Abstract
This article demonstrates a new smartphone-based reusable glucose meter. The glucose meter includes a custom-built smartphone case that houses a permanent bare sensor strip, a stylus that is loaded with enzyme-carbon composite pellets, and sensor instrumentation circuits. A custom-designed Android-based software application was developed to enable easy and clear display of measured glucose concentration. A typical test involves the user loading the software, using the stylus to dispense an enzymatic pellet on top of the bare sensor strip affixed to the case, and then introducing the sample. The electronic module then acquires and wirelessly transmits the data to the application software to be displayed on the screen. The deployed pellet is then discarded to regain the fresh bare sensor surface. Such a unique working principle allows the system to overcome challenges faced by previously reported reusable sensors, such as enzyme degradation, leaching, and hysteresis effects. Studies reveal that the enzyme loaded in the pellets are stable for up to 8 months at ambient conditions, and generate reproducible sensor signals. The work illustrates the significance of the pellet-based sensing system towards realizing a reusable, point-of-care sensor that snugly fits around a smartphone and which does not face issues usually common to reusable sensors. The versatility of this system allows it to be easily modified to detect other analytes for application in a wide range of healthcare, environmental and defense domains.
Keywords: electrochemical biosensors, glucose, smartphone, glucometer
1. Introduction
Diabetes mellitus is a serious global disease that affected nearly 8.8% (415 million) of adults worldwide in 2015 (“International Diabetes Foundation,” 2015), with projections to nearly 522 million by 2030 (Guariguata et al., 2011). The financial effect of this disease is immense, with most countries spending between 5–20% of total healthcare expenditure on diabetes and related health issues (Dieleman et al., 2016). Unfortunately, there is no cure, and as a result, diabetic patients must carefully manage their disease via periodic measurement of blood glucose levels (Guerci et al., 2003; Kirk and Stegner, 2010; Sönksen et al., 1978). Consequently, invasive, minimally invasive (Gilligan et al., 2004; Valdés-Ramírez et al., 2014; Wang et al., 2005; Zimmermann et al., 2003), and non-invasive (Vashist, 2012) glucose monitoring approaches have been a research topic for decades (Imani et al., 2016; Newman and Turner, 2005; Wang, 2008). In an ideal world, monitoring should be accomplished non-invasively; while there has been recent progress towards such an approach (Abellán-Llobregat et al., 2017; Alexeev et al., 2004; Bandodkar et al., 2015b; Tierney et al., 2001), challenges remain towards successful commercialization. Thus, most diabetic patients monitor their glucose levels by periodically extracting a small amount of blood by pricking a fingertip with a lancet, and transferring extracted blood to a glucose-sensitive test strip that is read via a handheld electrochemical glucometer. This method is both accurate, due to excellent selectivity and sensitivity of the functionalized test strips alongside electrochemical detection, and low-cost, since test strips can be economically screen printed at scale. However, as glucose levels need to be measured regularly, diabetic patients must carry the device and the test strips with them throughout the day. This adds additional responsibility onto the patient, and forgetting to carry the glucometer can potentially lead to sub-optimal diabetes control, and in some cases even more serious health issues (Ong et al., 2014; Patton, 2015). Thus, efforts should be made to facilitate more convenient monitoring of blood glucose levels in order to help improve healthcare outcomes.
With billions of smartphones around the globe, these devices have become an inseparable part of daily life and thus, integrating a blood glucose meter within a smartphone would help reduce the chances of a diabetic patient forgetting to carry their glucometer with them. In addition, the ability to autonomously store, process, and send blood glucose reading from the phone to a care provider or cloud service via the built-in wireless functionality is an attractive additional benefit. As a result, there have been several recent reports on integrating glucose measurement functionality into a smartphone-compatible platform (Dantu et al., 2014; “Dario ® Blood Glucose Monitoring System User Guide,” 2014; Jendrike et al., 2017; Keith-hynes et al., 2014; Ramchandani and Heptulla, 2012; Wu et al., 2015). However, many such approaches rely on colorimetric glucose detection, which unfortunately lacks in sufficient accuracy to support therapeutic intervention (e.g., administration of insulin injections) (Jendrike et al., 2017; Wu et al., 2015). Instead, electrochemical glucose detection, which is sufficiently accurate to be employed in existing commercial glucometers, has also been proposed for integration with smartphone platforms (“Dario ® Blood Glucose Monitoring System User Guide,” 2014). However, all prior-art requires separate dongles or test strips (Sun et al., 2016), which is not a fully-integrated solution, and thus does not achieve the desired level of increase in convenience. To make a non-negligible difference in use case, eliminating the need for patients to carry a separate glucometer device, whether implemented as a dongle or an altogether separate device, is necessary.
