart transplantation.139 The mechanisms behind the detrimental effect of TNF-α on the RV remain poorly understood. TNF-α seems to depress myocardial contractile function through uncoupling β-adrenergic signaling137 responsible for increasing cardiac nitric oxide and peroxinitrite140,141 and altering intracellular calcium homoeostasis.142 In vitro, stimulation of adult human cardiomyocytes by TNF-α provoked a hypertrophic growth response.143 It could also impair the coupling of the components of the beta-receptor-G protein system, thus leading to a negative ionotropic effect.144 It can also enhance nitric oxide (NO) production, increasing ionotropy.141 Also, we recently demonstrated that TNF-α may act as a second trigger along with the decrease in miR-208 to enhance MED13/NCoR1 upregulation and Mef2 suppression.117 Therefore, the role of TNF-α in heart failure appears to be important, although it still needs to be investigated.
Interleukin-6
IL-6 is known to regulate innate and acquired immune responses145 and is mainly produced by macrophages.146 This cytokine has pleiotropic effects, such as stimulation of T- and B-cell differentiation and macrophage activation,147 protection against cardiac ischemia-reperfusion injury,148 and regulation of cardiac lipid metabolism through its effect on peroxisome proliferator-activated receptors (PPARs).149 More specifically in the heart, IL-6 mediates fibrosis and cardiac hypertrophy, and promotes diastolic dysfunction.150 IL-6 plasmatic levels also correlate with systolic function151 and mortality152 in heart failure with reduced ejection fraction. In vitro, pretreatment with IL-6 activates the phosphatidylinositol-3-kinase (PI3K)/Akt pathway and induces activity of inducible NO synthase (iNOS), which results in protection of cardiomyocytes. In pressure-overload LVH, genetic deletion of IL-6 attenuates Ca2+-calmodulin protein kinase II (CaMKII)-dependent activation of STAT3, which then attenuates hypertrophy.151
Interleukin-1
IL-1 is a pro-inflammatory cytokine also implicated in heart failure that contains two active ligands, IL-1α and IL-1β.153 This cytokine can be produced by cardiomyocytes themselves in response to injury137 and its plasmatic levels are elevated in chronic heart failure.154 IL-1β induces intracellular reactive oxygen species (ROS) production and modifies L-type Ca2+ current in cardiomyocytes,155 but also decreases collagen synthesis in cardiac fibroblasts in vitro.156
Toll-like receptors
Toll-like receptors (TLRs) are an essential family of pattern recognition receptors (PRRs) that triggers innate immune responses.157 This family comprises ten members in humans (TLR1–TLR10) and each member recognizes a specific pathogen-associated molecular pattern (PAMP).158 Following the recognition of PAMPs, TLRs recruit Toll/IL-1 receptor (TIR) domain-containing adaptor proteins such as TRIF and MyD88, which initiate signal transduction pathways that end in the activation of NF-κB, interferon regulatory factors (IRFs), or MAP kinases. This activation aims to regulate the expression of chemokines, cytokines, and type I interferons (IFNs) that protect the host from microbial infection.159 Then, there are two TLRs-signaling pathways: TRIF-dependent158 and MyD88-dependent pathways.159
TLR2 and TLR4 are mostly expressed in cardiomyocytes,160 with TLR4 being upregulated in the failing human heart161,162 and TLR2 playing a role in the cardiac adaptive response to pressure overload.163 TLR4 acts as a signal-transducing receptor for lipopolysaccharide, while TLR2 distinguishes gram-positive bacterial lipoproteins and peptidoglycans.164 Both TLR2 and TLR4 can activate NF-κB in order to recruit inflammatory cytokines in the myocardial tissue.165 In addition, Boyd et al.166 demonstrated that TLR3, TLR5, TLR7, and TLR9 are constitutively expressed in cardiomyocyte cell lines and murine heart tissue. Interestingly, TLR9 has been associated with cardiac inflammation and heart failure.167,168 A recent experimental study of LVF in mice generated a novel concept of a TLR9-dependent failure component.169 In this model of chronic severe LV pressure overload, mitochondrial DNA from damaged heart cells generated a cardiac inflammatory response via TLR9, resulting in myocarditis and dilated cardiomyopathy.169
Animal studies
Maybe one of the best models to investigate inflammation in RVF is the PAB model. PAB is usually performed by placing a suture, clip, or inflatable ring around the main pulmonary trunk at proximity from the RV, thus representing a “pressure overload model.” This model preserves the pulmonary vasculature so that RV remodeling, and its potential for reversal, is independent from the changes in pulmonary vasculature.170 The banding can go to 21 days and RVH becomes maximal at 14 days.171–173 This model causes RVH and depression of myocardial contractility.171 Dewatche et al.174 observed that persistent RVF following PAB was associated with increased myocardial expression of IL-1β, IL-6, monocyte chemoattractant protein 1, pro-inflammatory IL-6/IL-10, concomitantly with neutrophil and macrophage infiltration; expression of IL-33 decreased, whereas macrophage inflammatory protein-1α expression remained unchanged. Thereby, they concluded that an acute afterload-induced RVF is associated with the activation of inflammatory processes.174 It has also been shown in mice that levels of IL-1, IL-6, granulocyte colony-stimulating factor (G-CSF), and monocytes induced by gamma interferon (MIG) were increased after PAB.175
Metabolic changes occurring during RVH and RVF
Switch toward glycolysis
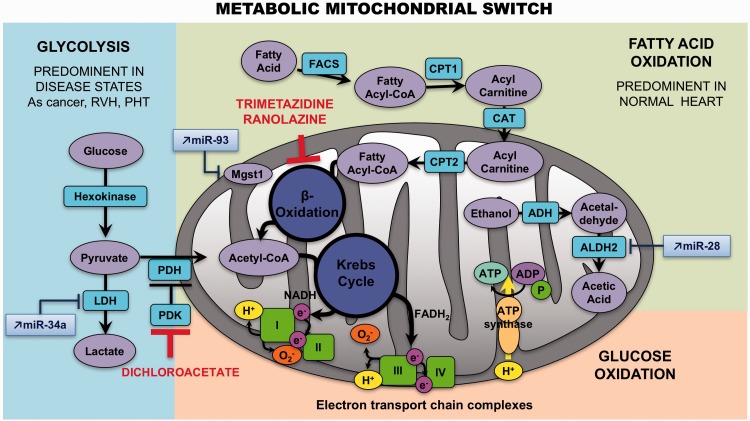
