Abstract
Objectives
The National Institutes of Health, led by the National Heart, Lung, and Blood Institute, organized a working group of experts to discuss the problem of weight regain after weight loss. A number of experts in integrative physiology and behavioral psychology were convened with the goal of merging their perspectives regarding the barriers to scientific progress and the development of novel ways to improve long-term outcomes in obesity therapeutics. The specific objectives of this working group were to: (1) identify the challenges that make maintaining a reduced weight so difficult; (2) review strategies that have been used to improve success in previous studies; and (3) recommend novel solutions that could be examined in future studies of long-term weight control.
Results
Specific barriers to successful weight loss maintenance include poor adherence to behavioral regimens and physiological adaptations that promote weight regain. A better understanding of how these behavioral and physiological barriers are related, how they vary between individuals, and how they can be overcome will lead to the development of novel strategies with improved outcomes.
Conclusions
Greater collaboration and cross-talk between physiological and behavioral researchers is needed to advance the science and develop better strategies for weight loss maintenance.
Introduction
Obesity or overweight afflicts two thirds of U.S. adults (1) and accounts for over 20% (200 billion dollars) of annual direct U.S. health-care costs (2,3). Although many strategies have proven useful for inducing weight loss and reducing comorbidities, recidivism rates continue to be disturbingly high. Research by diverse groups of scientists over the last two decades have significantly improved our understanding of the physiology and behavioral mechanisms underlying the difficulty in weight loss and maintenance (4-8). Despite these advances, weight regain after weight loss remains the most substantial problem in obesity therapeutics (9,10).
Recognizing this problem, the National Institutes of Health (NIH), led by the National Heart, Lung, and Blood Institute (NHLBI), organized a workshop to discuss the state of the science and develop interdisciplinary solutions that may be pursued to improve maintenance of weight loss. For this particular effort, the overarching objective was to merge two groups that have generally studied the problem from distinct and often isolated perspectives: integrative physiology and behavioral psychology. Experts recruited to this panel included basic and clinical scientists with expertise in energy homeostasis, neuroscience, exercise physiology, pharmaceutical development, food behaviors, cognitive function, and lifestyle management.
The specific objectives of this working group were to:
identify the challenges that make maintaining a reduced weight so difficult;
review strategies that have been used to improve success in previous studies; and
recommend novel solutions that could be examined in future studies of long-term weight control.
The panel was given the charge of focusing on ideas that could move the field forward in a significant way and hopefully make a substantial improvement in clinical care. All ideas were welcome, but the scope of this particular workshop was to focus on solutions and strategies that could be implemented at the level of the individual. This limitation was an attempt to avoid extensive discussions delving into policy changes and the built environment, which would be better addressed by a separate working group with more relevant expertise. Given these objectives and this scope of discussion, panel members participated in a number of pre-meeting activities and met in Bethesda, MD, in June of 2014.
A foundation for the discussion
Defining success with distinct outcomes
The first issue discussed was the lack of consistency in the definition of success in intervention studies. Panel members were unanimous in asserting that future studies should make following distinctions in study designs and terminology:
Long-term weight loss
Studies focusing on overall or long-term weight loss involve randomization prior to weight loss and examine overall weight change—from the start of the program to the end of the follow-up interval at 12-24 months, or longer (11,12). Typically these studies use overall mean weight loss or the percent of participants achieving a 5-10% weight loss at the end of the trial as the criteria of success, because weight losses of this magnitude have been shown to produce clinically significant improvements in health parameters. The group noted that if long-term weight loss is the aim, strategies that increase initial weight loss are likely to be important since larger initial weight loss are associated with larger long-term weight losses (13,14).
Maintenance of weight loss
The alternative approach is to randomize after a period of weight loss and focus on the weight change that occurs after the initial phase of treatment. This type of design is used very frequently in pharmacotherapy studies where participants who achieve a given amount of weight loss during the initial weight loss phase are randomly assigned to drug or placebo for maintenance (15,16). STOP Regain (17), the Weight Loss Maintenance trial (18), and the TOURS trial (19), are behavioral studies that also used this type of design. In these studies, participants who had achieved a preset criterion of success were randomly assigned to one of two active interventions or to a control group and followed for 12-30 months. A variety of different criteria of success were used in these studies, including mean weight regain, percent regaining a given amount, or percent still maintaining a given amount.
Both approaches have merit, but the working group emphasized that it is important for researchers to distinguish between the two in discussions about the problem of weight regain or the success and failure for specific outcomes. The consensus was that all studies report at least the following outcomes: (1) absolute weight loss at study end; (2) maximal observed weight loss and when it occurred; and (3) absolute weight regain from maximal observed weight loss. This common reporting would facilitate cross-study comparisons.