The key challenge to integrate electrochemical glucose sensors into smartphone platforms is convenient re-usability: such sensors operate by measuring the output of electrochemical reactions catalyzed by glucose-specific enzymes via functionalized electrodes, and, unfortunately, such functionalization is destroyed after only a few uses due to gradual leaching of sensor reagents (Bandodkar et al., 2015a). Hence, most prior-art solutions rely on screen-printing functionalized electrodes on paper strips that are inserted into the glucometer device (or smartphone dongle), and disposed of afterwards (Bandodkar and Wang, 2014; Kim et al., 2017; Ramchandani and Heptulla, 2012).
In this work, we present the design of a glucose sensing system that features integration of electrochemical electrodes directly onto the smartphone platform, eliminating the need for a separate glucometer dongle, while still leveraging the electronics and processing capabilities of the smartphone. Instead of functionalizing the permanently-attached electrodes during manufacturing for single-use applications, enzymes are applied to the electrodes on-demand by the end user via a stylus containing magnetic enzyme-loaded carbon composite pellets, enabling re-use of the electrodes.
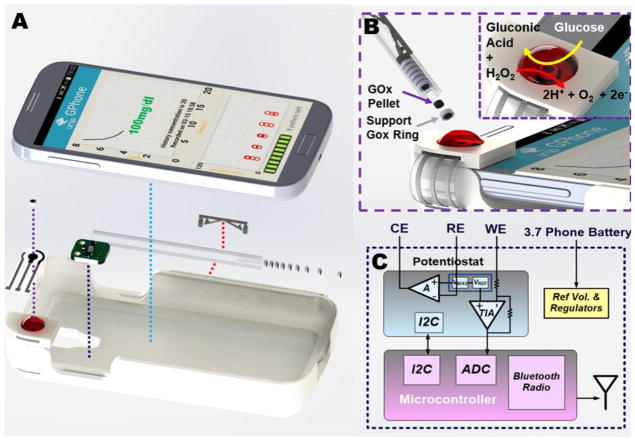
In order to demonstrate this concept, a custom smartphone case was built that comprised the permanent bare (without enzyme) three-electrode biosensor, a Bluetooth Low-Energy (BLE)-based wireless transceiver and potentiostat, and a stylus slot, all shown in Fig. 1A. The case snugly fits around the phone, adding negligible weight and size. Testing is accomplished by opening a custom-built application, dispensing a magnetic enzyme pellet onto the magnetic sensor surface as shown in Fig. 1B, and dropping a blood sample onto the sensor; within a few seconds, the blood glucose concentration is measured via the integrated potentiostat (Fig. 1C), and the results are displayed on the screen of the smartphone. Finally, the employed pellet is discarded and the sensor surface cleansed to reobtain a bare transducer surface for the next test with a fresh enzyme pellet, all with negligible test-to-test contamination.
Fig. 1.
(A) Exploded view of the smartphone-based glucose sensing system showing the smartphone case, permanently-attached passive sensor strip, enzyme-loaded pellet, pellet-dispending stylus, and electronic readout circuits. (B) Dispensing carbon-composite pellets loaded with GOx onto the bare carbon working electrode for electrochemical analysis. (C) Block diagram of the electronic readout circuits.
The following sections discuss the design rationale for the phone case and stylus, enzyme pellets, wireless potentiostat circuits, smartphone application, and the seamless integration of these to realize a practical standalone smartphone-based glucose sensor.
2. Materials and Methods
2.1 System design
The overarching goal of this work is to integrate glucose sensing functionality into a smartphone without requiring an external electronic dongle. While reusable glucose sensors are in principal possible to integrated into a phone, their long-term use is extremely limited due to gradual leaching of sensor reagents leading to erratic responses and carryover effect from previous tests.
In this work, we address this challenge through the development of a two-part glucose sensor. The first part comprises a permanently-installed passive sensor strip consisting of a conventional three electrode electrochemical measurement system, though in this case with the working electrode being carbon with no permanent functionalization. The second part involves a biocatalytic pellet that imparts selectivity towards glucose on top of the working electrode. The pellet, which is a small, solid, and circularly packed disc, contains the active sensor components such as glucose oxidase and rhodium within a carbon composite. A functional glucose sensor is realized when the replaceable pellet is applied to the permanent passive sensor strip. After each test, the enzyme-loaded pellet is replaced with a fresh active layer for the next test. By doing so, major issues relating to leaching and carryover effect are obviated.
