Abstract
Pulmonary hypertension (PH) is common in patients with chronic kidney disease (CKD) and associated with increased mortality but the hemodynamic profiles, clinical risk factors, and outcomes have not been well characterized. Our objective was to define the hemodynamic profile and related risk factors for PH in CKD patients. We extracted clinical and hemodynamic data from Vanderbilt’s de-identified electronic medical record on all patients undergoing right heart catheterization during 1998–2014. CKD (stages III–V) was defined by estimated glomerular filtration rate thresholds. PH was defined as mean pulmonary pressure ≥ 25 mmHg and categorized into pre-capillary and post-capillary according to consensus recommendations. In total, 4635 patients underwent catheterization: 1873 (40%) had CKD; 1518 (33%) stage 3, 230 (5%) stage 4, and 125 (3%) stage 5. PH was present in 1267 (68%) of these patients. Post-capillary (n = 965, 76%) was the predominant PH phenotype among CKD patients versus 302 (24%) for pre-capillary (P < 0.001). CKD was independently associated with pulmonary hypertension (odds ratio = 1.4, 95% confidence interval = 1.18–1.65). Mortality among CKD patients rose with worsening stage and was significantly increased by PH status. PH is common and independently associated with mortality among CKD patients referred for right heart catheterization. Post-capillary was the most common etiology of PH. These data suggest that PH is an important prognostic co-morbidity among CKD patients and that CKD itself may have a role in the development of pulmonary vascular disease in some patients.
Keywords: chronic renal insufficiency, pulmonary hypertension, survival, hemodynamics
Introduction
Chronic kidney disease (CKD) is common in the USA, affecting over 25 million people.1 Pulmonary hypertension (PH) and CKD often co-exist2–4 and prior studies suggest that PH is associated with increased mortality in patients with CKD.3,4 Moreover, the prevalence of PH increases across CKD stages in a dose-response manner, an observation that suggests a potential direct relationship.5
Despite increased recognition of PH as an important contributor to mortality among CKD patients, little is known about the etiology of PH in patients with CKD.6 Epidemiologic and long-term outcome data are lacking on the impact of PH, particularly among patients with early stage CKD.7,8 Potential mechanisms for the development of PH in patients with CKD include endothelial dysfunction, increased flow through arterio-venous shunts, exposure to dialysis membranes, and elevated left ventricular filling pressure.7 Most studies examining the link between CKD and PH have relied on Doppler echocardiography to identify PH.3–5,9,10 This approach is limited because echocardiography cannot reliably measure left ventricular filling pressure, which is critical to discriminate pre-capillary from post-capillary PH. In the largest series to date with invasive right heart catheterization (RHC) data, Navaneethan et al. reported an increased mortality in a cohort of PH patients with CKD,4 but did not investigate a population of CKD patients for the presence of PH. The hemodynamic profiles of PH (pre-capillary PH versus post-capillary PH) in the CKD population have not been fully explored. Therefore, studies employing invasive hemodynamics are needed to gain insight into the etiology of PH among CKD patients. Moreover, prior studies have lacked information about important co-morbidities and echo variables that may influence the relationship between PH and CKD.
In this study, we investigated the etiology of PH in a large referral population of CKD patients undergoing RHC. Furthermore, we evaluated the effect of PH on mortality. We hypothesized that the prevalence of PH and risk-adjusted mortality would increase across CKD stages and that the predominant etiology of PH among CKD patients would be post-capillary PH. Finally, we explored whether PH exists in the absence of established risk factors for pulmonary vascular disease (PVD) in patients with CKD, which would suggest a potential causal contribution. Some of the results of this study have been previously reported in the form of abstracts.11,12
Methods
Study population
This study was approved by the Vanderbilt University Institutional Review Board (#151223). We identified participants using the Vanderbilt Synthetic Derivative, a mirror-image, de-identified version of the electronic medical record containing data on approximately 2.5 million patients. The design, implementation, and content of the Synthetic Derivative have been described previously.13–15 We extracted clinical and hemodynamic data from all patients undergoing RHC at our institution during 1998–2014. The method of data extraction has been described in detail elsewhere.16–18 In brief, we collected laboratory data, clinical diagnoses, and echocardiographic data closest in time to, but no greater than six months before or after, the date of RHC. Only medications prescribed prior to RHC were extracted. Co-morbidities were defined by International Classification of Diseases-9 codes in the medical record prior to RHC or as defined by previously validated algorithms.18,19 Patients with inadequate RHC data (defined by the absence of right ventricular and pulmonary arterial pressures) (n = 150), acute myocardial infarction (n = 264), complex congenital heart disease (n = 151), chronic pulmonary embolism (n = 18), previous cardiac or lung transplantation (n = 362), or extreme physiology (n = 217), as previously defined,15,19 were excluded.
Definition and classification of chronic kidney disease
Renal function was determined from the estimated glomerular filtration rate (eGFR) using the CKD-EPI creatinine equation and the laboratory values closest to the procedure from any time before the RHC or within 30 days after. CKD stage was defined by accepted eGFR thresholds:20 CKD stages III, IV, and V were defined as GFR 30–59, 15–29, and < 15 mL/min/1.73 m2 or on dialysis, respectively. Recognizing that at the time of RHC many patients may have transient changes in their eGFR, we excluded patients with stage II CKD to improve our specificity for clinically relevant CKD. A random sample of 50 cases were manually reviewed to ensure that eGFR was being appropriately identified by our algorithm with a > 95% accuracy for correct identification of significant chronic renal dysfunction. These cases were reviewed to ensure that the eGFR was not reflective of a transient drop in renal function, but rather actual clinically significant chronic renal impairment. Of the 50 cases reviewed, only one was attributable to a transient change in renal function.