Merging disciplines with different perspectives
The interaction between biology, environment, and behavior is central to weight loss efforts and the problem of weight regain. These pressures interact and, ultimately, their collective input dictates a “steady state weight” in an adult human or animal. A significant change in any of these inputs has the potential to upset the existing balance, induce a change in weight, and evoke responses affecting a new “steady-state” weight. Such is the case when the environment is altered by dieting. In response to the decreased energy stores and negative energy balance, there is a coordinated decrease in energy expenditure (biology) and increase in responsivity to food-related cues (behavior). The potency of these metabolic and behavioral responses dictates the degree of weight loss, the duration of sustained weight loss at a lower steady state, and the ability of the individual to sustain the diet. These adaptive responses are designed to prevent continual weight loss but they also create the biological pressure to return the body to its original weight. One resounding recommendation from this panel is that to produce more effective weight loss maintenance therapies, we must improve our understanding of the mechanistic interactions between these pressures resulting from weight loss.
Barriers to success
Behavioral challenges
Behavioral approaches, which combine diet, exercise, and cognitive strategies, are recommended for dynamic and sustained weight loss, either alone or in combination with pharmacologic interventions (20). On average, behavioral approaches produce weight losses of approximately 8 kg (8%) during the initial phase of intervention (typically ∼6 months). Subsequently, participants tend to average weight regains of 1-2 kg/year, with faster weight regains in the earlier years. The working group was charged with identifying the most significant barriers to success.
Behavioral challenge 1: long-term adherence to regimens
The fact that weight regain occurs so consistently after about 6-9 months is believed to a large extent to reflect temporal decreases in adherence to prescribed regimens. Adherence to diet and physical activity prescriptions, group attendance, and completion of self-monitoring records are related to both initial and long-term weight loss (21). At the point of greatest weight loss success, the behavioral changes that led to early weight loss are predicted to have already substantially waned from their initial levels (22-25). Consistent with this assertion, in studies comparing different types of diet (e.g., low fat vs. low carbohydrate), the level of adherence to the prescribed regimen is a far stronger predictor of weight loss outcome than is the actual diet (26).
One explanation for the declining adherence is that the perceived costs of adherence gradually exceed the perceived benefits (27). Initially, the positive consequences of weight loss (e.g., sense of accomplishment; better fit of clothes) outweigh the cognitive and physical effort needed to lose weight. Later, when the goal is to maintain lost weight, the positive feedback is less compared to the effort required to keep adhering to the same regimen. Thus, the benefits no longer seem to justify the costs. Behavioral approaches that have been tested to facilitate long-term weight loss can be conceptualized as utilizing different approaches to change the cost: benefit ratio and thus promote longer-term adherence. Such approaches include strategies to (a) increase support from peers or professionals (18,19,28,29) and maximize motivation (30,31); (b) make it easier to follow the routine through provision of food or meal-replacement (32,33) or by reducing the boredom by varying the intervention (34); (c) facilitate development of self-regulating skills through self monitoring (17) and establishing this skill set prior to embarking on weight loss efforts (35), and (d) varying the dose, intensity, or behavioral support for physical activity (36). Many of these approaches have produced small, but statistically significant improvements in longer-term maintenance of weight, but no approach has worked to change the overall pattern of weight loss and regain. This lack of success would suggest that we need a better understanding of the motivating factors underlying adherence and how the cost: benefit ratio changes over time (Figure 1).
Figure 1.
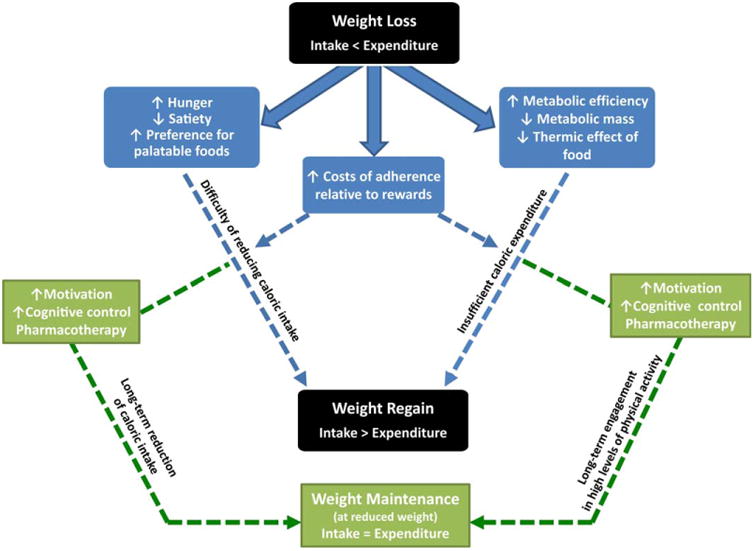
Weight loss leads to both physiological and psychological changes which promote subsequent weight regain (shown in blue). The path to overcome this propensity for regain (shown in green) may involve pharmaceutical and behavioral interventions that improve adherence, counter the physiological and behavioral adaptations, and re-establish the balance between intake and expenditure.