2.2 Smartphone case design
Ideally, the un-functionalized electrochemical electrodes, electronic instrumentation, and stylus would all be integrated directly into the housing of a smartphone. However, this requires intimate coordination with the smartphone manufacturing process; instead, a custom-designed phone case was developed to more easily demonstrate the proposed concept.
The phone case was designed using CAD software (Solidworks®, Dassault Systemes Solidworks Corp., France), and fabricated using an Acrylonitrile-Butadiene-Styrene based 3D printer (Mojo Desktop Printer, Stratasys, Eden Prairie, Minnesota) for prototyping purposes. The case could also be adopted to more affordable injection molding that is commonly used for smartphone accessories commercialized at scale. The case was carefully designed to include dedicated housing for the permanently-installed passive sensor strip, BLE electronics, and the pellet dispensing stylus. The slot where the sensor strip is located has a housing for a neodymium magnet (diameter = 2mm; height = 1mm) to facilitate reliable placement of magnetic pellets in the same location for each test. The phone case also has a pair of cylindrical magnets (diameter = 1mm; height = 0.5mm) affixed to its side wall for holding the stylus.
2.3 Pellet-dispensing stylus design
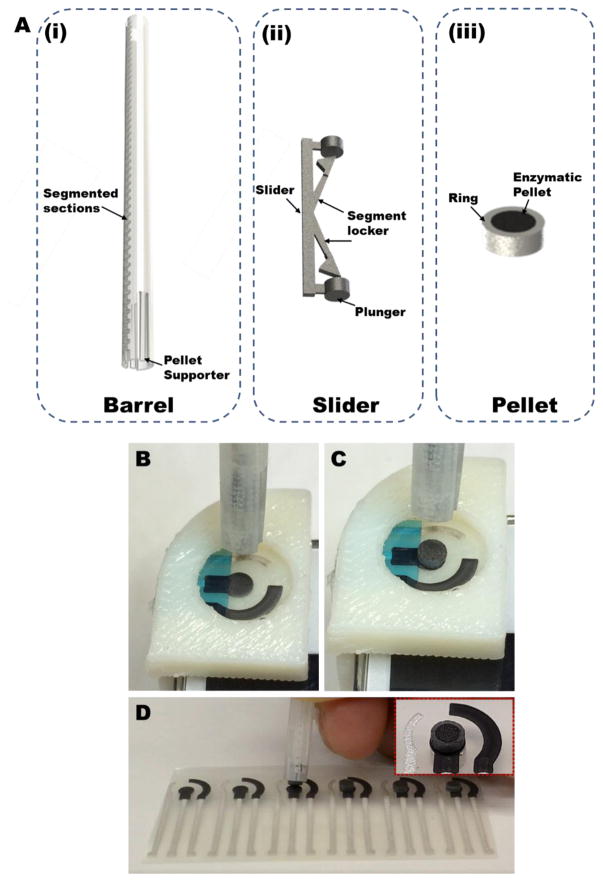
A custom-built 3D printed stylus has been designed to reproducibly dispense enzymatic pellets on top of the working electrode. The stylus consists of three main components: barrel (Fig. 2A(i)), slider (Fig. 2A(ii)) and pellet (Fig. 2A(iii)). The pellets are composed of soft, tacky materials, and hence reproducible release of single pellet from the stylus without damaging the pellet is challenging. Therefore, the pellet mixture was packed within cylindrical 3D printed hollow casings (Fig. 2A(iii)). Such a configuration served three purposes: to facilitate easy release of pellets from the stylus without damaging them, to control the amount of material in each pellet, and to ensure no residual pellet material attaches to the inner walls of the stylus to avoid potentially clogging which may hinder future pellet dispensing. The stylus consists of a hollow graduated cylindrical barrel (Fig. 2A(i)) wherein a stack of (~20) pellets (diameter=2.65mm, height=1mm) can be stored (Reviewer #3; Comment #5). The stylus also includes a slider (Fig. 2A(ii)) to push the pellets out of the barrel and onto the sensor. The slider is able to achieve its function due to the plunger and the segment locker. The slider can be moved by the user in steps along the segmented barrel, where each segment is equal to the height of a single pellet. The segment locker imparts a stepper motion to the slider that enables the user to dispense a single pellet each time.
Fig. 2.
(A) The custom-built 3D printed stylus designed to reproducibly dispense enzymatic pellets on top of the working electrode comprises of three main components: barrel (A(i)), slider (A(ii)) and pellet (A(iii)). (B, C, and D) Dispensing the enzymatic pellets on top of the working electrode.