Fatty acid oxidation (FAO) is the major source of ATP production (60–90%) in a normal adult heart, whereas glycolysis (GLY) or glucose oxidation (GO) are considered a secondary source, accounting for 10–40% of energy production.176 Changes in cardiac metabolism have long been recognized as problematic in chronic LVF.177,178 Akin to LVF, it has been postulated that RVH is characterized by abnormal energy metabolism and a certain degree of metabolic remodeling.179,180 Studies in Su/Hx and MCT rat models have demonstrated that RVH exhibits an increased expression of glycolysis-related genes116 and increased enzymatic glycolysis rate,181 suggesting that the primary energy source becomes GLY (which takes place in the cytoplasm) as opposed to the FAO (which takes place in the mitochondria) (Fig. 4).182 RV mitochondria become more hyperpolarized.183 There is also evidence of decreased ROS production and activation of nuclear factor of activated T-cells (NFAT) in cardiomyocytes from compensated RV. In addition, this remodeling appears to promote hypoxia inducible factor (HIF)-1α activation and to stimulate angiogenesis, since increased blood/oxygen supply is needed to support the growing myocardial mass.
Fig. 4.
Metabolic mitochondrial switch. In right ventricular hypertrophy, the primary energy source becomes glycolysis, as opposed to the fatty acid oxidation. Some miRNAs interact with metabolism enzymes: an increase of miR-34 a can repress the activity of the LDH, miR-93 can block Mgst1 activity, and miR-28 prevent mitochondrial aldehyde dehydrogenase activity. ALDH2, mitochondrial aldehyde dehydrogenase; LDH, lactate dehydrogenase; Mgst1, microsomal glutathione S-transferase 1.
Positron emission tomography (PET) studies have shown increased accumulation of the radioisotope-labeled glucose analogue 18F-2-deoxy-2-fluoro-D-glucose (18-FDG) in the RV of PAH patients,184–186 confirming the results found in animal models. Glucose transporters (GLUTs) authorize the entrance of 18F-FDG, which will be phosphorylated by hexokinase (HK) to 18F-FDG-6-P. Then, in the cell, it is impossible for the 18F-FDG-6-P to be metabolized via the glycolytic pathway, and because of its polar nature, becomes confined. Since this metabolic remodeling can be detected and quantified in vivo by FDG-PET and is restricted to the RV in PAH, RV-specific metabolic targeting therapies might be feasible. However, 18-FDG uptake studies have multiple limitations and the physiological interpretation is not straightforward (i.e. 18-FDG uptake studies do not directly measure glycolysis). RV oxygen consumption can also be measured by [11C]-acetate PET scanning.187 However, such studies are limited to a few specialized centers and are difficult to perform.185
Recent evidence suggests that this potentially adaptive switch appears to be reversed in RVF, proposing that the mitochondrial suppression was a selective and reversible event in RVH. The increase in glucose uptake (measured by PET imaging) and the activation of HIF-1α as well as the mitochondrial hyperpolarization are lost at this point.188 The trigger for the loss of this adoptive molecular program is unknown but it seems associated with the loss of the mitochondrial remodeling. The metabolic regulator dichloroacetate (DCA) has been shown to enhance oxidative phosphorylation through inhibition of the mitochondrial pyruvate dehydrogenase kinase,189 restoring electrical remodeling in RVH181 and K+ channel expression/function.190
On the other hand, little is known about the role of FAO in RVH and RVF. Multiple studies have shown that the rate of FAO is preserved or increased in LVH, and that it decreases only during the progression toward failure.191 Similar results have been reported in rats with PAB-induced RVH, which exhibit high rates of FAO.192 Due to the reciprocal relationship between FAO and GO, called the Randle cycle, inhibiting FAO might increase GO193 and enhance metabolic efficiency (FAO uses 12% more oxygen than GO to generate the same amount of ATP).194 Partial inhibitors of FAO (pFOXi) decreased FAO and restored pyruvate dehydrogenase (PDH) activity and GO in PAB, thereby increasing ATP levels. Fang et al.192 demonstrated that pFOXi such as trimetazidine and ranolazine restore PDH activity and GO in PAB, thereby increasing ATP levels, but also increase cardiac output and exercise capacity. Chronic administration of ranolazine reduces RVH and RV collagen deposition in MCT-induced PH and also improves PH.195 Besides, it diminishes levels of B-type natriuretic peptide and prevents ventricular arrhythmias.195 Trimetazidine inhibits apoptosis by increasing miR-21 expression in cardiomyocytes during hypoxia-reperfusion injury196 and reduces cardiac fibrosis by diminishing collagen accumulation and ROS production.197
Finally, glutaminolysis has been shown to be amplified in MCT induced-RVH. The use of a glutamine antagonist (6-diazo-5-oxo-l-norleucine or DON)198 caused an increase in GO in this model, restored PDH activity, and reduced RVH. Coherent with the increased glutaminolysis, expressions of glutamine transporters (SLC1A5 and SLC7A5) and mitochondrial malic enzyme were improved in the RV.198
Alterations in mitochondrial structure and dynamics
There is evidence to suggest important alterations of mitochondrial structure and dynamics during RVH and RVF. First, expressions of critical transcription factors involved in the regulation of mitochondrial biogenesis, including the mitochondrial transcription factor A (TFAm) and the PPAR-γ coactivator-1α (PGC-1α), have been described significantly downregulated in RVF.199 In accordance, RVF has also been described as exhibiting a net loss in the mitochondrial number per gram of RV tissue,200 the remaining mitochondria having an abnormal ultrastructure on electron microscopy.200 Another study showed in a PH animal model that the hypertrophied RV presents a diminution in mRNA expression of mitochondrial markers like sirtuin1 (SIRT1), PGC-1α, nuclear respiratory factor 1 (NRF1), and citrate-synthase.199
It has also been exposed that the dysregulation of mitochondrial dynamics occurs in RVH.