Definition and classification of pulmonary hypertension
PH was defined as a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg as measured by invasive RHC, and classified as either pre-capillary PH or post-capillary PH. Pre-capillary PH was defined as a mPAP ≥ 25 mmHg and a mean pulmonary artery wedge pressure (mPAWP)≤ 15 mmHg and post-capillary PH was defined as a mPAP ≥ 25 mmHg and mPAWP > 15 mmHg according to consensus statements21 and using previously published automated extraction methods.19 Co-morbid established PH risk factors including liver disease, human immunodeficiency virus, lupus, scleroderma, interstitial lung disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, and hypoventilation syndrome were identified in advance of our analysis.
Outcomes
The primary clinical outcome was all-cause mortality in patients with CKD. The Synthetic Derivative is linked to the social security death index to provide vital status. Survivors were censored at the time of last medical contact with the Vanderbilt system (e.g. date of last hospital discharge, clinic visit, or medication prescription) through 24 December 2015.
Statistical analysis
Data are reported as median (interquartile range [IQR]) or frequency (%), as appropriate. Clinical characteristics were compared between groups using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables. Multivariate logistic regression models were created to assess the association between CKD stage and PH adjusting for variables that were selected a priori based on clinical relevance. Kaplan–Meier curves of unadjusted mortality during the available follow-up period were calculated and censored at the time of last medical contact. Cox proportional hazard models were fitted to assess the association between CKD stage and mortality adjusting for pre-selected covariates.
Results
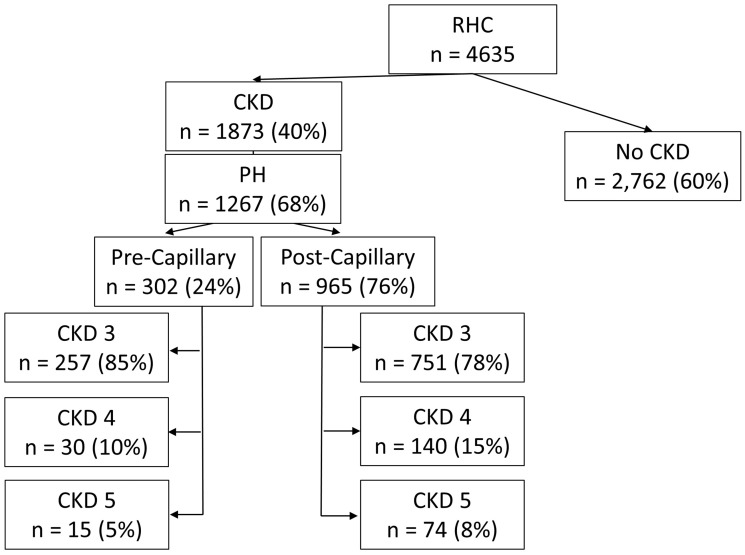
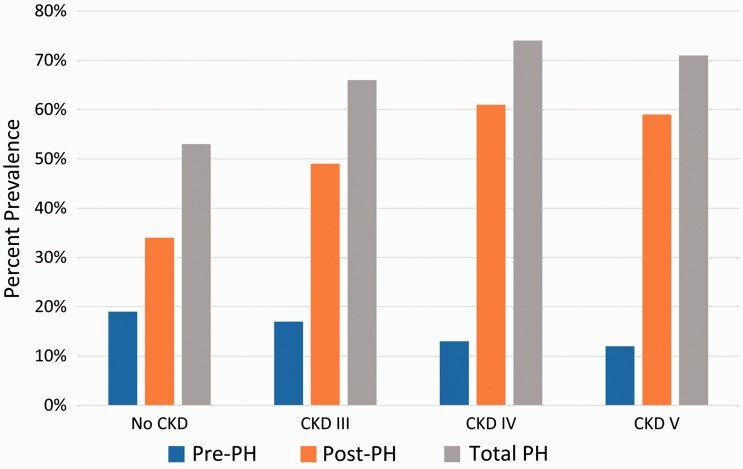
In total, our analysis included 4635 patients who underwent RHC (Fig. 1). The most common indications for RHC were ischemic heart disease, heart failure, PH, and aortic valve disease. The median interval between RHC and eGFR measurement was 0 days (IQR = 0–2 days). CKD stage III or greater was present in 1873 (40%) of patients, with a prevalence of CKD stage III, IV, and V individually being 1518 (33%), 230 (5%), and 125 (3%), respectively. PH was present in 1267 (68%) patients with CKD and the prevalence of PH increased with increasing CKD stage (P < 0.001; Fig. 2). Basic demographics and clinical characteristics of CKD patients with and without PH are compared in Table 1. Important differences between those with PH versus those without include younger age (64.9 years versus 67.0 years; P < 0.001), higher percentage of black race (14% versus 6%; P < 0.001), and higher BMI (29.1 versus 28.3; P = 0.002). Participants with PH had increased rates of co-morbidities including diabetes (25% versus 16%; P < 0.001), heart failure with reduced ejection fraction (28% versus 17%; P < 0.001), heart failure with preserved ejection fraction (33% versus 21%; P < 0.001), scleroderma (3% versus < 1%; P < 0.001), OSA (14% versus 9%; P < 0.004), and COPD (11% versus 8%; P = 0.02). Those with PH had higher right atrial pressure (11 mmHg versus 4.0 mmHg; P < 0.001) and higher brain natriuretic peptide (BNP) (582 versus 259; P < 0.001). Participants with PH were slightly less likely to have hypertension (HTN) (76% versus 83%; P = 0.001).
