Abstract
Background
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) has an emerging role in the treatment of peritoneal malignancies. The CRS-HIPEC approach has known treatment-related toxicities. This study sought to determine the predictors of major postoperative complications after CRS-HIPEC in a high-volume center.
Methods
From a single-institution database, this study investigated complications experienced by patients undergoing CRS-HIPEC. Multiple preoperative and operative factors were analyzed for their ability to predict 60-day Clavien grade 3 and greater (major) complications by logistic regression. A predictive model was created from preoperative factors using multivariate logistic regression. The model was tested by Akaike’s information criterion, the Hosmer and Lemeshow Goodness-of-Fit Test, the receiver operating characteristic, and the Youden Index.
Results
The study evaluated 247 patients undergoing CRS-HIPEC. The primary tumor site was the appendix in 166 cases (67.2 %), the colorectal area in 51 cases (20.6 %), the peritoneum (mesothelioma) in 22 cases (8.9 %), the ovary in 5 cases (2 %), and the small bowel in 3 cases (1.2 %). The median peritoneal cancer index was 14 (range 0–29), and 235 patients (95.1 %) had a complete (CC-0/1) cytoreduction. Major complications occurred for 41 patients (16.6 %), classified as grade 3 in 33 cases (13.4 %), grade 4 in 5 cases (2 %), and grade 5 (deaths) in 3 cases (1.2 %). The factors predictive of major complications in the multivariate analysis were a Charlson Comorbidity Index (CCI) score higher than 0 [odds ratio (OR), 2.505; p = 0.035], presence of preoperative symptoms (OR 1.951; p = 0.064), and prior resection status [no resection or prior CRS-HIPEC (OR 2.087) vs. prior resection without CRS-HIPEC (OR 3.209); p = 0.046]. These variables were used to create a tool predictive of postoperative complications.
Conclusion
Presence of symptoms, CCI, and prior resection status predict major complications and define a low-risk population after CRS-HIPEC.
Patients with peritoneal metastases have historically been considered as having incurable disease. In select patients, the peritoneum may represent the sole site of metastatic disease, prompting the use of cytoreductive surgery (CRS) and regional chemotherapy in the form of hyperthermic intraperitoneal chemotherapy (HIPEC). Mounting evidence points to the efficacy of CRS-HIPEC in the treatment of select patients with peritoneal metastases from cancers of the appendix,1–3 colon and rectum,4–6 small bowel,7,8 ovary,9 and peritoneum (mesothelioma).10,11
Whereas the magnitude of the benefit from CRS-HIPEC for peritoneal metastases is being better defined, treatment-related toxicities are well-known. Large series in the modern era have found a major morbidity rate of 24–34 % and a mortality rate of 2–4 % after CRS-HIPEC.2,5,12–15 Multiple independent predictors of morbidity and mortality have been identified including age, albumin, prior surgery, gastrectomy, operating time, intraoperative transfusion, extent of disease, HIPEC center, primary tumor site, grade, histology, number of anastomoses, presence of ascites, and HIPEC technique.2,5,12,14–16 Many of these variables are not known at the time of preoperative evaluation, when risk assessment is most valuable to both patient and physician for selection of the appropriate treatment. Therefore, we sought to determine the preoperative predictors of major postoperative complications after CRS-HIPEC in a high-volume center and to derive a prediction tool from these preoperative risk factors.