An alternative explanation for the decline in adherence is that the behaviors that generated the original overweight state comprise a series of habits that have been formed over prolonged periods of time and these habitual behaviors return after a period of successful control. Enhanced habit formation circuitry in people with obesity, perhaps involving dopamine signaling in the brain (37), may entrench old eating behaviors and drive weight regain.
Behavioral challenge 2: individual variability
There is tremendous variability in the weight loss outcomes with behavioral interventions; typically the standard deviation for the weight losses is as large as the mean, and this variability increases during maintenance. Clearly, behavioral approaches work more effectively for some persons than for others, but we have yet to elucidate why. However, pretreatment variables, including genetic, behavioral or psychological characteristics, have not been very successful in predicting weight loss outcomes. In contrast, early success in these programs is a strong, consistent predictor of overall outcome (38) and phenotypes present as a result of weight loss may also be predictive. In DPP those who met the 7% weight loss goal at the end of 16 weeks were three times as likely to meet this goal at 3 years than those who did not meet the goal initially (14). Even earlier results—as early as months 1 or 2 are predictive of subsequent outcomes (13). A better understanding of why some participants achieve greater success initially (and also long term) than others as well as why genetic and phenotypic correlates of weight regain (e.g., Fto and RMR) are not the same as those of weight loss (e.g., TFA2B and hunger) is clearly needed.
Behavioral challenge 3: designing studies on maintenance
Several designs have been used in this area. The most robust are the randomized trials comparing two or more approaches to long-term weight control. The group discussed several limitations in implementing these trials—one is the fact that the trials must be long (12-24 months) with a large number of subjects to detect modest effects. Thus, these studies are very costly. However, since these are the strongest designs, the later discussion focused on ways that might be used to enhance this approach. A second approach is to determine what behaviors are predictors of long-term success or to study those who have succeeded in maintaining weight loss and use these predictors to select optimal therapeutic interventions for individuals. The importance of considering a wider range of predictors of weight regain (physiological, behavioral, and psychological) and measuring these predictors more frequently to capture changes that occur prior to (not concurrent with) weight regain was stressed.
Physiological challenges
In its simplest description, the physiological system regulating body weight is a feedback loop between the brain and periphery, whereby neural circuitry largely centered in hypothalamus and hindbrain responds to peripheral signals regarding energy stores (adiposity) and energy balance (nutrient availability) by adjusting autonomic, neuroendocrine, behavioral, and metabolic systems. Negative energy balance and decreased energy stores (weight loss) induce changes in a number of critical central and peripheral regulatory nodes, which lead to increased appetite and disproportionately decreased energy expenditure (8,39), thus creating the optimal circumstance for weight regain following otherwise successful weight loss.
Physiological challenge 1: specific adaptations encouraging weight regain
Negative energy balance and reduced energy stores provoke changes in peripheral nutrient, hormonal (especially leptin and insulin), and other afferent neural signals (8,39), all of which converge on specific areas of the brain. The response in neural circuitry provides feed-forward input to enhance the incentive salience (rewarding value) of food (40,41) and to diminish satiation (42,43). Consequently, greater preference for more palatable foods and food seeking and ingestive behaviors may emerge. At the same time, efferent signals are sent to peripheral tissues to enhance metabolic efficiency (44-48) promote the preferential use of carbohydrate, rather than fat, for energy production (49-53), and maximize the capacity to absorb and store nutrients when they become available by establishing a positive fat balance (54). This preferential fuel utilization has been shown to predict subsequent weight gain (55). The loss of lean tissue and reduced physical activity levels (56) may also exacerbate the decline in metabolic requirements and further expand the energetic gap between appetite and expended energy.
The vast majority of these adaptive responses do not resolve or reset once this new steady-state weight is achieved (8,39) and may even strengthen with time during weight maintenance (57). Together they provide physiological and behavioral drives to regain lost weight (Figure 1). To improve weight loss maintenance, strategies that diminish or in some way over-ride this barrier to success will be needed.
Physiological challenge 2: individual variability
The systems governing body weight are built from a large number of genes that are expressed in numerous tissues of the body. Collectively their expression establishes the neural circuits, regulatory nodes, and communication pathways that sense and respond to environmental and behavioral stresses. The genetic diversity and broad range of epigenetic programming that occurs in humans yields a large amount of variability in how this homeostatic system functions and how it integrates with other systems involving metabolism, learned behavior, and cognitive function. This variability may explain, in part, why some people are more or less susceptible to an obesogenic environment. It also manifests in the adaptations to weight loss, the responses to behavioral interventions, and the sensitivity to pharmacotherapies. Unfortunately, interventions and therapies in obesity therapeutics are judged by the breadth of their effectiveness in non-targeted populations. It is clear that we need to individualize interventions or target specific populations with evidence-based strategies, but we currently lack the knowledge to do so.