Increased levels of mitophagy have been suggested in PAB-induced RVH with enhanced expression of autophagy/mitophagy marker Light Chain 3 (LC3)A/B and p62,201 whereas mitochondria biogenesis may be impaired because of the decreased expression of PGC-1α.199
Angiogenesis
The growing RV needs to be neo-vascularized in order to be adequately supplied and nourished, and it seems to be the case early during the progression of the disease. However, there is some evidence that the hypertrophied RV becomes relatively ischemic,202 not because of coronary disease, but potentially because of suppressed angiogenesis. Also, it has been described that while exposed to similar degrees of afterload,3 the RV has less susceptibility to fail in the PAB rat model3,203,204 compared with PH rat models (MCT or Sugen). This increased susceptibility to failure has been associated to a dysfunctional angiogenic signaling.3 This raises the intriguing hypothesis that the trigger for RVF may be an inability to sustain angiogenesis as the myocardial mass increases. Because angiogenesis is regulated by variations in O2 tension and metabolic factors,205 the use of metabolic modulators for the treatment of RVF in PAH is of potential interest. However, their efficiency could be limited by underlying mitochondrial abnormalities.
Conclusion
RVF is now a priority for preclinical and translational research in PAH, as well as numerous conditions for which RV function independently predicts morbidity and mortality. It is critical to improve our knowledge on the mechanisms underlying the transition from compensated RVH to maladaptive RVF in order to improve our ability to predict it and develop RV-specific therapies. The pathophysiology and pathobiology of RVF has to be approached in a comprehensive and specific manner in which its embryonic origin, its response to overload, its plasticity, and its relation with the pulmonary circulation are analyzed, avoiding systematic extrapolation of findings from the LV.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009; 119(16): 2250–2294. [DOI] [PubMed] [Google Scholar]
- 2.Pugliese SC, Poth JM, Fini MA, et al. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 2015; 308(3): L229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogaard HJ, Abe K, Vonk Noordegraaf A, et al. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009; 135(3): 794–804. [DOI] [PubMed] [Google Scholar]
- 4.Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008; 117(13): 1717–1731. [DOI] [PubMed] [Google Scholar]
- 5.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62(25 Suppl): D22–33. [DOI] [PubMed] [Google Scholar]
- 6.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009; 53(13): 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol 2007; 99(8): 1146–1150. [DOI] [PubMed] [Google Scholar]
- 8.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 2010; 121(2): 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaergaard J, Akkan D, Iversen KK, et al. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail 2007; 9(6–7): 610–616. [DOI] [PubMed] [Google Scholar]
- 10.Damy T, Goode KM, Kallvikbacka-Bennett A, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J 2010; 31(18): 2280–2290. [DOI] [PubMed] [Google Scholar]
- 11.Cappola TP, Felker GM, Kao WH, et al. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation 2002; 105(14): 1663–1668. [DOI] [PubMed] [Google Scholar]
- 12.Davlouros PA, Niwa K, Webb G, et al. The right ventricle in congenital heart disease. Heart 2006; 92(Suppl. 1): i27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Salvo TG, Mathier M, Semigran MJ, et al. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 1995; 25(5): 1143–1153. [DOI] [PubMed] [Google Scholar]
- 14.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37(1): 183–188. [DOI] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353(9162): 1386–1389. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SR, Eikelboom JW, Natarajan MK, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol 2001; 37(1): 37–43. [DOI] [PubMed] [Google Scholar]
- 17.Polak JF, Holman BL, Wynne J, et al. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol 1983; 2(2): 217–224. [DOI] [PubMed] [Google Scholar]
- 18.Gavazzi A, Berzuini C, Campana C, et al. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant 1997; 16(7): 774–785. [PubMed] [Google Scholar]
- 19.Mendes LA, Dec GW, Picard MH, et al. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J 1994; 128(2): 301–307. [DOI] [PubMed] [Google Scholar]
- 20.Meluzin J, Spinarova L, Hude P, et al. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr 2005; 18(5): 435–444. [DOI] [PubMed] [Google Scholar]
- 21.Juilliere Y, Barbier G, Feldmann L, et al. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J 1997; 18(2): 276–280. [DOI] [PubMed] [Google Scholar]
- 22.de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol 1998; 32(4): 948–954. [DOI] [PubMed] [Google Scholar]
- 23.Salomon NW, Stinson EB, Griepp RB, et al. Mitral valve replacement: long-term evaluation of prosthesis-related mortality and morbidity. Circulation 1977; 56(3 Suppl): II94–101. [PubMed] [Google Scholar]
- 24.Crawford MH, Souchek J, Oprian CA, Miller DC, Rahimtoola S, Giacomini JC, et al. Determinants of survival and left ventricular performance after mitral valve replacement. Department of Veterans Affairs Cooperative Study on Valvular Heart Disease. Circulation 1990; 81(4): 1173–81. [DOI] [PubMed] [Google Scholar]
- 25.Leavitt JI, Coats MH, Falk RH. Effects of exercise on transmitral gradient and pulmonary artery pressure in patients with mitral stenosis or a prosthetic mitral valve: a Doppler echocardiographic study. J Am Coll Cardiol 1991; 17(7): 1520–1526. [DOI] [PubMed] [Google Scholar]