METHODS
Design and Eligibility
This retrospective study investigated predictors of complications after CRS-HIPEC at a single, high-volume HIPEC center. Eligible patients were those undergoing CRS-HIPEC at the University of California, San Diego, from 3 August 2007 to 27 June 2014. Candidates for CRS-HIPEC at our institution are those with peritoneal metastases amenable to complete cytoreduction (CC-0/117) from appendix, colorectal, peritoneal (mesothelioma), ovarian, and small bowel cancers without extraperitoneal metastases and with sufficient physiologic reserve for a major operation. Study data were collected retrospectively from a prospectively maintained database using Research Electronic Data Capture (REDCap) electronic data capture tools.18
Preoperative Details
The preoperative variables collected included age, gender, smoking status, pack-year history, body mass index (BMI), albumin, Charlson Comorbidity Index (CCI, excluding the index malignancy), American Society of Anesthesiology classification of physical health (ASA), primary tumor site, tumor grade, most recent carcinoembryonic antigen (CEA) level, synchronous/metachronous peritoneal metastases, symptoms (including pain, abdominal distension, bowel obstruction, gastrointestinal bleeding or perforation), preoperative imaging evidence of disease and preoperative (imaging) peritoneal cancer index (PCI), prior chemotherapy, prior resection and operative status, and prior surgical score (PSS).19
Operative Details
All the patients underwent CRS followed immediately by HIPEC per the standardized technique performed at our institution.20 The extent of peritoneal disease was assessed at the time of surgery, measured according to the PCI.21 The completeness of cytoreduction (CC) score was used after CC to assess residual, unresected disease.17 Patients with appendiceal, colorectal, and small bowel primary tumors were given 10 mg/L perfusate of intraperitoneal mitomycin C. Patients with mesothelioma and ovarian cancer were dosed with 50 mg/m2 of cisplatin and 15 mg/m2 of doxorubicin. All HIPEC doses were administered during a 90-min perfusion with 42 °C hyperthermia. Two thirds of the total dose were given initially, and the final one third was administered after 45 min, followed by gastrointestinal reconstruction and definitive closure.
The operative variables collected included PCI, operative time, estimated blood loss (EBL), number of visceral resections, number of anastomoses, and CC score. Visceral resection was defined as colon resection, small bowel resection, appendectomy, anatomic hepatic resection (segmentectomy or lobectomy), pancreatectomy, cholecystectomy, hysterectomy and/or oophorectomy, partial or total gastrectomy, or splenectomy.
Postoperative Details
The postoperative variables collected included hospital length of stay (LOS) and complications. Complications were collected up to 60 days after CRS-HIPEC. All complication data were collected from inpatient medical records, outpatient medical records, outside medical records, or direct correspondence with referring physicians. Complications were graded per the Clavien classification.22 The patients with more than one complication had their worst 60-day complication recorded. Major complications were defined as Clavien grade 3 or greater.
Specific complications of interest after CRS-HIPEC also were recorded including enteric leak/fistula defined as anastomotic or bowel leak at the time of reoperation, enterocutaneous fistula, enteric contents from operative or postoperative drains or evidence of enteric contrast leak on imaging, ileus or delayed gastric emptying defined as no bowel function or inability to tolerate oral intake longer than 7 days after surgery, intraabdominal abscess defined as intraabdominal fluid collections with documented or highly suspected infection in those not amenable to sampling or drainage, deep venous thrombosis (DVT) or pulmonary embolus (PE) defined as any new DVT/PE documented by imaging (ultrasound or computed tomography), and blood product transfusion defined as any postoperative transfusion of packed red blood cells, fresh frozen plasma, or platelets.
Statistical Analysis
Univariate logistic regression was performed using preoperative and operative variables to identify predictors of 60-day major complications. Multivariate logistic regression was used to identify preoperative and operative variables with a p value of 0.20 or lower in univariate analysis, which independently predicted 60-day major complications. Multiple multivariate models were analyzed including use of all preoperative variables meeting univariate entry criteria, a reverse stepwise approach with removal of individual variables one by one starting with the highest p value until all variables had a p value lower than 0.10, and age and albumin variables forced into an all-inclusive or stepwise model.
The final model that minimized Akaike’s information criterion (AIC) was selected. Variables in the final model were tested for interaction by examination of interaction terms in the multivariate analysis. The Hosmer and Lemeshow Goodness-of-Fit Test was used to measure the fit of the final multivariate model.