Physiological challenge 3: translating relevant observations in animals to clinical applications
In obesity research, basic and clinical scientists often work in isolation and lack the motivation or means to coordinate their efforts. The reasons for this disconnect are complex, which makes effectively addressing this issue a significant challenge. Much of what we know about energy homeostasis in humans is based upon studies in animals, because we can pursue more extensive mechanistic investigations with more invasive measurements. However, humans exhibit greater genetic diversity, psychosocial influences, and other stressors, which are difficult to duplicate in nonhuman animal models. A broader appreciation of these perspectives is needed as we develop animal models for obesity research, examine the biological and behavioral mechanisms, and develop interventions that stem from this knowledge. An integrated effort from the basic and clinical sciences is needed to create a more effective bidirectional translation of knowledge.
Advancing the science to develop more effective therapies
Countering biology to reduce the gap between appetite and expenditure
Elevated appetite and suppressed energy expenditure are determinants of weight regain. Countering these responses to weight loss and the underlying adaptations that promote a positive energy imbalance could facilitate weight loss maintenance. The panel identified several gaps in knowledge that needed to be filled to advance the science, along with strategies that should be considered to reduce the gap between appetite and expenditure.
Deepening our understanding of exercise
The potential benefits of physical activity for weight loss maintenance were discussed at length. A number of studies correlate levels of physical activity to weight loss maintenance. However, it has been difficult to show the benefits of physical activity for preventing weight regain in randomized clinical trials due to the lack of long-term adherence to the exercise prescriptions (36). In order to establish a broader consensus about the effects of exercise and its utility for weight loss maintenance, further research was suggested in two important areas.
Countering biology with exercise
If exercise did no more than increase expended energy, the benefits of physical activity could be easily appreciated. However, exercise has other effects on both sides of the energy balance equation. Preclinical studies in weight reduced animal models of obesity have shown that both volitional and regimented exercise attenuate weight regain after weight loss (56,58) and counter the biological factors that promote weight regain by reducing intake and elevating expenditure (58,59). Consistent with these observations, higher physical activity during diet-induced weight loss has been associated with better compliance to dietary prescriptions (60). Even so, compensatory ingestive behaviors are commonly observed in normal weight humans who exercise (61). Similarly there may be variability in weight reduced subjects, with clear “responders” and “non-responders.” Even in animal studies these benefits of exercise on biological adaptations to weight loss may be sex and species dependent. Given that there is much that we do not know, it is critical that we deepen our understanding of how exercise affects appetite, food preference, and the components of energy expenditure in the weight-reduced state and during weight regain and under what conditions these effects are relevant or maximized in humans. Tied to both of these issues is whether certain types of exercise (e.g., aerobic, resistance, high intensity interval training) yield greater benefits or are more suited for weight loss or weight loss maintenance phases of a weight management program.
Enhancing adherence to exercise prescription
A number of approaches were discussed that could potentially address the issues of poor adherence to exercise prescriptions. The overarching consensus was that further study is needed of both the behavioral and biological factors that influence physical activity in the weight reduced state. Relevant questions that should be addressed in this pursuit include why some individuals choose to exercise but others do not (62) and whether physical activity can be increased by applying behavioral or pharmacological strategies. Alternatively, if exercise is not reinforcing, we need to determine if physical activity can be increased by “hiding” it in daily routines (e.g., by increasing the use of stand-up desks), or with pharmacotherapy targeting non-exercise activity thermogenesis.
Innovations with diet
Numerous studies have found that the magnitude of initial weight loss is related to the level of dietary adherence and the overall caloric deficit, rather than the macronutrient composition of the diet. Thus, the general consensus was that attention should focus on strategies that will lead to long-term selection of healthy, calorically appropriate dietary regimens. In addition, the committee felt that it is critical to develop dietary strategies that counter the adaptive responses that potentiate weight regain.
Engineering foods and modifying the response to food cues
Significant advances have been made over the last several decades in understanding how food components, energy density, and sensory properties can affect consumption and satiation. More recently, specific subtypes of macronutrients (resistant starches, polyunsaturated fats, certain amino acids, etc.), pre- and pro-biotics, and natural bioactive compounds have been developed and applied to decrease eating, reduce absorption efficiency, increase energy expenditure, or enhance the preferential use of fats for energy production. Much of this science and technology has yet to be applied specifically to questions of weight loss maintenance. Studies of the effects of dietary energy density provide one example of how the properties of foods could be used to improve outcomes (63). Enhancing collaboration between academicians and food manufacturers to encourage development of lower energy density foods that maximize palatability and satiation may help us to improve long-term adherence to a lower calorie regimen.