- 26.Zielinski T, Pogorzelska H, Rajecka A, et al. Pulmonary hemodynamics at rest and effort, 6 and 12 months after mitral valve replacement: a slow regression of effort pulmonary hypertension. Int J Cardiol 1993; 42(1): 57–62. [DOI] [PubMed] [Google Scholar]
- 27.Tunick PA, Freedberg RS, Gargiulo A, et al. Exercise Doppler echocardiography as an aid to clinical decision making in mitral valve disease. J Am Soc Echocardiogr 1992; 5(3): 225–230. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J 2011; 32(6): 751–759. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Davidson WR, Jr, Chambers CE, et al. Preoperative pulmonary hypertension is associated with postoperative left ventricular dysfunction in chronic organic mitral regurgitation: an echocardiographic and hemodynamic study. J Am Soc Echocardiogr 2006; 19(8): 1051–1055. [DOI] [PubMed] [Google Scholar]
- 30.Fawzy ME, Hassan W, Stefadouros M, et al. Prevalence and fate of severe pulmonary hypertension in 559 consecutive patients with severe rheumatic mitral stenosis undergoing mitral balloon valvotomy. J Heart Valve Dis 2004; 13(6): 942–947. discussion 7–8. [PubMed] [Google Scholar]
- 31.Hamada K, Nagai S, Tanaka S, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007; 131(3): 650–656. [DOI] [PubMed] [Google Scholar]
- 32.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005; 128(4): 2393–2399. [DOI] [PubMed] [Google Scholar]
- 33.Nathan SD, Shlobin OA, Ahmad S, et al. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest 2007; 131(3): 657–663. [DOI] [PubMed] [Google Scholar]
- 34.Lettieri CJ, Nathan SD, Barnett SD, et al. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129(3): 746–752. [DOI] [PubMed] [Google Scholar]
- 35.Whelan TP, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant 2005; 24(9): 1269–1274. [DOI] [PubMed] [Google Scholar]
- 36.Lederer DJ, Arcasoy SM, Wilt JS, et al. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174(6): 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaouat A, Kraemer JP, Canuet M, et al. [Pulmonary hypertension associated with disorders of the respiratory system]. Presse Med 2005; 34(19 Pt 2): 1465–1474. [DOI] [PubMed] [Google Scholar]
- 38.Weitzenblum E. Prognosis of pulmonary hypertension in chronic obstructive pulmonary disease. Cor Vasa 1980; 22(6): 418–427. [PubMed] [Google Scholar]
- 39.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107(5): 1193–1198. [DOI] [PubMed] [Google Scholar]
- 40.Kessler R, Oswald-Mammosser M. Does lung volume reduction surgery compromise the pulmonary circulation? Am J Respir Crit Care Med 1999; 160(4): 1429–1430. [DOI] [PubMed] [Google Scholar]
- 41.Leuchte HH, Baumgartner RA, Nounou ME, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med 2006; 173(7): 744–750. [DOI] [PubMed] [Google Scholar]
- 42.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115(5): 343–349. [DOI] [PubMed] [Google Scholar]
- 43.Sandoval J, Bauerle O, Palomar A, et al. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 1994; 89(4): 1733–1744. [DOI] [PubMed] [Google Scholar]
- 44.Mauritz GJ, Kind T, Marcus JT, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012; 141(4): 935–943. [DOI] [PubMed] [Google Scholar]
- 45.Wright LM, Dwyer N, Celermajer D. Follow-up of pulmonary hypertension with echocardiography. JACC Cardiovasc Imaging 2016; 9(6): 733–746. [DOI] [PubMed] [Google Scholar]
- 46.Maron BA, Ryan JJ. Treatment differences in pulmonary arterial hypertension management. Pulm Circ 2016; 6(4): 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zungu-Edmondson M, Suzuki YJ. Differential stress response mechanisms in right and left ventricles. J Rare Dis Res Treat 2016; 1(2): 39–45. [PMC free article] [PubMed] [Google Scholar]
- 48.Olivetti G, Ricci R, Lagrasta C, et al. Cellular basis of wall remodeling in long-term pressure overload-induced right ventricular hypertrophy in rats. Circ Res 1988; 63(3): 648–657. [DOI] [PubMed] [Google Scholar]
- 49.Smith SH, Bishop SP. Regional myocyte size in compensated right ventricular hypertrophy in the ferret. J Mol Cell Cardiol 1985; 17(10): 1005–1011. [DOI] [PubMed] [Google Scholar]
- 50.Werchan PM, Summer WR, Gerdes AM, et al. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am J Physiol 1989; 256(5 Pt 2): H1328–1336. [DOI] [PubMed] [Google Scholar]
- 51.Zierhut W, Zimmer HG, Gerdes AM. Effect of angiotensin converting enzyme inhibition on pressure-induced left ventricular hypertrophy in rats. Circ Res 1991; 69(3): 609–617. [DOI] [PubMed] [Google Scholar]
- 52.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56(1): 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capasso JM, Fitzpatrick D, Anversa P. Cellular mechanisms of ventricular failure: myocyte kinetics and geometry with age. Am J Physiol 1992; 262(6 Pt 2): H1770–1781. [DOI] [PubMed] [Google Scholar]
- 54.Beltrami CA, Finato N, Rocco M, et al. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol 1995; 27(1): 291–305. [DOI] [PubMed] [Google Scholar]
- 55.Gerdes AM, Kellerman SE, Moore JA, et al. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation 1992; 86(2): 426–430. [DOI] [PubMed] [Google Scholar]
- 56.Mann DL. Basic mechanisms of left ventricular remodeling: the contribution of wall stress. J Card Fail 2004; 10(6 Suppl): S202–206. [DOI] [PubMed] [Google Scholar]
- 57.Katsumi A, Orr AW, Tzima E, et al. Integrins in mechanotransduction. J Biol Chem 2004; 279(13): 12001–12004. [DOI] [PubMed] [Google Scholar]
- 58.Ross RS, Pham C, Shai SY, et al. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res 1998; 82(11): 1160–1172. [DOI] [PubMed] [Google Scholar]
- 59.Torsoni AS, Constancio SS, Nadruz W, Jr, et al. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res 2003; 93(2): 140–147. [DOI] [PubMed] [Google Scholar]