The preoperative predictors from the final multivariate model were used to create individual risk groups comprising every possible combination of the predictors. A receiver operating characteristic (ROC) curve was created from these risk groups to evaluate the overall model by measuring the area under the curve (AUC) compared with an AUC of 0.5. A cutoff between low- and high-risk groups then was selected based on the distribution of the predicted probabilities of major complications, with the Youden Index used to maximize the J statistic.23 A predictive model for 60-day major complications then was created using this low- versus high-risk cutoff, with points assigned to each risk factor corresponding to the rounded odds ratio from the multivariate analysis. Comparison of means between subgroups was performed using Student’s t test for comparison of two groups or analysis of variance (ANOVA) (with Tukey’s post hoc comparison) for comparison of more than two groups.
RESULTS
Patient Baseline and Operative Details
The analysis included 247 CRS-HIPEC procedures performed for 231 patients. The baseline and operative details are listed in Table 1. The ASA class was determined from the clinical record by the anesthesiologist, and the majority of patients were class 3 or higher due to having metastatic (peritoneal) disease.
TABLE 1.
Baseline and operative details
| Variable | n (%) |
|---|---|
| Baseline variables | |
| Median age: years (range) | 53 (20–86) |
| Gender | |
| Male | 117 (47.4) |
| Female | 130 (52.6) |
| Current smoker | 8 (3.2) |
| Median smoking pack-year history: years (range) | 0 (0–50) |
| Median BMI: kg/m2 (range) | 26.4 (18.6–48.0) |
| Median albumin: g/dL (range) | 4.3 (2.4–5.2) |
| CCI: median (range) | 0 (0–8) |
| ASA | |
| 2 | 25 (10.1) |
| 3 | 126 (51.0) |
| 4 | 3 (1.2) |
| NA | 93 (37.7) |
| Primary site | |
| Appendix | 166 (67.2) |
| Colorectal | 51 (20.6) |
| Mesothelioma | 22 (8.9) |
| Ovarian | 5 (2.0) |
| Small bowel | 3 (1.2) |
| Median CEA (ng/ml) | 4.0 (0.2–144.0) |
| Symptomatic | 99 (40.0) |
| Imaging evidence of disease | 212 (85.8) |
| Median preoperative PCI | 6 (0–38) |
| Prior chemotherapy | 111 (44.9) |
| Prior resection | |
| None | 62 (25.1) |
| Yes without prior CRS-HIPEC | 166 (67.2) |
| Yes with prior CRS-HIPEC | 19 (7.7) |
| Operative variables | |
| PCI: median (range) | 14 (0–29) |
| Median operative time: min (range) | 403.5 (196–799) |
| Median EBL: mL (range) | 250 (10–4000) |
| No. of visceral resections: median (range) | 2 (0–10) |
| No. of anastomoses: median (range) | 1 (0–6) |
| CC score | |
| 0 | 199 (80.6) |
| 1 | 36 (14.6) |
| 2 | 11 (4.5) |
| 3 | 1 (0.04) |
| Median LOS: days (range) | 10 (4–45) |
BMI Body mass index, CCI Charlson comorbidity index, ASA American Society of Anesthesiology classification of physical health, NA not available, CEA carcinoembryonic antigen, PCI peritoneal cancer index, CRS-HIPEC cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, EBL estimated blood loss, CC completeness of cytoreduction, LOS hospital length of stay
Complications
Figure 1 illustrates 60-day postoperative complications by Clavien grade (Fig. 1a) and 60-day major complications by complication type (Fig. 1b). Of the 247 patients, 99 (40.1 %) had no 60-day complications. Of the 148 patients (59.9 %) who had 60-day complications, 134 (90.5 % of all the patients with a complication) experienced complications during their inpatient stay after CRS-HIPEC, and 14 (9.5 %) experienced complications after discharge (28 patients also had a complication after discharge that was less severe than their inpatient complication).
FIG. 1.