In addition to controlled release of pellets, controlling the location where the pellet is attached to the sensor is also critical, as misalignment can lead to erratic response. Also, the pellet should bond desirably well so that there is no creeping of the sample between the volume underneath the pellet and the bare working electrode. These conditions were met by including nickel microparticles within each pellet in order to impart magnetic properties in them, followed by placing a permanent neodymium magnet (diameter = 1 mm; height = 0.5 mm) underneath the working electrode. By doing so, the pellet released from the stylus is attracted only towards the working electrode, and the magnetic attraction enables firm attachment of the pellet to the working electrode. Factors such as the size and type of the magnet and the amount of nickel in each pellet was considered to obtain a system wherein the pellet selectively and firmly attaches itself to the working electrode, but with an attraction force that is sufficiently weak to facilitate easy removal of the pellet after each test without leaving any residue on the working electrode. (Reviewer#3; Comment #1) Supplemental video 1 shows the reproducible dispensing of single pellets from the stylus, and illustrates the ease of using the system.
2.4 Formulation of enzyme-based magnetic pellets
Three different pellet compositions (P1 to P3) were tested for maximizing sensor stability under ambient conditions. The constituents for each composition, as shown in table 1, were thoroughly mixed with a mortar. All three compositions include magnetic materials to facilitate a simple and reliable pellet placement procedure.
Table 1.
Composition of various enzyme-loaded carbon composite pellets.
| Composition Type | Chemical Constituent | Weight (in mg) |
|---|---|---|
| P1 | Glucose oxidase | 30 |
| Trehalose | 30 | |
| Rh-C | 27 | |
| Nickel | 121 | |
| Graphite | 122 | |
| Silicon oil | 135 | |
| P2 | Glucose oxidase and Trehalose (1:1 wt ratio) lyophilized by first dissolving in PBS buffer (pH 7.0) | 60 |
| Rh-C | 27 | |
| Nickel | 121 | |
| Graphite | 122 | |
| Silicon oil | 135 | |
| P3 | Glucose oxidase and Trehalose (1:1 wt ratio) lyophilized by first dispersing in polyethylene glycol (60wt% in water). | 233 |
| Rh-C | 27 | |
| Nickel | 121 | |
| Graphite | 122 | |
| Silicon oil | 135 |
Each composition was firmly packed in a hollow cylindrical tube to form the active portion of the working electrode, while an external Ag/AgCl electrode and platinum wire was utilized as the reference and counter electrode, respectively. For benchtop testing and characterization, a stainless-steel wire was inserted into the cylindrical electrodes at one end to serve as an electrical contact while the other end of the cylinder defined the active area of the electrode. The active side of the electrodes were delicately polished with a weighing paper to obtain a smooth uniform surface. The electrodes were stored at ambient room conditions. All experiments were conducted at ambient temperature (~25 °C) using a benchtop potentiostat (CH Instruments, model 630C) at 0.4V (vs. Ag/AgCl). (Reviewer #1; Comment #3; Reviewer #3; Comment #2) The sensor relies on the selective oxidation of glucose to produce gluconic acid and hydrogen peroxide. Rh-C acts as a catalyst and oxidizes the H2O2 to generate electrons and oxygen. The reaction mechanism is shown in Fig. 1B. (Reviewer #3; Comment #2) After each characterization test, the active side of the electrodes were cut transversely by a width of approximately 1mm, and the excised part was discarded to obtain a fresh electrode surface for the next test. The stability study comprised of triplicate tests conducted periodically for each electrode variant.
2.5 Design of wireless potentiostat
A Texas Instruments LMP91000 Analog Front End (AFE) was used as a potentiostat to drive the electrochemical biosensor to 0.4V, and read out the corresponding current (i.e., constant-potential amperometry). The output of the LMP91000 can, in principal, be directly digitized by analog-to-digital converters readily available internal to most smartphones. However, in this implementation we did not have access to smartphone internals, and thus instead we developed a custom printed circuit board (PCB) featuring a Texas Instruments CC2541 BLE System-on-Chip for sensor digitization, processing, and communication of sensed data to the smartphone. A block diagram of the PCB is shown in Fig. 1C, which occupied 1.8cmx1.9cm. The CC2541 was loaded with firmware that set configuration registers on the LMP91000 via I2C. A Johanson Technology 2.45GHz antenna (2450AT42A100) and impedance matched balun (24550BM15A0002) were employed for wireless transmission. The PCB was powered directly from the battery of the smartphone, with on-board low-drop out regulators LP5907 and LM4120 respectively providing the main DC voltage supply and the reference voltage for the instrumentation circuitry.
2.6 Development of Android-based smartphone application
To provide the user with a friendly interface, we built an Android-based smartphone application to display blood glucose concentration in real time. The application also has the ability to log measurements.