Dietary strategies to counter the biological responses
In addition to looking at food components, there was some enthusiasm for determining if particular dietary strategies (e.g., intermittent or alternate day fasting) or meal patterns (single vs. multiple daily meals) could mitigate the biological changes that occur with weight loss that subsequently promote weight regain. Animal models were considered as potentially useful in this area. Finally, the group recognized the need to consider preexisting individual differences in dietary preferences, genetic background, or metabolic profiles, in order to investigate how best to match specific types of diet strategies to individuals.
Innovations in behavioral strategies
Despite the progress that has been made with behavioral strategies to improve success in both initial and long-term weight loss outcomes, further research is needed to understand how to utilize cognitive and behavioral skills to overcome the physiological pressures for weight regain.
A deeper understanding of cognitive function
We need to enhance our understanding of how cognitive functions (learning and memory) integrate with other systems that influence ingestive behaviors and physical activity. It is possible that maladaptations in the processes of learning and memory linked to a predisposition for obesity may present unrecognized challenges to weight loss maintenance objectives. A better understanding of how cognitive function is altered in concert with homeostatic and hedonic systems during weight loss maintenance may help us to develop behavioral strategies with greater, more sustained effects.
Behavioral skill development
Improvements in weight loss maintenance may be facilitated by considering how best to overcome the biological challenges imposed by weight loss. The working group discussed approaches to decreasing the rewarding value and cravings for foods by modifying memories related to food (64,65) and teaching individuals to delay gratification or improving impulse control. Further study of the potential success of establishing behavioral skills that are related to weight maintenance in the period prior to starting on weight loss and in teaching self-regulation using frequent monitoring of weight to know when behavior changes are needed were encouraged.
Innovations in pharmacotherapy
The main problem with pharmacotherapy to date has been that effective doses often yield adverse side effects. This may be remedied if separate consideration is given to developing independent therapies/dosing for weight loss (negative energy imbalance) and weight loss maintenance (energy balance).
Leptin and leptin sensitizers
The decline of blood leptin levels is a hallmark of the homeostatic response to weight loss (66), and the failure of leptin to improve weight loss in clinical trials perplexed the scientific community. It is now clear that the effects of exogenous leptin administration may prove to be more effective after weight loss or in combination with drugs that enhance the sensitivity of tissues to the actions of leptin. In weight-reduced individuals, leptin alone has shown to counter many of the adaptive responses to weight loss (40,67-69). For these reasons, the use of leptin alone after weight loss or leptin in combination with a leptin sensitizer even during weight loss could prove valuable if targeted to the right individuals.
Novel combinations or dosing regimens to safely improve efficacy
A number of drugs that have been developed, like leptin, have been cast aside because of their minimal effects at safe doses. Animal studies have shown that lower doses could be used for weight loss maintenance (70). The unique aspects of the weight reduced state should be considered strategically as combinations are developed for weight loss maintenance. Two or more drugs could be used to target one aspect of this feedback system (e.g., leptin with a leptin sensitizer) or a combination could target different aspects of this feedback system to overcome redundancy (e.g., a combination of a centrally acting and peripherally-acting agents). The right combination may allow the use of lower doses to minimize side-effects. Practically, this pursuit of effective combinations for weight loss maintenance may require some refinement of the drug development process.
New targets and behavioral pairings on the horizon
Along with pairing drugs with other drugs, there is the potential to pair certain combinations of drugs with specific behavioral therapies to take advantage of synergistic effects, target individual needs, or counter a broader range of the homeostatic and hedonic adaptations. These types of strategies will only be realized with a better understanding of the interactions between biology and behavior in body weight control, extensive collaborations between physiologists and behavioral psychologists, and a coordinated effort of both fields. The working group also envisioned novel targets for pharmacotherapy that could be considered with this effort, which include the motivation to be physically active and changing food preferences.
Mechanisms of weight loss and weight regain following bariatric surgery
Bariatric surgery is becoming more common as a treatment option for obesity, particularly among the severely obese and those with obesity-associated conditions such as sleep apnea and type 2 diabetes. Despite the often large and sustained weight loss following bariatric surgery, many patients experience significant weight regain over time. Interrogating the mechanisms for weight loss and weight regain following bariatric surgery could help identify targets for pharmacotherapy. Moreover, many of the same guiding principles that the working group identified to be important for weight loss maintenance and prevention of weight regain following lifestyle interventions can most certainly be applied to patients post bariatric surgery.