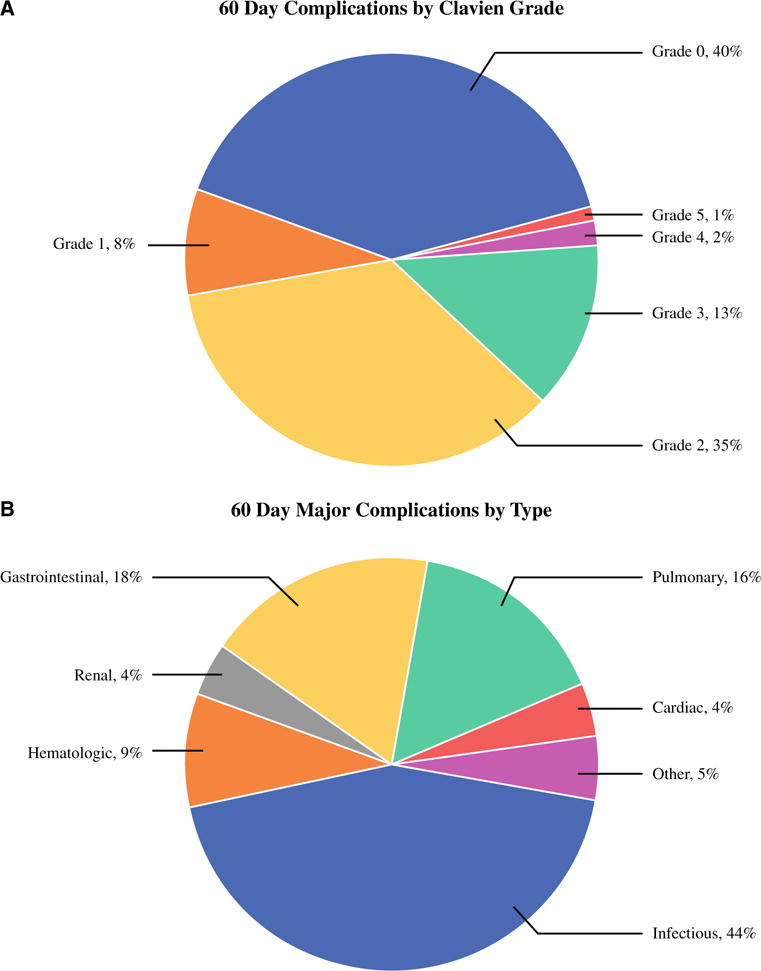
Complications. a The 60-day complications by Clavien grade among all the patients (n = 247). b The 60-day major complications by type among all the major (Clavien grade ≥3) complications (n = 55)
Among the 20 patients (8.1 %) with grade 1 complications, the most common complication was superficial wound infection for 5 patients and mildly increased creatinine for 3 patients. For the 87 patients (35.2 %) with grade 2 complications, the most common complication was postoperative blood product transfusion (29 patients) and prolonged ileus/delayed gastric emptying requiring intravenous nutrition (19 patients, in addition to 5 patients with both complications). A total of 55 major complications were experienced by 41 patients (16.6 %) including grade 3 complications by 33 patients (13.4 %), grade 4 complications by 5 patients (2 %), and grade 5 complications (death) by 3 patients (1.2 %).
The most common single major complication was infected or potentially infected intraabdominal or intrathoracic fluid collection requiring percutaneous drainage, experienced by 26 patients (6 of whom had additional major complications). The three postoperative deaths, all due to pneumonia, were caused by respiratory failure and sepsis (n = 1), sudden death from PE after discharge of a patient with an inferior vena cava filter (n = 1), and sepsis from an intraabdominal abscess (n = 1). Seven patients (2.8 %) returned to the operating room during their postoperative admission. Table 2 shows the 60-day specific complications of any grade.
TABLE 2.
The 60-day specific complications among all the patients (n = 247)
| 60-Day specific complications | n (%) |
|---|---|
| Enteric leak/fistula | 4 (1.6) |
| Ileus or DGE >7 days | 31 (12.6) |
| Intraabdominal abscess | 24 (9.7) |
| Wound infection | 17 (6.9) |
| DVT/PE | 8 (3.2) |
| Blood product transfusion | 47 (19.0) |
DGE Delayed gastric emptying, DVT/PE deep venous thrombosis/pulmonary embolism
Predictors of Major Complications
Uni- and multivariate analyses were performed to identify factors predictive of 60-day major complications (Table 3). Univariate analysis identified CCI higher than 0, symptomatic disease, prior resection status (previous resection with CRS-HIPEC or no history of resections), greater number of visceral resections, and greater EBL to be significant predictors of major complications. Age, BMI, albumin, and preoperative PCI also were tested as categorical variables and not found to be significant predictors of major complications.