- 60.Bendig G, Grimmler M, Huttner IG, et al. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev 2006; 20(17): 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willey CD, Palanisamy AP, Johnston RK, et al. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci 2008; 4(3): 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babbitt CJ, Shai SY, Harpf AE, et al. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol 2002; 118(6): 431–439. [DOI] [PubMed] [Google Scholar]
- 63.Lowes BD, Minobe W, Abraham WT, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 1997; 100(9): 2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 2002; 90(11): 1150–1152. [DOI] [PubMed] [Google Scholar]
- 65.Harris DE, Work SS, Wright RK, et al. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. J Muscle Res Cell Motil 1994; 15(1): 11–19. [DOI] [PubMed] [Google Scholar]
- 66.Holubarsch C, Goulette RP, Litten RZ, et al. The economy of isometric force development, myosin isoenzyme pattern and myofibrillar ATPase activity in normal and hypothyroid rat myocardium. Circ Res 1985; 56(1): 78–86. [DOI] [PubMed] [Google Scholar]
- 67.Sugiura S, Kobayakawa N, Fujita H, et al. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circ Res 1998; 82(10): 1029–1034. [DOI] [PubMed] [Google Scholar]
- 68.van Rooij E, Sutherland LB, Qi X, Richardson JA, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007; 316(5824): 575–579. [DOI] [PubMed] [Google Scholar]
- 69.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D34–41. [DOI] [PubMed] [Google Scholar]
- 70.Vallabhajosula S, Radhi S, Cevik C, et al. Hyperthyroidism and pulmonary hypertension: an important association. Am J Med Sci 2011; 342(6): 507–512. [DOI] [PubMed] [Google Scholar]
- 71.Boerrigter G, Burnett JC., Jr Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease. Cardiovasc Drug Rev 2007; 25(1): 30–45. [DOI] [PubMed] [Google Scholar]
- 72.Graettinger JS, Muenster JJ, Selverstone LA, et al. A correlation of clinical and hemodynamic studies in patients with hyperthyroidism with and without congestive heart failure. J Clin Invest 1959; 38(8): 1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman T, Borow KM, Sarne DH, et al. Myocardial mechanics in hyperthyroidism: importance of left ventricular loading conditions, heart rate and contractile state. J Am Coll Cardiol 1986; 7(5): 967–974. [DOI] [PubMed] [Google Scholar]
- 74.Klein IO, Ojamaa K. The cardiovascular system in hypothyroidism. In: Braverman LE, Utiger RD. (eds). Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text, 8th ed Philadelphia, PA: Lippincott Williams & Wilkins, 2000, pp. 777–782. [Google Scholar]
- 75.Klein I, Ojamaa K. Thyrotoxicosis and the heart. Endocrinol Metab Clin North Am 1998; 27(1): 51–62. [DOI] [PubMed] [Google Scholar]
- 76.Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 2008; 118(3): 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viragh S, Challice CE. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res 1973; 42(1): 1–24. [DOI] [PubMed] [Google Scholar]
- 78.Verzi MP, McCulley DJ, De Val S, et al. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 2005; 287(1): 134–145. [DOI] [PubMed] [Google Scholar]
- 79.Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007; 232(7): 866–880. [PubMed] [Google Scholar]
- 80.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature 2000; 407(6801): 221–226. [DOI] [PubMed] [Google Scholar]
- 81.Christoffels VM, Habets PE, Franco D, et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol 2000; 223(2): 266–278. [DOI] [PubMed] [Google Scholar]
- 82.Zaffran S, Kelly RG, Meilhac SM, et al. Right ventricular myocardium derives from the anterior heart field. Circ Res 2004; 95(3): 261–268. [DOI] [PubMed] [Google Scholar]
- 83.Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 2003; 5(6): 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang ZP, Young Seok H, Zhou B, et al. CIP, a cardiac Isl1-interacting protein, represses cardiomyocyte hypertrophy. Circ Res 2012; 110(6): 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dodou E, Verzi MP, Anderson JP, et al. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 2004; 131(16): 3931–3942. [DOI] [PubMed] [Google Scholar]
- 86.Naya FJ, Black BL, Wu H, et al. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med 2002; 8(11): 1303–1309. [DOI] [PubMed] [Google Scholar]
- 87.Peng X, Wu X, Druso JE, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci U S A 2008; 105(18): 6638–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morin S, Charron F, Robitaille L, et al. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J 2000; 19(9): 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srivastava D, Thomas T, Lin Q, et al. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet 1997; 16(2): 154–160. [DOI] [PubMed] [Google Scholar]
- 90.Bar H, Kreuzer J, Cojoc A, et al. Upregulation of embryonic transcription factors in right ventricular hypertrophy. Basic Res Cardiol 2003; 98(5): 285–294. [DOI] [PubMed] [Google Scholar]
- 91.Brade T, Gessert S, Kuhl M, et al. The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev Biol 2007; 311(2): 297–310. [DOI] [PubMed] [Google Scholar]
- 92.Tao Y, Wang J, Tokusumi T, et al. Requirement of the LIM homeodomain transcription factor tailup for normal heart and hematopoietic organ formation in Drosophila melanogaster. Mol Cell Biol 2007; 27(11): 3962–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yelon D, Ticho B, Halpern ME, et al. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 2000; 127(12): 2573–2582. [DOI] [PubMed] [Google Scholar]
- 94.Huang JB, Liang J, Zhao XF, et al. Epigenetics: novel mechanism of pulmonary hypertension. Lung 2013; 191(6): 601–610. [DOI] [PubMed] [Google Scholar]