Fig. 1.
Flow-diagram of study population. PH, pulmonary hypertension; CKD, chronic kidney disease; RHC, right heart catheterization.
Fig. 2.
Prevalence of pulmonary hypertension by CKD stage. Bar height represents PH prevalence by CKD stage with blue bars representing pre-capillary PH (Pre-PH), orange bars representing post-capillary PH (Post-PH), and gray bars representing the total PH prevalence. The prevalence of PH increases with worsening CKD stage and the proportion of PH attributable to post-capillary PH also increases. CKD, chronic kidney disease; PH, pulmonary hypertension.
Table 1.
Characteristics of CKD Patients with and without PH.
| CKD no PH (n = 606) | CKD w/ PH (n = 1267) | P value | |
|---|---|---|---|
| Follow-up (years) | 2.4 (0.7–5.7) | 1.9 (0.3–3.7) | < 0.001 |
| Age | 67.0 (58.6–76.3) | 64.9 (55.0–73.0) | < 0.001 |
| Male | 301 (50%) | 608 (48%) | 0.495 |
| Race | < 0.001 | ||
| Black | 36 (6%) | 174 (14%) | |
| White | 542 (89%) | 1042 (82%) | |
| Other | 28 (5%) | 51 (4%) | |
| CKD stage | 0.052 | ||
| CKD III | 510 (84%) | 1008 (80%) | |
| CKD IV | 60 (10%) | 170 (13%) | |
| CKD V | 36 (6%) | 89 (7%) | |
| Dialysis | 44 (7%) | 153 (12%) | 0.001 |
| Diabetes | 97 (16%) | 319 (25%) | < 0.001 |
| BMI | 28.3 (24.2–33.0) | 29.1 (24.9–34.2) | 0.002 |
| HTN | 501 (83%) | 966 (76%) | 0.002 |
| COPD | 48 (8%) | 137 (11%) | 0.02 |
| ILD | 11 (2%) | 34 (3%) | 0.251 |
| SLE | 10 (2%) | 22 (2%) | 0.893 |
| Scleroderma | 3 (<1%) | 36 (3%) | < 0.001 |
| Heart failure | 266 (44%) | 844 (67%) | < 0.001 |
| HFrEF (EF < 40%) | 105 (17%) | 349 (28%) | < 0.001 |
| HFpEF | 126 (21%) | 414 (33%) | < 0.001 |
| Any valve dz. | 53 (9%) | 98 (8%) | 0.45 |
| Rheumatic valve dz. | 22 (4%) | 33 (3%) | 0.219 |
| Aortic valve dz. | 16 (3%) | 37 (3%) | 0.732 |
| Mitral valve dz. | 23 (4%) | 49 (4%) | 0.94 |
| Tricuspid valve dz. | 12 (2%) | 21 (2%) | 0.619 |
| Pulmonic valve dz. | 0 (0%) | 5 (<1%) | 0.122 |
| CAD | 174 (52%) | 339 (50%) | 0.655 |
| OSA | 55 (9%) | 174 (14%) | 0.004 |
| HIV | 6 (1%) | 13 (1%) | 0.942 |
| Liver dz. | 58 (10%) | 117 (9%) | 0.815 |
| PA mean (mmHg) | 19 (15–22) | 36 (30–44) | < 0.001 |
| Pre-Cap PH | – | 302 (24%) | |
| Wedge (mmHg) | 9 (6–13) | 19 (15–25) | < 0.001 |
| PVR (WU) | 1.73 (1.26–2.3) | 3.05 (2.01–4.85) | < 0.001 |
| CO (L/min) | 5.58 (4.67–6.77) | 4.99 (3.95–6.32) | < 0.001 |
| RAP (mmHg) | 4.0 (2.0–6.75) | 11.0 (7.0–16.0) | < 0.001 |
| BNP | 259 (105–691) | 582 (269–1283) | < 0.001 |
| Creatinine | 1.34 (1.16–1.65) | 1.45 (1.22–1.90) | < 0.001 |
| Hgb A1c | 5.8 (5.4–6.3) | 5.9 (5.5–6.6) | < 0.001 |
| Hgb | 12.5 (11.1–13.9) | 11.8 (10.3–13.5) | < 0.001 |
| Iron | 53.0 (34.5–87.0) | 41.0 (26.0–62.0) | < 0.001 |
Categorical variables are presented as n (%). % is reflective of those with available data; continuous variables are presented as mean (lower, upper quartile).
HFrEF plus HFpEF do not add up to total heart failure because echocardiogram data were not available for all patients.
CAD, coronary artery disease; CKD, chronic kidney disease; CO, cardiac output (calculated by Fick); COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HTN, hypertension; ILD, interstitial lung disease; SLE, systemic lupus erythematosus; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; OSA, obstructive sleep apnea; PA, pulmonary artery; PVR, pulmonary vascular resistance (in woods units); RAP, mean right atrial pressure.