TABLE 3.
Predictors of major complications
| Variable (n) | Major complications (%) | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|---|
| OR | p value | OR | p value | ||
| Preoperative variables | |||||
| Overall (247) | 16.6 | ||||
| Age | 1.007 | 0.613 | |||
| Gender | 0.378 | ||||
| Male (117) | 18.8 | ||||
| Female (130) | 14.6 | 0.739 | |||
| Current smoker | 0.533 | ||||
| No (236) | 16.5 | 0.594 | |||
| Yes (8) | 25.0 | ||||
| Smoking pack-year history | 1.010 | 0.613 | |||
| BMI | 1.016 | 0.612 | |||
| Albumin | 0.851 | 0.653 | |||
| CCI | 0.044 | ||||
| CCI 0 (212) | 14.6 | ||||
| CCI >0 (35) | 28.6 | 2.335 | 2.505 | 0.035 | |
| ASA | 0.819 | ||||
| 2 (25) | 0.0 | 0.000 | 0.998 | ||
| 3 (126) | 16.7 | 0.400 | 0.463 | ||
| 4 (3) | 33.3 | Ref | |||
| Primary | 0.521 | ||||
| Appendix (166) | 14.5 | Ref | |||
| Colorectal (51) | 19.6 | 1.443 | 0.378 | ||
| Peritoneum (22) | 18.2 | 1.315 | 0.646 | ||
| Ovary (5) | 40.0 | 3.944 | 0.144 | ||
| Small bowel (3) | 33.3 | 2.958 | 0.383 | ||
| CEA | 1.009 | 0.251 | |||
| Symptoms | 0.024 | ||||
| No (148) | 12.2 | ||||
| Yes (99) | 23.2 | 2.186 | 1.951 | 0.064 | |
| Imaging evidence of disease | 0.188 | ||||
| No (32) | 25.0 | 1.808 | |||
| Yes (212) | 15.6 | ||||
| Preoperative PCI | 1.014 | 0.505 | |||
| Prior chemotherapy | 0.221 | ||||
| No (136) | 14.0 | 0.657 | |||
| Yes (111) | 19.8 | ||||
| Prior resections | 0.021 | 0.046 | |||
| None (62) | 24.2 | 2.330 | 0.026 | 2.087 | 0.063 |
| Resection without CRS-HIPEC (166) | 12.0 | Ref | Ref | ||
| Resection with CRS-HIPEC (19) | 31.6 | 3.369 | 0.027 | 3.209 | 0.036 |
| Operative variables | |||||
| No. of visceral resections | 1.434 | 0.002 | |||
| No. of anastomoses | 1.349 | 0.067 | |||
| EBL | 1.001 | 0.030 | |||
| PCI | 1.011 | 0.702 | |||
| CC score | 0.966 | ||||
| 0 (199) | 16.1 | Ref | |||
| 1 (36) | 19.4 | 1.260 | 0.618 | ||
| 2 (11) | 18.2 | 1.160 | 0.854 | ||
| 3 (1) | 0.00 | 0.000 | 1.000 | ||
OR Odds ratio, BMI body mass index, CCI Charlson Comorbidity Index, ASA American Society of Anesthesiology classification of physical health, CEA carcinoembryonic antigen, PCI peritoneal cancer index, CRS-HIPEC cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, EBL estimated blood loss, CC completeness of cytoreduction
Additional variables analyzed for their ability to predict major complication in the univariate analysis included tumor grade/differentiation, synchronous versus metachronous peritoneal metastases, number of preoperative chemotherapy cycles, preoperative chemotherapy treatment response, number of prior tumor resections, number of prior abdominal surgeries, and PSS. None of these variables were significant predictors of major complications.