Individualized and targeted strategies to maximize effectiveness
As individual variability was identified by both physiologists and behavioral psychologists as a significant challenge to developing more effective strategies, considerable discussion was devoted to the path that would best overcome this obstacle to progress.
More comprehensive understanding of weight loss maintenance versus regain
We still know very little about the interaction between the physiological and behavioral factors that influence if and when weight regain will occur or about the actual phenomenon/process of weight regain. Further information is needed regarding the physiological, behavioral, and environmental changes that relate prospectively to the transition from weight loss to weight plateaus and subsequently to weight regain. We also have limited understanding of the up-stream contributors to weight regain, such as the biological and behavioral effects of stress or “triggers” which may affect weight loss maintenance. These concerns (coupled with the individual variability in treatment outcomes) led to the recommendation to more carefully document the behavioral, physiological, and environmental predictors of weight regain in combination with each other and the responses to various treatment regimens.
A focus on individual responses
The general consensus was that, whether studying biological responses or behavioral interventions, we need to pay less attention to group means and greater attention to the individual variability that emerges in both the process and outcomes. Although individual genes, phenotypes, and behaviors provide little predictive power for success on their own, there may be value in developing predictive models that are based upon a large number of unrelated parameters (71-82). Matching genetic with other behavioral characteristics, like current activity or eating patterns, may help match patients to more effective treatments. Consideration of an individual's preferences for certain diet-exercise regimens was discussed, although preference has not always been related to outcome. Since initial weight loss is the best predictor of long-term success examining predicting parameters of early responses may be a cost effective way to begin modeling targeted treatments.
Advances in technology, constructs, and definitions
The working group noted that the limitations of the tools and constructs that are regularly used in studies of weight loss maintenance and energy balance are hindering advancement. Overcoming these obstacles to progress may require a concerted effort to recruit individuals with expertise in engineering, information technology, and other relevant disciplines.
Measuring components of energy balance and related parameters
An important limitation to research on maintenance of weight loss is the inability to accurately determine what and how much people are consuming in the free living environment (83). The working group stressed the need to further develop and validate new approaches to objectively measure food and energy intake and dietary patterns and to improve measurement of constructs such as hunger and satiety. Although recent technology allows for objective assessment of physical activity patterns and doses, there continue to be important limitations in using these instruments to estimate the total energy expended (84).
Constructs and mechanisms underlying “motivation”
There is great interest in understanding what “motivates” an individual to adhere to weight loss prescription (62,85), but we need to have better defined constructs and measures for studying “motivation” in the context of adherence to a diet and exercise prescriptions, pursuing a healthy lifestyle, or changing one's environment. Too often, terms like reward, reinforcement, and value are used as generalized, undefined explanations of eating and appetitive behavior. Clear constructs with better definitions are needed to improve our understanding of how the homeostatic, hedonic, and cognitive mechanisms that underlie eating and activity-related behaviors influence body weight regulation.
Information technology and social networking
With the establishment of the internet and the development of readily available communication, the opportunity to utilize these technologies in subject recruitment, data acquisition, and compliance monitoring has emerged. New technologies such as wireless scales and ecological momentary assessments (reporting behaviors, moods, and other parameters at the time when they occur, rather than days or weeks later) may allow us to document the process of weight regain and the attitudinal, behavioral, and physiological changes that precede regain. The potential also exists to use this technology to deliver personalized interventions in the situations where they are most needed and to use social networking approaches to expand the reach and efficacy of these approaches (86). These new technologies are currently underutilized and have great potential to affect research and clinical outcomes in weight loss maintenance.
Novel research designs
There is need for new designs in this area to help reduce the large sample size and long duration of human weight loss maintenance trials. In some cases, it may be possible to use animal studies, which can be conducted in a shorter time frame (57,58,70). Certain types of clinical studies in controlled environments could also provide information about long-term interventions. The use of MOST (Multiphase Optimization Strategy) (87) and SMART (Sequential multiple assignment, randomized trial) (88) designs, where a variety of different strategies can be tested in one study, was recommended. Finally, it may be possible to develop paradigms where relapse would be expected to occur sooner, thus reducing the time period needed for maintenance intervention trials.
Conclusion
The overwhelming conclusion from this meeting was that greater collaboration and cross-talk between physiological and behavioral researchers is needed. The development of intervention strategies to improve maintenance of weight loss must be informed by an understanding of the physiological changes that occur with weight loss and promote weight regain. Including ancillary studies in intervention trials to examine physiological questions and behavioral measures in human physiological studies is critical. Training in both areas for early-stage obesity researchers may lead to a more sophisticated understanding of factors that influence relapse and development of methods to enhance long-term maintenance.