- 95.Saco TV, Parthasarathy PT, Cho Y, et al. Role of epigenetics in pulmonary hypertension. Am J Physiol Cell Physiol 2014; 306(12): C1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim GH, Ryan JJ, Marsboom G, et al. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ 2011; 1(3): 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huston JH, Ryan JJ. The emerging role of epigenetics in pulmonary arterial hypertension: an important avenue for clinical trials (2015 Grover Conference Series). Pulm Circ 2016; 6(3): 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003; 33(Suppl.): 245–254. [DOI] [PubMed] [Google Scholar]
- 99.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer 2004; 4(12): 988–993. [DOI] [PubMed] [Google Scholar]
- 100.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev 2011; 25(10): 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet 2013; 14(3): 204–220. [DOI] [PubMed] [Google Scholar]
- 102.Peaston AE, Whitelaw E. Epigenetics and phenotypic variation in mammals. Mamm Genome 2006; 17(5): 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gilsbach R, Preissl S, Gruning BA, et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun 2014; 5: 5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haas J, Frese KS, Park YJ, et al. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol Med 2013; 5(3): 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Movassagh M, Choy MK, Knowles DA, et al. Distinct epigenomic features in end-stage failing human hearts. Circulation 2011; 124(22): 2411–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cho YK, Eom GH, Kee HJ, et al. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J 2010; 74(4): 760–770. [DOI] [PubMed] [Google Scholar]
- 107.Zhao L, Chen CN, Hajji N, et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 2012; 126(4): 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bogaard HJ, Mizuno S, Hussaini AA, et al. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med 2011; 183(10): 1402–1410. [DOI] [PubMed] [Google Scholar]
- 109.Cavasin MA, Demos-Davies K, Horn TR, et al. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res 2012; 110(5): 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 2012; 52: 303–319. [DOI] [PubMed] [Google Scholar]
- 111.Delgado-Olguin P, Huang Y, Li X, et al. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet 2012; 44(3): 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reddy S, Zhao M, Hu DQ, et al. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 2012; 44(10): 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 2006; 103(48): 18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang N, Zhou Z, Liao X, et al. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life 2009; 61(6): 566–571. [DOI] [PubMed] [Google Scholar]
- 115.Cheng Y, Ji R, Yue J, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 2007; 170(6): 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Drake JI, Bogaard HJ, Mizuno S, et al. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 2011; 45(6): 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paulin R, Sutendra G, Gurtu V, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 2015; 116(1): 56–69. [DOI] [PubMed] [Google Scholar]
- 118.Potus F, Ruffenach G, Dahou A, et al. Downregulation of microRNA-126 Contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 2015; 132(10): 932–943. [DOI] [PubMed] [Google Scholar]
- 119.Michela P, Velia V, Aldo P, et al. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 2015; 768: 71–76. [DOI] [PubMed] [Google Scholar]
- 120.Joshi SR, Dhagia V, Gairhe S, et al. MicroRNA-140 is elevated and mitofusin-1 is downregulated in the right ventricle of the Sugen5416/hypoxia/normoxia model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2016; 311(3): H689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuhn KP, Byrne DW, Arbogast PG, et al. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 2003; 167(4): 580–586. [DOI] [PubMed] [Google Scholar]
- 122.Overbeek MJ, Lankhaar JW, Westerhof N, et al. Right ventricular contractility in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. Eur Respir J 2008; 31(6): 1160–1166. [DOI] [PubMed] [Google Scholar]
- 123.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179(2): 151–157. [DOI] [PubMed] [Google Scholar]
- 124.Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123(2): 344–350. [DOI] [PubMed] [Google Scholar]
- 125.Overbeek MJ, Mouchaers KT, Niessen HM, et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010; 2010: 604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma R, Bolger AP, Li W, et al. Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol 2003; 92(2): 188–193. [DOI] [PubMed] [Google Scholar]
- 127.Paulus WJ. How are cytokines activated in heart failure? Eur J Heart Fail 1999; 1(4): 309–312. [DOI] [PubMed] [Google Scholar]
- 128.Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002; 283(3): H1253–1262. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 2016; 97: 245–262. [DOI] [PubMed] [Google Scholar]
- 130.Waehre A, Vistnes M, Sjaastad I, et al. Chemokines regulate small leucine-rich proteoglycans in the extracellular matrix of the pressure-overloaded right ventricle. J Appl Physiol (1985) 2012; 112(8): 1372–1382. [DOI] [PubMed] [Google Scholar]
- 131.Moreth K, Brodbeck R, Babelova A, et al. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest 2010; 120(12): 4251–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 2010; 277(19): 3864–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herrera Garza E, Cubillos Garzon A, Stetson SJ, et al. [Tumor necrosis factor-alpha: a mediator in the pathogenesis of cardiac insufficiency]. Arch Inst Cardiol Mex 1999; 69(5): 462–468. [PubMed] [Google Scholar]
- 134.Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 1997; 81(4): 627–635. [DOI] [PubMed] [Google Scholar]
- 135.Sun M, Chen M, Dawood F, et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 2007; 115(11): 1398–1407. [DOI] [PubMed] [Google Scholar]