Multivariate logistic regression was performed using preoperative and operative variables separately. Of the operative factors predictive of major complications in the univariate analysis, only the number of visceral resections was significant in the multivariate analysis [hazard ratio (HR) 1.434; p = 0.002]. Subgroup analysis of individual visceral resections and the associated risk of major complications was performed using Pearson’s Chi square test. Pancreatectomy, splenectomy, cholecystectomy, partial and total colectomy, appendectomy, partial gastrectomy, small bowel resection, and hysterectomy and/or oophorectomy were not significantly associated with a higher risk for major complications. However, anatomic hepatic resections were significantly associated with major complications (40.0 vs. 18.5 % major complications with anatomic hepatic resection versus nonanatomic or no hepatic resection, respectively; p = 0.042). Very few patients (n = 10) had ostomies created as part of their CRS-HIPEC, and although none of these patients had major complications, this rate did not differ significantly from those without ostomy creation (17.3 % major complication rate in those without ostomies; p = 0.150).
The most predictive multivariate model using preoperative variables (AIC = 51.66) identified CCI higher than 0, symptoms, and prior resection status (no resection or resection with prior CRS-HIPEC) as independent predictors of major complications. There were no significant interactions among these variables. The Hosmer and Lemeshow Test was not significant (p = 0.117), indicating that the model was adequately fit. From all possible combinations of these three variables (CCI, symptoms, and prior resection status), there were 12 possible risk groups from which predicted and observed rates of major complications were derived (Supplementary Fig. 1). An ROC curve was created from these risk groups (Supplementary Fig. 2), which had an AUC >0.5 (p<0.001). A high-risk cutoff (>10 % predicted probability of a 60-day major complication) was established as any patient with the presence of any risk factor (CCI> 0 and/or any symptom and/or no prior resection or prior resection with CRS-HIPEC). This allowed the risk groups to be divided into low- and high-risk groups with 0–10 and 10–60 % risk of 60-day major complications, respectively. A tool predictive of major complications was created from this model (Table 4).
TABLE 4.
Final predictive model of major complications with two risk groups
| Preoperative factor | Points |
|---|---|
| CCI | |
| 0 | 0 |
| >0 | 3 |
| Symptoms | |
| No | 0 |
| Yes | 2 |
| Prior resection | |
| Yes without CRS-HIPEC | 0 |
| None | 2 |
| Yes with CRS-HIPEC | 3 |
| Total | 8 (max) |
|
| |
| Group | Risk of major complication |
|
| |
| 0 | Low risk (0–10 %) |
| 2–8 | High risk (10–60 %) |
CCI Charlson Comorbidity Index, CRS-HIPEC cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, max maximum
We further investigated subgroups based on the predictive preoperative variables. The patients with a CCI higher than 0 did not differ significantly in number of anastomoses or resections or EBL from those with a CCI of 0. The patients with symptoms had greater numbers of anastomoses (1.0 vs. 0.77; p = 0.054), a greater number of visceral resections (2.07 vs. 1.64; p = 0.017), and greater EBL (538 vs. 301 mL; p = 0.001) than those without symptoms. Those with no prior resections had a greater number of anastomoses (1.08 vs. 0.73; p = 0.0038), a greater EBL (543 vs. 346 mL; p = 0.017), and a trend of more visceral resections (2.13 vs. 1.72; p = 0.113) than those with prior resections (without CRS-HIPEC).
DISCUSSION
This study found that 60-day major complications after CRS-HIPEC from a high-volume HIPEC center occurred for 16.6 % of the patients and that CCI, the presence of symptoms, and prior resection status were independent preoperative predictors of major complications. From these variables, we identified a group of patients with no high-risk factors (39 % in this study) who were at a lower than average risk for major complications after CRS-HIPEC. Although intraoperative variables also were predictive of major complications in the univariate analysis (number of visceral resections and EBL), using only variables known at the time of preoperative evaluation has the most utility in risk assessment.