Hemodynamic etiology of PH
Post-capillary PH was the predominant PH phenotype among CKD patients referred for RHC: 965 (76%) versus 302 (24%) for pre-capillary PH (P < 0.001). The prevalence of combined PH, defined as post-capillary PH with a diastolic pressure gradient (DPG) of ≥ 7 mmHg, was 201/965 (21%). The prevalence of post-capillary PH increased with worsening CKD stage whereas pre-capillary PH decreased with escalating CKD stage (Fig. 2). Mean right atrial pressure was greater (13.1 ± 6.7 mmHg versus 8.1 ± 5.4 mmHg, P < 0.001) in the post-capillary PH group compared with the pre-capillary PH group. The demographics and clinical characteristics of CKD patients with pre-capillary PH versus post-capillary PH are compared in Table 2. Pre-capillary PH patients were more likely to be women and have other established pre-capillary PH risk factors such as intrinsic lung disease, lupus, and scleroderma. A total of 176/302 (58%) patients with CKD and pre-capillary PH had no established risk factors for PVD including no evidence of liver disease, human immunodeficiency virus, lupus, scleroderma, interstitial lung disease, COPD, obstructive sleep apnea, and hypoventilation syndrome. CKD patients with pre-capillary PH and no established risk factors, were more likely to be women (75% versus 49%; P < 0.001), have less coronary artery disease (32% versus 55%; P < 0.001), and have a higher hemoglobin (13.0 gm/dL versus 12.2 gm/dL; P < 0.001) when compared with other CKD patients without known risk factors for PVD.
Table 2.
Characteristics of CKD Patients with PH: Pre-Capillary versus Post-Capillary.
| CKD w/ pre-capillary PH (n = 302) | CKD w/ post-capillary PH (n = 965) | P value | |
|---|---|---|---|
| Follow-up (years) | 2.0 (0.5–4.0) | 1.8 (0.3–3.7) | 0.11 |
| Age | 64.5 (52.4–71.9) | 65.1 (56.0–73.4) | 0.032 |
| Male | 82 (27%) | 526 (55%) | <0.001 |
| Race | 0.864 | ||
| Black | 42 (14%) | 132 (14%) | |
| White | 247 (82%) | 795 (82%) | |
| Other | 13 (5%) | 38 (4%) | |
| CKD stage | <0.001 | ||
| CKD III | 257 (85%) | 751 (78%) | |
| CKD IV | 30 (10%) | 140 (15%) | |
| CKD V | 15 (5%) | 74 (8%) | |
| Dialysis | 21 (7%) | 132 (14%) | 0.002 |
| Diabetes | 41 (14%) | 278 (29%) | < 0.001 |
| BMI | 28.6 (24.6–32.6) | 29.5 (25.1–34.6) | 0.022 |
| HTN | 207 (69%) | 759 (79%) | < 0.001 |
| COPD | 37 (12%) | 134 (14%) | 0.47 |
| ILD | 19 (6%) | 15 (2%) | < 0.001 |
| SLE | 11 (4%) | 11 (1%) | 0.004 |
| Scleroderma | 29 (10%) | 7 (1%) | < 0.001 |
| Heart failure | 167 (55%) | 677 (70%) | < 0.001 |
| HFrEF (<40%) | – | 317 (33%) | – |
| HFpEF | – | 295 (31%) | – |
| Any valve dz. | 20 (7%) | 78 (8%) | 0.41 |
| Rheumatic valve dz. | 7 (2%) | 26 (3%) | 0.72 |
| Aortic valve dz. | 10 (3%) | 27 (3%) | 0.64 |
| Mitral valve dz. | 6 (2%) | 43 (4%) | 0.052 |
| Tricuspid valve dz. | 4 (1%) | 17 (2%) | 0.60 |
| Pulmonic valve dz. | 0 (0%) | 5 (1%) | 0.21 |
| CAD | 50 (30%) | 289 (57%) | < 0.001 |
| OSA | 40 (13%) | 134 (14%) | 0.78 |
| HIV | 5 (2%) | 8 (1%) | 0.21 |
| Liver dz. | 24 (8%) | 93 (10%) | 0.38 |
| PA mean (mmHg) | 35 (28–48) | 36 (30–44) | 0.62 |
| Wedge (mmHg) | 11 (7–13) | 22 (18–27) | < 0.001 |
| PVR (WU) | 4.76 (3.0–8.8) | 2.7 (1.8–4.3) | < 0.001 |
| CO (L/min) | 5.0 (3.7–6.4) | 5.0 (4.0–6.3) | 0.31 |
| RAP (mmHg) | 7.0 (4.0–11.0) | 13.0 (8.0–17.0) | < 0.001 |
| BNP | 399 (164–850) | 656 (327–1402) | < 0.001 |
| Creatinine | 1.3 (1.1–1.6) | 1.5 (1.3–2.0) | < 0.001 |
| Hgb A1c | 5.8 (5.4–6.3) | 6.0 (5.5–6.8) | < 0.001 |
| Hgb | 12.8 (11.4–14.4) | 11.5 (10.1–13.1) | < 0.001 |
| Iron | 41.5 (27–58.8) | 40.0 (25.0–63.0) | 0.734 |
Categorical variables are presented as n (%). % is reflective of those with available data; continuous variables are presented as mean (lower, upper quartile).
CAD, coronary artery disease; CKD, chronic kidney disease; CO, cardiac output (calculated by Fick); COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HTN, hypertension; ILD, interstitial lung disease; SLE, systemic lupus erythematosus; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; OSA, obstructive sleep apnea; PA, pulmonary artery; PVR, pulmonary vascular resistance (in woods units); RAP, mean right atrial pressure.