- 136.Herrera-Garza EH, Stetson SJ, Cubillos-Garzon A, et al. Tumor necrosis factor-alpha: a mediator of disease progression in the failing human heart. Chest 1999; 115(4): 1170–1174. [DOI] [PubMed] [Google Scholar]
- 137.Chung MK, Gulick TS, Rotondo RE, et al. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ Res 1990; 67(3): 753–763. [DOI] [PubMed] [Google Scholar]
- 138.Deswal A, Petersen NJ, Feldman AM, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001; 103(16): 2055–2059. [DOI] [PubMed] [Google Scholar]
- 139.van Riemsdijk-van Overbeeke IC, Baan CC, et al. The TNF-alpha system in heart failure and after heart transplantation: plasma protein levels, mRNA expression, soluble receptors and plasma buffer capacity. Eur Heart J 1999; 20(11): 833–840. [DOI] [PubMed] [Google Scholar]
- 140.Cheng XS, Shimokawa H, Momii H, et al. Role of superoxide anion in the pathogenesis of cytokine-induced myocardial dysfunction in dogs in vivo. Cardiovasc Res 1999; 42(3): 651–659. [DOI] [PubMed] [Google Scholar]
- 141.Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992; 257(5068): 387–389. [DOI] [PubMed] [Google Scholar]
- 142.Yokoyama T, Vaca L, Rossen RD, et al. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest 1993; 92(5): 2303–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yokoyama T, Nakano M, Bednarczyk JL, et al. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997; 95(5): 1247–1252. [DOI] [PubMed] [Google Scholar]
- 144.Bristow MR, Hershberger RE, Port JD, et al. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 1990; 82(2 Suppl): I12–25. [PubMed] [Google Scholar]
- 145.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 2005; 175(6): 3463–3468. [DOI] [PubMed] [Google Scholar]
- 146.Kishimoto T, Akira S, Narazaki M, et al. Interleukin-6 family of cytokines and gp130. Blood 1995; 86(4): 1243–1254. [PubMed] [Google Scholar]
- 147.Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 2011; 13(11): 1161–1171. [DOI] [PubMed] [Google Scholar]
- 148.McGinnis GR, Ballmann C, Peters B, et al. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol 2015; 308(11): H1423–1433. [DOI] [PubMed] [Google Scholar]
- 149.Bonda TA, Szynaka B, Sokolowska M, et al. Interleukin 6 modulates PPARalpha and PGC-1alpha and is involved in high-fat diet induced cardiac lipotoxicity in mouse. Int J Cardiol 2016; 219: 1–8. [DOI] [PubMed] [Google Scholar]
- 150.Melendez GC, McLarty JL, Levick SP, et al. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010; 56(2): 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gabriel AS, Martinsson A, Wretlind B, et al. IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. Eur J Intern Med 2004; 15(8): 523–528. [DOI] [PubMed] [Google Scholar]
- 152.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000; 102(25): 3060–3067. [DOI] [PubMed] [Google Scholar]
- 153.March CJ, Mosley B, Larsen A, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature 1985; 315(6021): 641–647. [DOI] [PubMed] [Google Scholar]
- 154.von Haehling S, Schefold JC, Lainscak M, et al. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail Clin 2009; 5(4): 549–560. [DOI] [PubMed] [Google Scholar]
- 155.El Khoury N, Mathieu S, Fiset C. Interleukin-1beta reduces L-type Ca2+ current through protein kinase C activation in mouse heart. J Biol Chem 2014; 289(32): 21896–21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 2000; 86(12): 1259–1265. [DOI] [PubMed] [Google Scholar]
- 157.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124(4): 783–801. [DOI] [PubMed] [Google Scholar]
- 158.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 2014; 5: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kawai T, Akira S. TLR signaling. Cell Death Differ 2006; 13(5): 816–825. [DOI] [PubMed] [Google Scholar]
- 160.Coggins M, Rosenzweig A. The fire within: cardiac inflammatory signaling in health and disease. Circ Res 2012; 110(1): 116–125. [DOI] [PubMed] [Google Scholar]
- 161.Liu L, Wang Y, Cao ZY, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med 2015; 19(12): 2728–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frantz S, Kobzik L, Kim YD, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 1999; 104(3): 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Higashikuni Y, Tanaka K, Kato M, et al. Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1beta upregulation via nuclear factor kappaB activation. J Am Heart Assoc 2013; 2(6): e000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388(6640): 394–397. [DOI] [PubMed] [Google Scholar]
- 165.Vallejo JG. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011; 121(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 166.Boyd JH, Mathur S, Wang Y, et al. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res 2006; 72(3): 384–393. [DOI] [PubMed] [Google Scholar]
- 167.Knuefermann P, Schwederski M, Velten M, et al. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res 2008; 78(1): 26–35. [DOI] [PubMed] [Google Scholar]
- 168.Lohner R, Schwederski M, Narath C, et al. Toll-like receptor 9 promotes cardiac inflammation and heart failure during polymicrobial sepsis. Mediators Inflamm 2013; 2013: 261049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012; 485(7397): 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guihaire J, Bogaard HJ, Flecher E, et al. Experimental models of right heart failure: a window for translational research in pulmonary hypertension. Semin Respir Crit Care Med 2013; 34(5): 689–699. [DOI] [PubMed] [Google Scholar]
- 171.Rouleau JL, Kapuku G, Pelletier S, et al. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation 2001; 104(8): 939–944. [DOI] [PubMed] [Google Scholar]
- 172.Thompson JT, Rackley MS, O’Brien TX. Upregulation of the cardiac homeobox gene Nkx2-5 (CSX) in feline right ventricular pressure overload. Am J Physiol 1998; 274(5 Pt 2): H1569–1573. [DOI] [PubMed] [Google Scholar]