The application launches with a welcome page with application name: GPhone Glucose Meter. It starts a background thread that scans supported BLE devices and automatically establishes a connection. Upon achieving a successful connection, the welcome page is hidden and the application starts to communicate with the BLE device. If no wireless connection could be obtained, a message is displayed to inform the user.
The communication rules between the application and the BLE device is based on Universally Unique Identifier (UUID), which enables every object in a distributed system to be distinguishable from the others without the help of a central controller. In this way, the application only needs to know the UUIDs that it should write commands to and read data from. The following table shows the UUIDs employed in the present design:
| Action | UUID | Content |
|---|---|---|
| Write | 00002902-0000-1000-8000-00805f9b34fb | Empty |
| Read | 0000fff1-0000-1000-8000-00805f9b34fb | High digits byte data |
| 0000fff2-0000-1000-8000-00805f9b34fb | Low digits byte data |
The application calculates the value of the current with a group of high and low digits (byte) data. As communication begins, another thread that holds the UI display is started. There is also a handler and a timer inside this thread. The handler listens to three messages:
| Message ID | Condition | Action |
|---|---|---|
| 0x123 | A current value is received | Plot a data point |
| 0x124 | 5 seconds since last current value received | Show glucose concentration |
| 0x125 | 28 seconds since last current value received | Update history |
The background thread sends a calculated current value in a message with ID 0x123 to this handler. The timer increases a counter after each second. The handler resets the counter so that the counter saves the number of seconds since last current value was received.
The layout of the UI is shown in Fig. 1A and consists of three blocks. The upper part plots the current value versus time since a round of measurement started. It visualizes how the current lands in a stable value at the end of a 20-second measurement. After one round of measurement finishes and the counter reaches 5, the timer sends a message with ID 0x124 to the handler. Then, the handler renders the glucose concentration in the plot area. Based on the measured current value at time t=10 seconds, we place the glucose concentration at upper or lower part in the plot area to avoid overlapping with the curve. The glucose concentration text has different colors in different range according to the following table (units in mg/dL).
| C <= 60 or C > 125 | Red (Danger) |
| 110 < C <= 125 or 60 < C <= 70 | Orange (Warning) |
| 70 < C <= 110 | Green (Safe) |
When the counter reaches 28, the timer sends a message with ID 0x125 to the handler. The handler records the glucose concentration result in a log file. The logged results are shown in the lower part of the interface, where each result is represented as a white circle. The maximum value on the Y axis of the history graph changes automatically so that all the data points can fit within. When the user touches a white circle, a pop up window reveals the corresponding concentration value and time of the record. The canvas is colored red, orange, and green according to the definitions in the table above so that each data point sits in the region with its corresponding color. This part visualizes the distribution of glucose concentration over time. Finally, the bar at the bottom shows the remaining number of pellets in the dispenser.
3. Results & Discussion
3.1 Enzymatic pellet composition and testing
In our previous work, we explored a similar 2-part glucose sensing approach where the user draws the active sensor layer onto a sensor strip using a roller pen filled with enzymatic ink (Bandodkar et al., 2015a). However, there are certain limitations to the aqueous-media-based enzymatic ink approach. For example, evaporation of aqueous media, long-term stability of enzyme and other reagents, and reproducibility in applying a fixed amount of active sensor material to the sensor strip are all serious limitations. In the present work, we overcome these shortcomings by developing a silicon oil-based solid enzymatic pellet system. Such a system has several attractive attributes: the enzyme stability is much higher in a hydrophobic environment than in aqueous media; each pellet has a fixed quantity of active sensor reagents and hence the pellet approach has better sensor response reproducibility than the previously explored enzymatic ink route; evaporation, which is a major problem for ink-based system, is not an issue for the pellets.