Acknowledgments
The authors acknowledge the following NIH collaborators who convened the working group and made important contributions to the meeting: Catherine Loria, PhD, NHLBI (Chair); Tanya Agurs-Collins, PhD, NCI; Susan Czajkowski, PhD, NHLBI; Christine Hunter, PhD, NIDDK; Susan Yanovski, MD, NIDDK.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 4.Leibel R, Rosenbaum M. Metabolic response to weight perturbation. In: Clément K, editor. Novel Insights into Adipose Cell Functions, Research and Perspectives in Endocrine Interactions. Heidelberg: Springer-Verlag; 2010. pp. 121–133. [Google Scholar]
- 5.Thomas J, Bond D, Phelan S, Hill J, Wing R. Weight-loss maintenance for 10 years in the national weight control registry. Am J Prev Med. 2014;46:17–23. doi: 10.1016/j.amepre.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Sweet L, Hassenstab J, McCaffery J, et al. Brain responses to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity. 2012;20:2220–2225. doi: 10.1038/oby.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci. 2013;124:231–241. doi: 10.1042/CS20120223. [DOI] [PubMed] [Google Scholar]
- 8.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–R600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dombrowski S, Knittle K, Avenell A, Araujo-Soares V, Sniehotta F. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loveman E, Frampton G, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15:1–18. doi: 10.3310/hta15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unick JL, Hogan PE, Neiberg RH, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring) 2014;22:1608–1616. doi: 10.1002/oby.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 17.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 18.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 19.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22(S2):S1–S410. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 23.Hall KD. Predicting metabolic adaptation, body weight change and energy intake in humans. Am J Physiol. 2010;298:E449–E466. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow CC, Hall KD. Short and long-term energy intake patterns and their implications for human body weight regulation. Physiol Behav. 2014;134:60–65. doi: 10.1016/j.physbeh.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perri MG. The maintenance of treatment effects in the long-term management of obesity. Clin Psychol Sci Pract. 1998;5:526–543. [Google Scholar]
- 28.Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity: an evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol. 1984;52:404–413. doi: 10.1037//0022-006x.52.3.404. [DOI] [PubMed] [Google Scholar]
- 29.Perri MG, McAdoo WG, McAllister DA, et al. Effects of peer support and therapist contact on long-term weight loss. J Consult Clin Psychol. 1987;55:615–617. doi: 10.1037/0022-006X.55.4.615. [DOI] [PubMed] [Google Scholar]
- 30.West DS, Gorin AA, Subak LL, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes (Lond) 2011;35:259–269. doi: 10.1038/ijo.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67:132–138. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 32.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 33.Ditschuneit HH, Flechtner-Mors M. Value of structured meals for weight management: risk factors and long-term weight maintenance. Obes Res. 2001;9(Suppl 4):284S–289S. doi: 10.1038/oby.2001.132. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery RW, Levy RL, Langer SL, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Prev Med. 2009;49:384–389. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiernan M, Brown SD, Schoffman DE, et al. Promoting healthy weight with “stability skills first”: a randomized trial. J Consult Clin Psychol. 2013;81:336–346. doi: 10.1037/a0030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. discussion 1559-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry. 2014;19:1078–1084. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res. 2010;1350:95–102. doi: 10.1016/j.brainres.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornier MA, Salzberg AK, Endly DC, et al. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PloS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 43.Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–147. doi: 10.1111/j.1365-277X.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 45.Knuth ND, Johannsen DL, Tamboli RA, et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity. 2014;22:2563–2569. doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLean PS, Higgins JA, Johnson GC, et al. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1306–R1315. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 48.Dulloo AG, Calokatisa R. Adaptation to low calorie intake in obese mice: contribution of a metabolic component to diminished energy expenditures during and after weight loss. Int J Obes. 1991;15:7–16. [PubMed] [Google Scholar]
- 49.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 50.Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587:4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 52.Corpeleijn E, Mensink M, Kooi ME, Roekaerts PM, Saris WH, Blaak EE. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity (Silver Spring) 2008;16:1025–1032. doi: 10.1038/oby.2008.24. [DOI] [PubMed] [Google Scholar]
- 53.Astrup A, Buemann B, Christensen NJ, Toubro S. Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am J Physiol. 1994;266:E592–E599. doi: 10.1152/ajpendo.1994.266.4.E592. [DOI] [PubMed] [Google Scholar]