A simple two-risk-group predictive tool also maximizes clinical utility. Validation with larger multi-institutional data sets may allow adequate discrimination of additional risk groups from the current model, but the current data set most accurately defines two-risk groups. We believe this tool may be useful in specifically outlining the risks of CRS-HIPEC, particularly those in the low-risk group, to better inform patients, families, or referring physicians who have understandable concerns about CRS-HIPEC-related morbidity and mortality. Furthermore, we found that additional risk factors for complications (e.g. age, albumin, BMI, preoperative imaging PCI) were not predictive in our model, suggesting that outside of extremes in these variables, they can be deemphasized in risk assessment for a patient. Other postoperative complication risk assessment tools, such as the National Surgical Quality Improvement Program (NSQIP) risk calculator,24 are not CRS-HIPEC specific and allow only one procedure to be risk assessed, whereas CRS-HIPEC typically includes multiple simultaneous procedures.
The rate of major complications for patients after CRS-HIPEC in the current study was similar to or lower than the rate in most other large published series.2,5,13,14,16 This major complication rate is lower than that for other large, open abdominal surgical oncology procedures (17 % after hepatectomy for colorectal liver metastases,25 27 % after pancreaticoduodenectomy,26 28 % after gastrectomy27). In addition, two publications from the American College of Surgeons NSQIP have reported the rate for NSQIP-defined complications as 31–32.9 % after CRS-HIPEC.12,28
Some advantages of our series over the aforementioned reports include ability to determine the severity and etiology of individual complications and measurement of complications beyond 30 days. The most common major complications from other large studies include bleeding, sepsis, enteric leak, wound infection, and urinary tract infection.5,12,16,28 In our series, abscess was the most common major morbidity, whereas grade 3 bleeding or greater, sepsis, enteric leak, wound infection, and urinary tract infections were relatively uncommon. This heterogeneity may be explained by differences in the grading and recording of major complications (Clavien score in our series vs. NCI Common Terminology Criteria for Adverse Events or NSQIP definitions in other studies), differences in the threshold for percutaneous drainage of potentially infected fluid collections, and differences in patient populations or operative techniques. The enteric leak rate in our series also was much lower (1.6 %) than in other studies (4.5–15 %),4,5,16 which may have resulted in the low mortality rate also reported (1.2 %) because this complication is a common cause of postoperative mortality.13
Given that prior studies have shown a correlation between the number of anastomoses and increasing major morbidity and mortality, we tried to limit the number of bowel resections or excisions (the median number of anastomoses was 1 in our series) at our center. Patients with extensive bowel serosal involvement also are often excluded from consideration for CRS-HIPEC because they typically are not candidates for complete CC.
Multiple preoperative and operative variables are reported to predict complications after CRS-HIPEC.2,5,12,14–16 Preoperative variables are known during operative planning and discussion, and complication prediction models that use only preoperative variables are more clinically useful and do not significantly degrade the predictive ability compared with models that include operative variables.29 Preoperative variables predictive of major complications from other large series include PSS, number of prior operations, age, albumin, and primary tumor site.2,12,15 We did not find these variables to be predictive of major complications in our series. In this series, CCI was predictive, which was somewhat expected because it is a valid risk assessment tool30 that has been independently validated in cancer31 and surgical32 populations. Symptoms and prior resection status were somewhat unexpected predictors of major complications. Patients with symptoms and no prior resection had higher numbers of visceral resections and greater EBL than those with no symptoms or with a prior resection, which may explain their higher rates of major complications because a greater number of visceral resections and greater EBL were operative predictors of major complications. Patients with no prior resections required at least one visceral resection of their primary tumor, and those with symptoms required more extensive CC than those without symptoms.
The limitations of the current study included the nuances of a single-center series. Our findings also have not been validated to date in an independent data set. The high-risk group in our model included patients with both average and high risk of major complications, limiting the utility of the high-risk group but preserving the clinical utility of the low-risk group. Finally, determining the precise etiology of complications, whether from CRS or HIPEC, was not possible in this series because all the patients received both procedures. However, a randomized controlled trial examining gastric cancer treated by CRS alone versus CRS with HIPEC found similar major morbidity in both groups, suggesting that HIPEC may not add significant morbidity to CRS.33
In conclusion, CRS-HIPEC can be performed with acceptable morbidity and mortality rates at high-volume centers, and a low-risk group can be predicted based on preoperative variables. Use of a predictive tool for major complications from these preoperative variables may be useful for informed consent of patients, particularly by defining a low-risk group, if independent validation can be obtained.