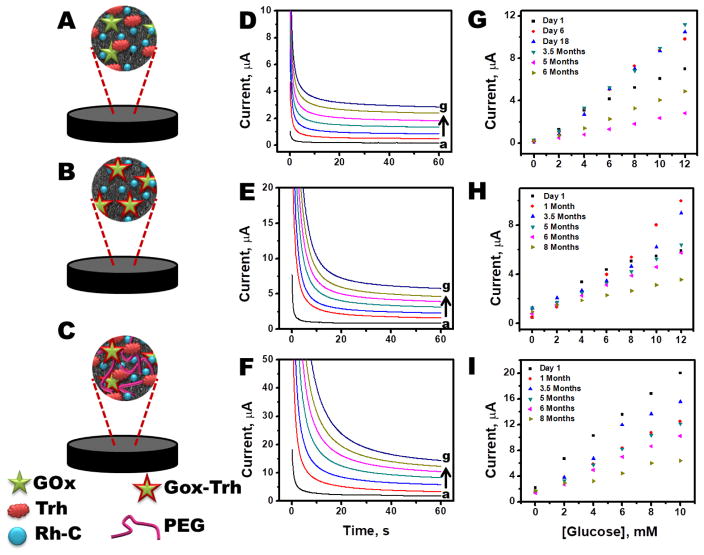
Three different routes for pellet preparation were explored to obtain the optimal configuration. A typical pellet comprised of glucose oxidase (for selectivity), rhodium on carbon (for catalytic oxidation of hydrogen peroxide), trehalose (for enzyme stability), graphite (for conductivity), nickel (for magnetic attachment) and silicon oil (binder). It has been well reported that an enzyme’s activity relies on its three dimensional conformation, and that activity deteriorates chiefly due to increased collisions with solvent molecules. Polyols, such as, trehalose, strongly interact with the enzyme and protect the enzyme from colliding solvent molecules, thus enhancing its stability (Combes and Monsan, 1984; Colaco et al., 1992)). (Reviewer#3; Comment #3) Nickel nanoparticles were loaded within the pellets to impart magnetic properties to the pellets. Different methods of enzyme stabilization were carried out wherein the trehalose was mechanically mixed (pellet composition P1), lyophilized with the enzyme dissolved in phosphate buffer (pellet composition P2), or lyophilized with the enzyme dissolved in polyethylene glycol suspension (pellet composition P3). The stability of each pellet composition was tested for 8 months, as illustrated in Fig. 3. For each pellet composition, the relative standard deviation (R.S.D.) of the measured current was lower than 15%. (Reviewer#1; Comment #2) An underlying reason for P1 to show poorer stability than P2 and P3 could be attributed to mechanical mixing of the trehalose within the mixture. In the case of P1, only a fraction of the trehalose is in close proximity of the enzyme to form a stabilizing layer around it and protect it from denaturing. However, in case of P2, the stabilizer, trehalose, was first dispersed with the enzyme solution so that it could form a protective layer around the enzyme, and then it was lyophilized to obtain a dry powder. PEG, which can also act as a stabilizer, was utilized as an additional stabilizer while preparing P3. However, the resulting lyophilized powder was quite sticky, and thus easy dispersion to obtain P3 was challenging. Hence, the sensor response for P3 varied more than P2 due to possible inhomogeneity of enzyme dispersion in P3. (Reviewer#3; Comment #3) As a result of these issues, composition P2 was observed to offer the highest stability with highest reproducibility over the course of the study. Therefore, composition P2 was employed for all further studies.
Fig. 3.
(A) Schematic illustrating different components of pellet composition (A) P1, (B) P2 and (C) P3. Typical chromoamperometric response for increasing concentration of glucose for (D) P1, (E) P2 and (F) P3. Calibration plots acquired during stability of study for (G) P1, (I) P2 and (J) P3. In all cases highest R.S.D. <=15%. (Reviewer #1; Comment #2)
3.2 Smartphone sensor testing
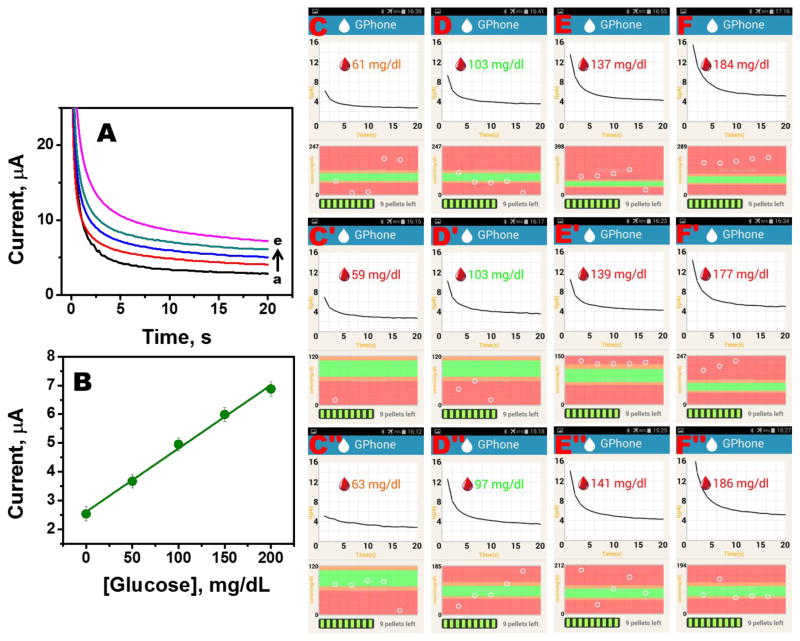
Sensor reproducibility is an important parameter for practical applications. In the present reusable sensor system, factors such as homogeneity of the composition, reproducible packing of the composition within each pellet casing, repeatable release and attachment of the pellet from the stylus to the unfunctionalized working electrode, and its removal after each test without leaving any residue behind on the electrode will affect sensor reproducibility. The effect of these parameters was initially tested by performing reproducibility studies for pellets using a benchtop electrochemical analyzer (CH Instruments, Model 630C) as shown in Fig. 4A and B. In this study, a fresh pellet was dispensed onto an unfunctionalized three electrode system and data was recorded for different glucose concentrations. Thereafter, the pellet was discarded and a fresh one was dispensed on top of the unfunctionalized working electrode and the test was repeated for 6 different pellets. Fig. 4A illustrates the typical chronoamperometric plots obtained for a pellet in physiologically relevant glucose concentrations, while, the calibration plot for this study appears in Fig. 4B (n = 6; R.S.D <= 2.5%). Thereafter, the complete system was tested for reproducibility wherein the ease and repeatability of dispensing a pellet onto the unfunctionalized working electrode affixed to the phone case, wireless data transmission from the sensor to the phone, and accuracy of the application software to display the test result was analyzed. In this study, a smartphone with an Andriod OS was placed in the phone