Outcomes
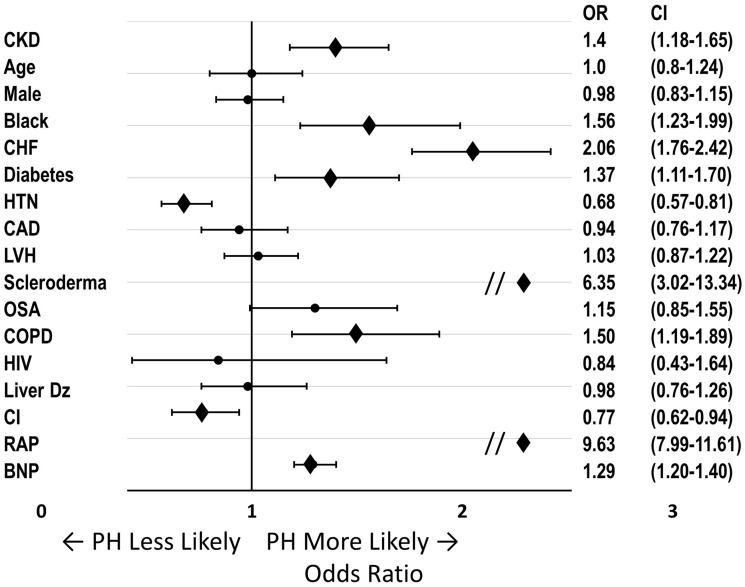
In the multivariate analysis of the entire cohort adjusting for 24 clinically relevant covariates, CKD stage III or greater was independently associated with PH (odds ratio [OR] = 1.4, 95% confidence interval [CI] = 1.18–1.65; Fig. 3). Other variables independently associated with PH in our model included black race (OR = 1.56, 95% CI = 1.23–1.99), congestive heart failure (OR = 2.06, 95% CI = 1.76–2.42), diabetes (OR = 1.37, 95% CI = 1.11–1.70), scleroderma (OR = 6.35, 95% CI = 3.02–13.34), COPD (OR = 1.5, 95% CI = 1.19–1.89), right atrial pressure (OR = 9.63, 95% CI = 7.99–11.61), and BNP (OR = 1.29, 95% CI = 1.20–1.40).
Fig. 3.
Association of clinical features with PH. Multivariate logistic regression analysis of all patients undergoing RHC. ORs for the presence of PH for all covariates. ORs and 95% CIs shown to the right. For continuous variables, effects comparing 25th and 75th percentiles were calculated. CKD and African American status were independently associated with PH, along with known PH risk factors congestive heart failure, Scleroderma, COPD, RAP, and BNP.
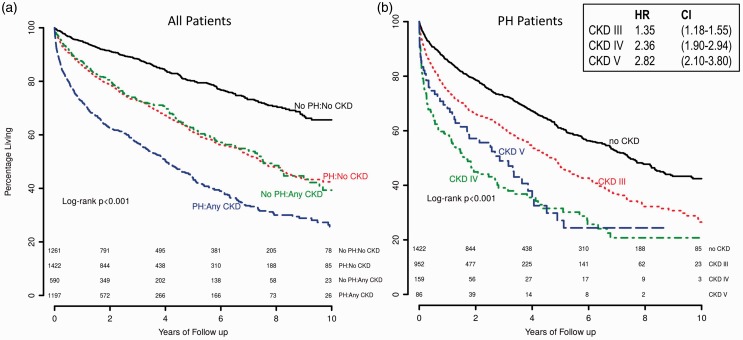
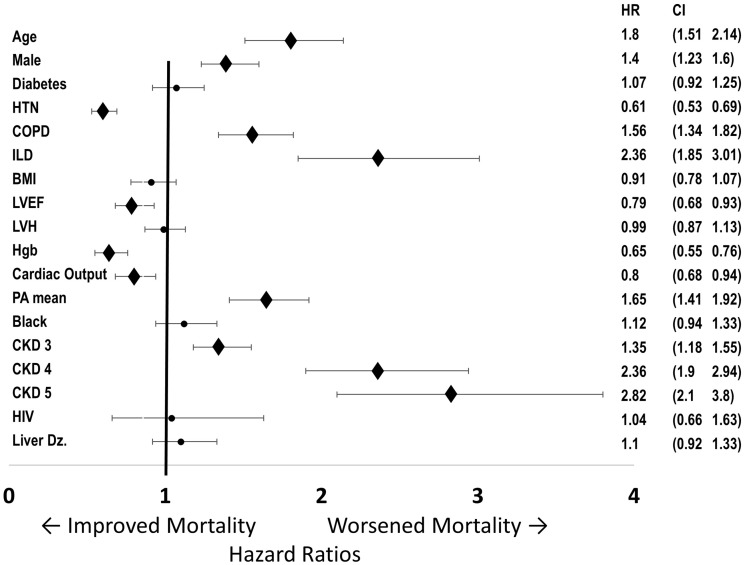
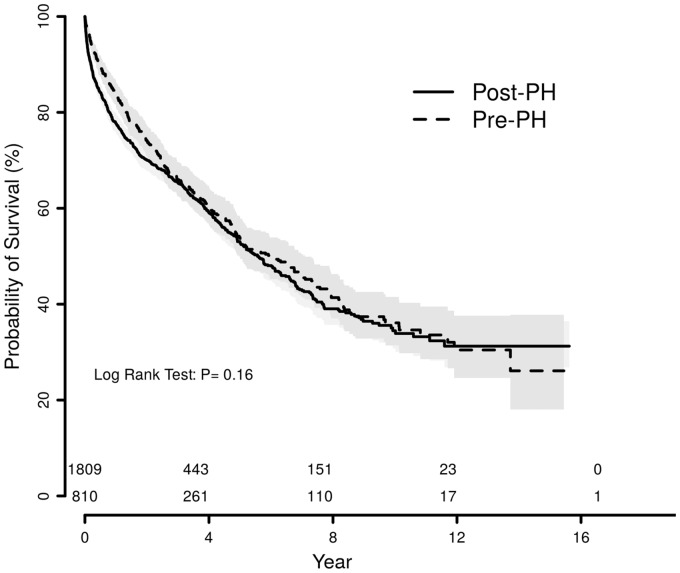
Mortality among CKD patients was significantly increased by PH status. In unadjusted analyses, CKD or PH individually had a nearly identical impact on mortality (Fig. 4a). The combination of both CKD and PH was associated with the highest mortality. Among patients with PH, mortality was lowest in those with normal renal function and increased in a graded fashion with CKD stage (Fig. 4b). In an adjusted Cox regression analysis of all patients undergoing RHC, CKD was independently associated with mortality (CKD III hazard ratio [HR] = 1.35, 95% CI = 1.18–1.54; CKD IV HR = 2.3, 95% CI =1.85–2.87; CKD V HR = 2.7, 95% CI = 2.01–3.64) in addition to age, male sex, mPAP, COPD, and interstitial lung disease (Fig. 5). When the adjusted analysis was restricted to participants with CKD stage III or greater, age (HR = 1.55, 95% CI = 1.22–1.96), male sex (HR = 1.44, 95% CI = 1.20–1.73), COPD (HR = 1.54, 95% CI =1.25–1.91), interstitial lung disease (HR = 2.52, 95% CI = 1.59–4.0), and mPAP (HR = 1.6, 95% CI = 1.23–2.10) were associated with mortality. There was no mortality difference between those with CKD and post-capillary PH versus CKD and pre-capillary PH (P = 0.16) (Fig. 6). There was also no mortality difference between post-capillary PH and pre-capillary PH when CKD stages were considered individually (P ≥ 0.4 for all comparisons).