- 54.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes. 2008;32(Suppl 7):S109–S119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 56.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R771–R778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 57.MacLean PS, Higgins JA, Jackman MR, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1577–R1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- 58.MacLean PS, Higgins JA, Wyatt HR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol. 2009;297:R793–R802. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steig AJ, Jackman MR, Giles ED, et al. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R656–R667. doi: 10.1152/ajpregu.00212.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. Effect of physical activity on weight loss, energy expenditure, and energy intake during diet induced weight loss. Obesity (Silver Spring) 2014;22:363–370. doi: 10.1002/oby.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med. 2014;48:1472–1476. doi: 10.1136/bjsports-2012-091721. [DOI] [PubMed] [Google Scholar]
- 62.Herring MP, Sailors MH, Bray MS. Genetic factors in exercise adoption, adherence and obesity. Obes Rev. 2014;15:29–39. doi: 10.1111/obr.12089. [DOI] [PubMed] [Google Scholar]
- 63.Lowe MR, Butryn ML, Thomas JG, Coletta M. Meal replacements, reduced energy density eating, and weight loss maintenance in primary care patients: a randomized controlled trial. Obesity. 2014;22:94–100. doi: 10.1002/oby.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunstrom JM, Burn JF, Sell NR, et al. Episodic memory and appetite regulation in humans. PloS One. 2012;7:e50707. doi: 10.1371/journal.pone.0050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgs S. Memory for recent eating and its influence on subsequent food intake. Appetite. 2002;39:159–166. doi: 10.1006/appe.2002.0500. [DOI] [PubMed] [Google Scholar]
- 66.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 67.Kissileff HR, Thornton JC, Torres MI, et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95:309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 69.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skowronski AA, Morabito MV, Mueller BR, et al. Effects of a novel MC4R agonist on maintenance of reduced body weight in diet-induced obese mice. Obesity. 2014;22:1287–1295. doi: 10.1002/oby.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batra P, Das S, Robinson I, et al. Eating behaviors as predictors of weight loss in a 6 month weight loss intervention. Obesity. 2013;21:2256–2263. doi: 10.1002/oby.20404. [DOI] [PubMed] [Google Scholar]
- 72.Delahanty L, Pan Q, Jablonski K, et al. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diab Care. 2012;35:363–366. doi: 10.2337/dc11-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Field A, Cook N, Gillman M. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munro I, Bore M, Nunro D, Garg M. Using personality as a predictor of diet induced weight loss and weight management. Int J Behav Nutr Phys Act. 2011;8:129. doi: 10.1186/1479-5868-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price D, Ma Y, Rubin R, et al. Depression as a predictor of weight regain among successful weight losers in the diabetes prevention program. Diab Care. 2013;36:216–221. doi: 10.2337/dc12-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strohacker K, McCaffery J, MacLean P, Wing R. Adaptations of leptin, ghrelin, or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes. 2014;38:388–396. doi: 10.1038/ijo.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogels N, Diepvens K, Westerterp-Plantenga M. Predictors of long-term weight maintenance. Obes Res. 2005;13:2162–2168. doi: 10.1038/oby.2005.268. [DOI] [PubMed] [Google Scholar]
- 78.Wang P, Holst C, Wodzig W, et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int J Obes. 2012;36:1545–1551. doi: 10.1038/ijo.2011.278. [DOI] [PubMed] [Google Scholar]
- 79.Womble L, Williamson D, Greenway F, Redmann S. Psychological and behavioral predictors of weight loss during drug treatment for obesity. Int J Obes. 2001;25:340–345. doi: 10.1038/sj.ijo.0801551. [DOI] [PubMed] [Google Scholar]
- 80.Hansen D, Astrup A, Toubro S, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi-centre STORM trial. Sibutramine Trial of Obesity Reduction and Maintenance. Int J Obes. 2001;25:496–501. doi: 10.1038/sj.ijo.0801481. [DOI] [PubMed] [Google Scholar]
- 81.Labayen I, Ortega F, Ruiz J, Lasa A, Simon E, Margareto J. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab. 2011;96:E996–E1000. doi: 10.1210/jc.2010-3006. [DOI] [PubMed] [Google Scholar]
- 82.Shih L, Liou T, Chao J, et al. Leptin, superoxide dismutase, and weight loss: initial leptin predicts weight loss. Obesity. 2006;14:2184–2192. doi: 10.1038/oby.2006.256. [DOI] [PubMed] [Google Scholar]
- 83.Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C. Measuring food intake with digital photography. J Hum Nutr Diet. 2014;27(Suppl 1):72–81. doi: 10.1111/jhn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin Nutr Metab Care. 2007;10:597–603. doi: 10.1097/MCO.0b013e328285d883. [DOI] [PubMed] [Google Scholar]
- 85.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res. 2014;16:e40. doi: 10.2196/jmir.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellegrini CA, Hoffman SA, Collins LM, Spring B. Optimization of remotely delivered intensive lifestyle treatment for obesity using the Multiphase Optimization Strategy: Opt-IN study protocol. Contemp Clin Trials. 2014;38:251–259. doi: 10.1016/j.cct.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]