Supplementary Material
Acknowledgments
This study was supported by a CTRI Grant (1UL1RR031980-01).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-5012-3) contains supplementary material, which is available to authorized users.
DISCLOSURES None of the authors of this manuscript have any disclosures or relevant conflicts of interest to declare.
References
- 1.Austin F, Mavanur A, Sathaiah M, Steel J, Lenzner D, Ramalingam L, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–93. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 3.Lord AC, Shihab O, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur J Surg Oncol. 2015;41:396–9. doi: 10.1016/j.ejso.2014.08.476. [DOI] [PubMed] [Google Scholar]
- 4.Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 5.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 6.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ., III Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–62. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Shen P, Stewart JH, Russell GB, Levine EA. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. Am Surg. 2013;79:644–8. [PMC free article] [PubMed] [Google Scholar]
- 8.van Oudheusden TR, Lemmens VE, Braam HJ, et al. Peritoneal metastases from small bowel cancer: results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery. 2015;157:1023–7. doi: 10.1016/j.surg.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Bakrin N, Classe JM, Pomel C, Gouy S, Chene G, Glehen O. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J Visc Surg. 2014;151:347–53. doi: 10.1016/j.jviscsurg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 11.Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:1686–93. doi: 10.1245/s10434-014-3978-x. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett EK, Meise C, Roses RE, Fraker DL, Kelz RR, Karakousis GC. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol. 2014;21:1494–500. doi: 10.1245/s10434-013-3223-z. [DOI] [PubMed] [Google Scholar]
- 13.Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20:4224–30. doi: 10.1245/s10434-013-3145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polanco PM, Ding Y, Knox JM, Ramalingam L, Jones H, Hogg ME, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol. 2015;22:1673–9. doi: 10.1245/s10434-014-4111-x. [DOI] [PubMed] [Google Scholar]
- 15.Macri A, Arcoraci V, Belgrano V, Caldana M, Cioppa T, Costantini B, et al. Short-term outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: preliminary analysis of a multicentre study. Anticancer Res. 2014;34:5689–93. [PubMed] [Google Scholar]
- 16.Desantis M, Bernard JL, Casanova V, Cegarra-Escolano M, Benizri E, Rahili AM, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) Langenbeck’s Arch Surg. 2015;400:37–48. doi: 10.1007/s00423-014-1253-z. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43:S15–25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner JM, Tobin L, Heavey SF, Kelly KJ, Roeland EJ, Lowy AM. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;22:1716–21. doi: 10.1245/s10434-014-3985-y. [DOI] [PubMed] [Google Scholar]
- 21.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98:228–31. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 23.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 24.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–42. e831–3. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–52. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 27.Selby LV, Vertosick EA, Sjoberg DD, Schattner MA, Janjigian YY, Brennan MF, et al. Morbidity after total gastrectomy: analysis of 238 patients. J Am Coll Surg. 2015;220:863–71. e862. doi: 10.1016/j.jamcollsurg.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ, Carmichael JC, Mills SD, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the American College of Surgeons National Surgical Quality Improvement Program. JAMA Surg. 2013;149:170–5. doi: 10.1001/jamasurg.2013.3640. [DOI] [PubMed] [Google Scholar]
- 29.Dahlke AR, Merkow RP, Chung JW, Kinnier CV, Cohen ME, Sohn MW, et al. Comparison of postoperative complication risk prediction approaches based on factors known preoperatively to surgeons versus patients. Surgery. 2014;156:39–45. doi: 10.1016/j.surg.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol. 2012;65:924–33. doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Kork F, Balzer F, Krannich A, Weiss B, Wernecke KD, Spies C. Association of comorbidities with postoperative in-hospital mortality: a retrospective cohort study. Medicine. 2015;94:e576. doi: 10.1097/MD.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–81. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