Fig. 4.
Survival according to CKD and PH status. (a) Kaplan–Meyer curves of unadjusted mortality in years based on the presence or absence of PH and any degree of CKD. The number at risk is displayed beneath the curves. (b) Kaplan–Meyer curves of unadjusted mortality in years among PH patients based on the presence or absence of CKD III or CKD IV/V. The number at risk is displayed beneath the curves. Adjusted HRs and 95% CIs for CKD stages III–V are shown in the box. Full multivariate analysis with all covariates displayed in Fig. 5.
Fig. 5.
Adjusted HRs for mortality. Multivariate regression analysis of all patients undergoing RHC. HRs for mortality for all covariates in the multivariate regression. HR and 95% CIs shown to the right. For continuous variables, effects comparing 25th and 75th percentiles were calculated. After adjustment for clinical values, CKD stage has a strong association with increased mortality, along with age, male sex, COPD, interstitial lung disease, and mPAP.
Fig. 6.
Survival by pre-capillary or post-capillary PH status among CKD patients. Kaplan–Meyer curves of unadjusted mortality in years among CKD patients based on pre-capillary vs. post-capillary PH status. There was no difference in mortality between groups. Pre-PH, pre-capillary PH; Post-PH, post-capillary PH.
Discussion
We examined the prevalence, etiology, and outcome of PH among CKD patients in a large electronic medical record (EMR)-based population referred for RHC . We found that PH is common among patients with CKD and independently associated with higher mortality. The HR of 1.4 is relatively modest; however, CKD represents a large population that is at risk. PH prevalence and the hazard for death increased as the severity of CKD worsened. Consistent with smaller studies,10 post-capillary PH was the predominant PH phenotype among CKD patients; however, we also identified a significant number of CKD patients with pre-capillary PH without any known risk factors for PH. This observation is important because it suggests a possible direct pathophysiologic relationship between CKD and PH. Our study provides novel information compared with prior studies in this area by demonstrating the prevalence of PH within a large CKD population using invasive data and demonstrating an independent association between CKD and PH after controlling for 17 relevant clinical variables. This has yet to be shown with invasive data allowing a robust characterization of the hemodynamic phenotype as well as associated clinical variables in CKD patients with PH.
Using invasive hemodynamic data provides some important advantages over Doppler echocardiography. First, Doppler echocardiography can both under- and overestimate pulmonary pressures due to technical limitations.22 Second, invasive catheterization provides a more accurate estimation of left heart filling pressures, which allowed us to distinguish between pre-capillary PH and post-capillary PH. Accurate discrimination of these two phenotypes is important because the treatment and underlying etiologies differ. In our cohort, post-capillary PH was by far the most common PH phenotype among CKD patients and the post-capillary proportion increased across CKD stages. This has been attributed to the high frequency of co-morbidities contributing to both CKD and post-capillary PH (e.g. hypertension and diabetes). CKD has long been understood to co-exist with systemic hypertension and heart disease23 and has been associated with a high frequency of atherosclerosis and left sided structural remodeling.23–25 Similarly, age, left-ventricular hypertrophy, hypertension, and left ventricular systolic dysfunction have also been associated with PH.3,21,26,27 Given the degree of overlap between CKD and left-sided heart disease, the predominance of post-capillary PH did not come as a surprise. In addition, many patients with advanced CKD have a chronic volume overloaded state that exacerbates these conditions.
Our data include a large percentage of patients with moderate CKD (stage III; n = 1518) which has been less well described in the literature.5 Navaneethan et al. studied CKD stage III and IV patients within a cohort of PH and found increased mortality as well as a relationship between right atrial pressure and CKD.4 Our study aimed to build off of this work by describing the hemodynamic etiology of PH among a cohort of CKD patients referred for RHC, as this has treatment implications. Our findings confirm the effect of PH on the mortality of CKD patients despite the mPAPs being only modestly increased across CKD groups; stage III 29 mmHg, stage IV 33 mmHg, and stage V 31 mmHg. These findings warrant careful observation for modest elevations in pulmonary pressures even in relatively early stage CKD.