- 173.Bishop JE, Rhodes S, Laurent GJ, et al. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 1994; 28(10): 1581–1585. [DOI] [PubMed] [Google Scholar]
- 174.Dewachter C, Belhaj A, Rondelet B, et al. Myocardial inflammation in experimental acute right ventricular failure: Effects of prostacyclin therapy. J Heart Lung Transplant 2015; 34(10): 1334–1345. [DOI] [PubMed] [Google Scholar]
- 175.Vistnes M, Waehre A, Nygard S, et al. Circulating cytokine levels in mice with heart failure are etiology dependent. J Appl Physiol 1985. 2010; 108(5): 1357–1364. [DOI] [PubMed] [Google Scholar]
- 176.Stanley WC, Lopaschuk GD, Hall JL, et al. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 1997; 33(2): 243–257. [DOI] [PubMed] [Google Scholar]
- 177.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 2007; 356(11): 1140–1151. [DOI] [PubMed] [Google Scholar]
- 178.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation 2004; 110(8): 894–896. [DOI] [PubMed] [Google Scholar]
- 179.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 2012; 185(3): 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol 2010; 661: 171–185. [DOI] [PubMed] [Google Scholar]
- 181.Piao L, Fang YH, Cadete VJ, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010; 88(1): 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Haddad F, Ashley E, Michelakis ED. New insights for the diagnosis and management of right ventricular failure, from molecular imaging to targeted right ventricular therapy. Curr Opin Cardiol 2010; 25(2): 131–140. [DOI] [PubMed] [Google Scholar]
- 183.Nagendran J, Gurtu V, Fu DZ, et al. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg 2008; 136(1): 168–78. [DOI] [PubMed] [Google Scholar]
- 184.Can MM, Kaymaz C, Tanboga IH, et al. Increased right ventricular glucose metabolism in patients with pulmonary arterial hypertension. Clin Nucl Med 2011; 36(9): 743–748. [DOI] [PubMed] [Google Scholar]
- 185.Oikawa M, Kagaya Y, Otani H, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005; 45(11): 1849–1855. [DOI] [PubMed] [Google Scholar]
- 186.Fang W, Zhao L, Xiong CM, et al. Comparison of 18F-FDG uptake by right ventricular myocardium in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with congenital heart disease. Pulm Circ 2012; 2(3): 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wong YY, Ruiter G, Lubberink M, et al. Right ventricular failure in idiopathic pulmonary arterial hypertension is associated with inefficient myocardial oxygen utilization. Circ Heart Fail 2011; 4(6): 700–706. [DOI] [PubMed] [Google Scholar]
- 188.Sutendra GD, Dromparis P, Paulin R, et al. A metabolic remodeling in right ventricular hypertrophy is associated w ith decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013; 91(11): 1315–1327. [DOI] [PubMed] [Google Scholar]
- 189.Stacpoole PW, Nagaraja NV, Hutson AD. Efficacy of dichloroacetate as a lactate-lowering drug. J Clin Pharmacol 2003; 43(7): 683–691. [PubMed] [Google Scholar]
- 190.Michelakis ED, McMurtry MS, Wu XC, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 2002; 105(2): 244–250. [DOI] [PubMed] [Google Scholar]
- 191.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 2011; 90(2): 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fang YH, Piao L, Hong Z, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med (Berl) 2012; 90(1): 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Randle PJ, Priestman DA, Mistry SC, et al. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem 1994; 55(Suppl): 1–11. [DOI] [PubMed] [Google Scholar]
- 194.Abozguia K, Clarke K, Lee L, et al. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med 2006; 3(9): 490–498. [DOI] [PubMed] [Google Scholar]
- 195.Liles JT, Hoyer K, Oliver J, et al. Ranolazine reduces remodeling of the right ventricle and provoked arrhythmias in rats with pulmonary hypertension. J Pharmacol Exp Ther 2015; 353(3): 480–489. [DOI] [PubMed] [Google Scholar]
- 196.Li XW, Wang XM, Li S, et al. [Effects of rutaecarpine on right ventriclar remodeling in rats with monocrotaline-induced pulmonary hypertension]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014; 30(5): 405–410. [PubMed] [Google Scholar]
- 197.Park AM, Wong CM, Jelinkova L, et al. Pulmonary hypertension-induced GATA4 activation in the right ventricle. Hypertension 2010; 56(6): 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Piao L, Fang YH, Parikh K, et al. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013; 91(10): 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Enache I, Charles AL, Bouitbir J, et al. Skeletal muscle mitochondrial dysfunction precedes right ventricular impairment in experimental pulmonary hypertension. Mol Cell Biochem 2013; 373(1–2): 161–170. [DOI] [PubMed] [Google Scholar]
- 200.Gomez-Arroyo J, Mizuno S, Szczepanek K, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail 2013; 6(1): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qipshidze N, Tyagi N, Metreveli N, et al. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. Am J Physiol Heart Circ Physiol 2012; 302(3): H688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gomez A, Bialostozky D, Zajarias A, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 2001; 38(4): 1137–1142. [DOI] [PubMed] [Google Scholar]
- 203.Faber MJ, Dalinghaus M, Lankhuizen IM, et al. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am J Physiol Heart Circ Physiol 2006; 291(4): H1580–1586. [DOI] [PubMed] [Google Scholar]
- 204.Bogaard HJ, Mizuno S, Al Hussaini AA, et al. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med 2011; 183: 1402–1410. [DOI] [PubMed] [Google Scholar]
- 205.Fraisl P, Mazzone M, Schmidt T, et al. Regulation of angiogenesis by oxygen and metabolism. Dev Cell 2009; 16(2): 167–179. [DOI] [PubMed] [Google Scholar]