case. A fresh pellet was dispensed from the stylus onto the sensor strip housed in the phone case and then the custom-built application software was loaded. Thereafter, a solution of known glucose concentration (60, 100, 140 or 180 mg/dl) was dropped onto the sensor strip. The glucose concentration was then recorded by the application software. The “used” pellet was discarded after the glucose concentration was displayed on the phone screen. Supplementary video 2 shows the entire procedure. These steps were repeated multiple times for different concentrations. Fig. 4C-F illustrates the phone screenshots for this study. Data plotted in Fig. 4 highlight the reproducibility of system. Additionally, the supplementary video 2 illustrates the ease with which the system can be used, which is an important requirement since such devices are expected to be used by consumers who have varied levels of technological savviness.
Fig. 4.
Sensor reproducibility study (A) Chronoamperograms for different glucose concentrations measured by a benchtop electrochemical analyzer (B) Calibration plot of the study (R.S.D. <= 2.5%)(Reviewer #1; Comment #2). (C-F) Screenshot plots of the Android Application software for different concentrations (60, 100, 140 or 180 mg/dl) using a new pellet for each test.
4. Conclusions
In this work we have demonstrated a reusable smartphone based glucose meter consisting of a custom-built smartphone case that includes a permanent bare sensor strip and a stylus for housing and dispensing enzymatic pellets directly onto the sensor strip. The case also has a slot for attaching a custom-built Bluetooth electronic module for data acquisition and transmission. A user-friendly Andriod based application software has also been developed for clear display of glucose level. The user performs a glucose test by loading the software on their phone, followed by dispensing a pellet from the stylus onto the sensor strip and introducing the sample. The pellet and the sample are discarded after the test is over to reobtain a fresh sensor surface for the next test. Such a reusable system overcomes the challenges faced by previously reported reusable sensors, such as gradual enzyme leaching, enzyme degradation, and hysteresis effect. Future work will focus on further optimizing the system by analyzing the sensor performance at different ambient temperatures, studying the effects of varying sample volumes, testing for the effects of common electroactive species that can potentially affect the sensor response, and characterizing and optimizing the reversible magnetic bonding of the pellets to the sensor surface. (Reviewer #1; Comment #3 Reviewer#3; Comment #1, #4)
Supplementary Material
Video 1: Video illustrating reproducibility of dispensation of pellets on an array of working electrodes.
Video 2: Video illustrating each step involved in using the smartphone-based reusable glucose sensor.
Highlights.
Integration of electrochemical glucose sensing electrodes directly onto a smartphone platform
Electrode re-functionalization via deposited enzyme pellets
Integrated instrumentation bioelectronics
Applications to more convenient diabetes management
Acknowledgments
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering of NIH (under Award number R21EB019698. US NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abellán-Llobregat A, Jeerapan I, Bandodkar A, Vidal L, Canals A, Wang J, Morallón E. Biosens Bioelectron. 2017;91:885–891. doi: 10.1016/j.bios.2017.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeev VL, Das S, Finegold DN, Asher SA. Clin Chem. 2004;50:2350–2360. doi: 10.1373/clinchem.2004.039701. [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Jia W, Ramírez J, Wang J. Adv Heal Mater. 2015a:1215–1224. doi: 10.1002/adhm.201400808. [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Jia W, Yardımcı C, Wang X, Ramirez J, Wang J. Anal Chem. 2015b;87:394–8. doi: 10.1021/ac504300n. [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Wang J. Trends Biotechnol. 2014;32:363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Colaco C, Sen S, Thangavelu M, Pinder S, Roser B. Biotechnol (Nature Publ Company) 1992;10:1007–1011. doi: 10.1038/nbt0992-1007. [DOI] [PubMed] [Google Scholar]
- Combes D, Monsan P. Ann N Y Acad Sci. 1984;434:061–063. [Google Scholar]
- Dantu V, Vempati J, Srivilliputhur S. IEEE Eng Med Biol Soc Conf. 2014:3695–3698. doi: 10.1109/EMBC.2014.6944425. [DOI] [PubMed] [Google Scholar]
- Dario ® Blood Glucose Monitoring System User Guide, 2014.
- Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, Lavado R, Lomsadze L, Reynolds A, Squires E, Campbell M, Decenso B, Dicker D, Flaxman AD, Gabert R, Highfill T, Naghavi M, Nightingale N, Templin T, Tobias MI, Vos T, Murray CJL. JAMA. 2016;316:2627–2646. doi: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan BC, Shults M, Rhodes RK, Jacobs PG, Brauker JH, Pintar TJ, Updike SJ. Diabetes Technol Ther. 2004;6:378–386. doi: 10.1089/152091504774198089. [DOI] [PubMed] [Google Scholar]
- Guariguata L, Whiting D, Weil C, Unwin N. Diabetes Res Clin Pract. 2011;94:322–332. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Guerci B, Drouin P, Grangé V, Bougnères P, Fontaine P, Kerlan V, Passa P, Thivolet C, Vialettes B, Charbonnel B. Diabetes Metab. 2003;29:587–594. doi: 10.1016/s1262-3636(07)70073-3. [DOI] [PubMed] [Google Scholar]
- Imani S, Mercier PP, Bandodkar AJ, Kim J, Wang J. IEEE Int Symp Circuits Syst. 2016:1122–1125. [Google Scholar]
- International Diabetes Foundation. [accessed 5.22.17]; URL http://www.diabetesatlas.org.
- Jendrike N, Baumstark A, Chen C, Rittmeyer D, Haug C, Freckmann G. J Diabetes Sci Technol. 2017:1–3. doi: 10.1177/1932296817706594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith-hynes P, Mize B, Robert A, Place J. electronics. 2014;3:609–623. [Google Scholar]
- Kim J, Kumar R, Bandodkar AJ, Wang J. Adv Electron Mater. 2017;3:1–15. doi: 10.1002/aelm.201600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JK, Stegner J. J Diabetes Sci Technol. 2010;4:435–439. doi: 10.1177/193229681000400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JD, Turner APF. Biosens Bioelectron. 2005;20:2435–2453. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Ong WM, Chua SS, Ng CJ. Patient Prefer. Adherence. 2014:237–246. doi: 10.2147/PPA.S57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR. J Diabetes Sci Technol. 2015;9:668–675. doi: 10.1177/1932296814567709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani N, Heptulla RA. Int J Pediatr Endocrinol. 2012;28:1–10. doi: 10.1186/1687-9856-2012-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönksen PH, Judd SL, Lowy C. Lancet. 1978;311:729–732. doi: 10.1016/s0140-6736(78)90854-1. [DOI] [PubMed] [Google Scholar]
- Sun AC, Yao C, AGV, Hall DA. Sensors Actuators B Chem. 2016;235:126–135. doi: 10.1016/j.snb.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S. Biosens Bioelectron. 2001;16:621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- Valdés-Ramírez G, Li YC, Kim J, Jia W, Bandodkar AJ, Nuñez-Flores R, Miller PR, Wu SY, Narayan R, Windmiller JR, Polsky R, Wang J. Electrochem commun. 2014;47:58–62. [Google Scholar]
- Vashist SK. Anal Chim Acta. 2012;750:16–27. doi: 10.1016/j.aca.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Wang J. Chem Rev. 2008;108:814–25. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- Wang PM, Cornwell M, Prausnitz MR. Diabetes Technol Ther. 2005;7:131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- Wu Y, Boonloed A, Sleszynski N, Koesdjojo M, Armstrong C, Bracha S, Remcho VT. Clin Chim Acta. 2015;448:133–138. doi: 10.1016/j.cca.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Fienbork D, Stoeber B, Flounders AW, Liepmann D. TRANSDUCERS, 12th IEEE International Conference On Solid-State Sensors, Actuators and Microsystems; 2003. pp. 99–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Video illustrating reproducibility of dispensation of pellets on an array of working electrodes.
Video 2: Video illustrating each step involved in using the smartphone-based reusable glucose sensor.