Several potential mechanisms have been put forward to explain the co-existence of PH and CKD. Fibroblast growth factor 23 (FGF-23) is a marker of worsening renal function and has also recently been implicated as a causative factor in left heart disease.28 FGF-23 has also been shown to be elevated and predict prognosis in patients with CKD and pre-capillary PH.29,30 Therefore, FGF-23 may have a direct effect on pulmonary vascular remodeling or an indirect effect by promoting left heart disease, which in turn leads to pulmonary vascular remodeling in those that are genetically predisposed.
Elevated right atrial pressure, which was strongly associated with PH in our data, may also be an important mediator of this relationship. Elevated right atrial pressure leading to renal vein hypertension is increasingly recognized as a contributor to CKD.31–33 In turn, CKD may contribute to PH via increased renin-angiotensin-aldosterone-system activation34 and inflammatory response35 which have both been shown to be elevated in CKD and contribute to pulmonary vascular remodeling.36–40 Worsening PH may then lead to further elevated right atrial pressure and worsened CKD in a reciprocal relationship. However, this is speculative and the elevated right atrial pressure may also be a result of, rather than a cause of, PH and CKD or both.
There was an apparent protective effect of HTN on the risk of PH in our CKD cohort. This association has been noted in a prior report by our group.18 We suspect this is likely to be a treatment effect due to those with diagnosed HTN receiving more aggressive therapies compared to those with undiagnosed HTN. Our model also already partially corrects for the negative effects of HTN by controlling for other associated variables (e.g. LVH, diabetes, age).
The majority (58%) of CKD patients with pre-capillary PH did not have any identifiable risk factors for pre-capillary PH physiology.41 This group has a striking preponderance of women (75%) compared with the CKD cohort referred for RHC at large (49%). This implies a gender-specific mechanism for the pulmonary vascular remodeling. The influence of sex on renin–angiotensin–aldosterone pathway activation has also been well documented via a number of mechanisms.42 The rates of angiotensin-converting-enzyme inhibitor and angiotensin receptor blocker use in women compared with men in the CKD and entire cohort, respectively, were: 37% versus 47% (P < 0.001) and 31% versus 41% (P < 0.001). It is plausible that gender differences in the balance of renin–angiotensin–aldosterone system activation versus blockade may contribute to the increased rate of pre-capillary PH physiology in women with CKD, though this is speculative.
Limitations
Our findings should be interpreted in the context of several limitations. First, our cohort is subject to bias because all patients were referred for a clinically indicated RHC. Despite this limitation, EMR-based cohorts provide pragmatic advantages compared with creating similar cohorts prospectively, which can be expensive and impractical.13 Although this approach limits our ability to report the true prevalence of PH in the CKD population, the use of a referral population may make our results more generalizable. Direct, invasive measurement of pulmonary pressures is the gold standard for the diagnosis of PH;21 however, it can be subject to error.43,44 The RHC was performed at the discretion of the treating physician and therefore we are unable to standardize the technique and pressure tracings are not currently available for manual review in Vanderbilt’s Synthetic Derivative. Notably, discrepancies between the computer-generated and manual mPAWP determination at end-expiration have been reported;45–47 however, we have previously shown strong agreement between the computer-generated mPAWP values in our dataset and manually reviewed mPAWP values on a population level.19 Furthermore, the large size of the dataset and multiple operators should compensate for any systematic error in data acquisition.
Additionally, as with all retrospective and EMR-based studies, accurate identification of co-morbidities with ICD-9 codes with or without supportive laboratory data is imperfect. However, we did utilize previously published methods to extract this data17,18 and the majority of our definitions were designed to be sensitive but not specific, which would bias our findings to the null hypothesis.
CKD patients with pre-capillary PH and no other risk factors for PVD were an interesting group in our study. One concern was that dialysis, particularly if done just before the RHC, may produce transient low mPAWP pressures compared with PAP giving the impression of pre-capillary PH physiology, when in fact high left ventricular filling pressures were present at other times. Only eight (4.5%) individuals in this group were on dialysis, making artificially low mPAWP due to recent dialysis an insignificant contributor to the pre-capillary PH cohort.
Conclusions
In this single-center EMR-based cohort, we found that PH is common among patients with CKD who were referred for RHC and that PH is independently associated with mortality. Although post-capillary PH is the predominant PH etiology, many CKD patients, particularly women, with pre-capillary PH have no identifiable risk factors suggesting the possibility of a contributory relationship. Further research is warranted to determine if CKD has any direct impact on the pulmonary vasculature that may explain the association between PH and CKD. Prospective studies utilizing biological samples would allow more accurate phenotyping, biomarker analysis and genotyping. This information could be used to design mechanistic studies and, potentially, lead to novel therapeutic targets.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding
NIH #1 U01 HL125212-01 (Dr. Hemnes); American Heart Association Fellow to Faculty Grant #13FTF16070002, Pulmonary Hypertension Association Proof-of-Concept Award, Actelion Entelligence Young Investigator Award, and Gilead PAH Scholars Award Program (Dr. Brittain); NIH #5T32HL087738-08 (Dr. Assad). The dataset used in the analyses described were obtained from Vanderbilt University Medical Centers BioVU which is supported by institutional funding and by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 2012; 27: 3908–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaneethan SD, Roy J, Tao K, et al. Prevalence, predictors, and outcomes of pulmonary hypertension in CKD. J Am Soc Nephrol 2016; 27: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navaneethan SD, Wehbe E, Heresi GA, et al. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol 2014; 9: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Liang X, Liu S, et al. Pulmonary hypertension: epidemiology in different CKD stages and its association with cardiovascular morbidity. PLoS One 2014; 9: e114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawar B, Ellam T, Jackson C, et al. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol 2013; 37: 281–290. [DOI] [PubMed] [Google Scholar]
- 7.Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary hypertension in CKD. Am J Kidney Dis 2013; 61: 612–622. [DOI] [PubMed] [Google Scholar]
- 8.Yang QM, Bao XR. Pulmonary hypertension in patients with stage 1-3 chronic kidney disease. Genet Mol Res 2014; 13: 5695–5703. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Liu S, Liang X, et al. Pulmonary hypertension as an independent predictor of cardiovascular mortality and events in hemodialysis patients. Int Urol Nephrol 2014; 46: 141–149. [DOI] [PubMed] [Google Scholar]
- 10.Pabst S, Hammerstingl C, Hundt F, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS One 2012; 7: e35310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Leary JM, Assad TR, Xu M, et al. Pulmonary hypertension in patients with chronic kidney disease: invasive hemodynamic etiology and outcomes in a large electronic medical record-based cohort [abstract]. J Am Coll Cardiol 2016; 13(Suppl. 5): 2048. [Google Scholar]
- 12.O’Leary JM, Assad TR, Hemnes AR, et al. Influence of gender on pulmonary hypertension phenotype in chronic kidney disease [abstract]. Circulation 2016; 134: A20657. [Google Scholar]
- 13.Bowton E, Field JR, Wang S, et al. Biobanks and electronic medical records: enabling cost-effective research. Sci Transl Med 2014; 6: 234cm3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells QS, Farber-Eger E, Crawford DC. Extraction of echocardiographic data from the electronic medical record is a rapid and efficient method for study of cardiac structure and function. J Clin Bioinforma 2014; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assad TR, Hemnes AR, Doss LN, et al. Determining the clinical and hemodynamic profile of combined pulmonary hypertension in a large de-identified database. Am J Respir Crit Care Med 2015; 191: A5522. [Google Scholar]
- 16.Brittain EL, Chan SY. Integration of complex data sources to provide biologic insight into pulmonary vascular disease (2015 Grover Conference Series). Pulm Circ 2016; 6: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ 2016; 6: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol 2016; 68: 2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and pre-capillary pulmonary hypertension. Pulm Circ 2016; 6: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266. [PubMed] [Google Scholar]
- 21.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–50. [DOI] [PubMed] [Google Scholar]
- 22.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32: S112–119. [DOI] [PubMed] [Google Scholar]
- 24.Jungers P, Massy ZA, Nguyen Khoa T, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrol Dial Transplant 1997; 12: 2597–2602. [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47: 186–192. [DOI] [PubMed] [Google Scholar]
- 26.Guha A, Amione-Guerra J, Park MH. Epidemiology of pulmonary hypertension in left heart disease. Prog Cardiovasc Dis 2016; 59: 3–10. [DOI] [PubMed] [Google Scholar]
- 27.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser R, Seiler S, Held M, et al. Prognostic impact of renal function in precapillary pulmonary hypertension. J Intern Med 2014; 275: 116–126. [DOI] [PubMed] [Google Scholar]
- 30.Imazu M, Takahama H, Amaki M, et al. Use of serum fibroblast growth factor 23 vs. plasma B-type natriuretic peptide levels in assessing the pathophysiology of patients with heart failure. Hypertens Res 2017; 40: 181–188. [DOI] [PubMed] [Google Scholar]
- 31.Dini FL, Demmer RT, Simioniuc A, et al. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 2012; 14: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Lullo L, Floccari F, Rivera R, et al. Pulmonary hypertension and right heart failure in chronic kidney disease: new challenge for 21st-century cardionephrologists. Cardiorenal Med 2013; 3: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navaneethan SD, Dweik RA. Elevated pulmonary pressure: A novel risk marker in kidney disease? Kidney Int 2015; 88: 7–9. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Gatto P, Giusti G, et al. Pathophysiology of cardiorenal syndrome in decompensated heart failure: role of lung-right heart-kidney interaction. Int J Cardiol 2013; 169: 379–384. [DOI] [PubMed] [Google Scholar]
- 35.Colombo PC, Ganda A, Lin J, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev 2012; 17: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein IH, Ligtenberg G, Neumann J, et al. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol 2003; 14: 3239–3244. [DOI] [PubMed] [Google Scholar]
- 37.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 38.Ciarka A, Doan V, Velez-Roa S, et al. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 181: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 39.Caglar K, Yilmaz MI, Saglam M, et al. Serum fetuin-a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract 2008; 108: c233–240. [DOI] [PubMed] [Google Scholar]
- 40.Friedman D, Szmuszkovicz J, Rabai M, et al. Systemic endothelial dysfunction in children with idiopathic pulmonary arterial hypertension correlates with disease severity. J Heart Lung Transplant 2012; 31: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. [DOI] [PubMed] [Google Scholar]
- 42.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 2002; 53: 672–677. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan JJ, Rich JD, Thiruvoipati T, et al. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 2012; 163: 589–594. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs G, Naeije R, Olschewski H. Reply: reading of pulmonary artery pressure tracings: the best compromise of accuracy and clinical pertinence. Am J Respir Crit Care Med 2014; 190: 1198–1199. [DOI] [PubMed] [Google Scholar]
- 46.LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J 2014; 44: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boerrigter BG, Waxman AB, Westerhof N, et al. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014; 43: 1316–1325. [DOI] [PubMed] [Google Scholar]