Summary
The availability of effective, simple, well tolerated oral direct acting antiviral (DAA) hepatitis C regimens have raised optimism for HCV elimination at the population level. HCV reinfection in key populations such as people who inject drugs (PWID) and HIV-infected men who have sex with men (MSM) however threatens the achievement of this goal both from a patient, provider and population perspective. The goal of this review is to synthesize our current understanding of estimated rates and factors associated with HCV reinfection. This review also proposes interventions to aid understanding of and reduce hepatitis C reinfection among PWID and HIV-infected MSM in the oral direct acting antiviral era.
Keywords: hepatitis C, reinfection, people who inject drugs, men who have sex with men
Introduction
Hepatitis C is a major public health challenge that affects 70 million people and is a leading cause of morbidity and mortality globally (1, 2). The availability of simple and effective oral direct acting antiviral (DAA) therapies of short duration with minimal side effects make HCV cure a possibility for those who have access to treatment. The ability to cure hepatitis C has also fueled optimism regarding the potential for HCV elimination. Indeed, the World Health Organization has set lofty HCV elimination targets of 90% diagnosed, 80% treated, and 65% reduction in mortality by 2030 (3).
The hepatitis C epidemic disproportionally affects people who inject drugs (PWID) and HIV infected men who have sex with men (MSM). These two populations are the core of the epidemic and sustain ongoing HCV transmission in many countries in which the incidence of healthcare related HCV transmission is low. As such, any effort to eliminate hepatitis C through treatment must address these two populations.
Effective HCV treatments lead to HCV cure in over 95% of patients treated with these oral DAA regimens (4). However, because HCV infection does not confer protective immunity, individuals who have cleared a previous infection either spontaneously or after treatment induced clearance remain at risk for reinfection. Reinfection is defined as the reoccurrence of HCV viremia after a previously cleared infection (5).
The potential for HCV reinfection remains a major obstacle to achieving the HCV elimination goals outlined by the WHO. From a patient perspective, concern for HCV reinfection remains a major driver for provider and health system level barriers to HCV treatment of persons perceived to be at high risk for reinfection after cure (6). The perception by many providers and payers is that PWID in particular will simply get reinfected after HCV treatment. As such, HCV treatment of PWID is often not considered worthwhile; as a consequence, many PWID in care, who could benefit from HCV treatment and cure are not offered these treatments. From a population perspective, elimination is dependent on treating enough infected individuals so as to reduce overall population prevalence and thus reduce the pool of individuals who can sustain the epidemic by transmitting infection to others. As such, even if HCV treatment were to penetrate populations of high risk individuals including PWID and HIV- infected MSM engaged in high risk sexual practices, reinfection represents a major obstacle to control. To achieve the WHO elimination goals, it is imperative that we have a clear understanding of rates and predictors of reinfection as this information can guide focused interventions to reduce this risk of reinfection in high risk groups. Most of the data available on HCV reinfection is from the interferon era and suggest that these rates are low. More recent data suggest that HCV reinfection rates may be higher in some subpopulations. Some experts speculate that easier DAA treatment may be associated with higher rates of reinfection due, in part, to an ability to offer HCV treatments to a wider pool of patients, who had hitherto not been candidates for HCV treatment in the interferon era. In this review, we summarize rates of HCV reinfection among PWID and HIV-infected MSM and characterize the subgroups most at risk. We also propose interventions to mitigate the risk of reinfection in these groups
Incidence of HCV Reinfection
The risk of HCV reinfection after sustained virologic response (SVR) has been reported in a number of studies including two recent meta-analyses published in the peer reviewed literature (7–21) (Table 1). Rates of HCV reinfection in the British Columbia Hepatitis Testers Cohort which includes all individuals tested for HCV or HIV at the British Columbia Center for Disease Control Public Health Lab from 1990–2013 were recently published by Islam and colleagues (20). This retrospective study represents the largest cohort to date (5915 individuals) reporting rates of HCV reinfection. Spontaneous clearance was defined as two consecutive negative HCV PCR tests (at least 28 days apart) after HCV diagnosis without treatment while SVR was defined as two consecutive negative HCV PCR tests at least 28 days apart, 12 weeks after HCV treatment. Reinfection was defined as a positive HCV PCR after initial HCV clearance. This cohort included 3690 individuals who achieved spontaneous HCV clearance and 2225 individuals who achieved SVR after interferon based HCV treatment. Overall, there were 452 reinfections for a reinfection rate of 1.27/100 person-years of follow-up. Among 2225 individuals who achieved SVR following treatment, reinfection was detected in 50 individuals. The post-SVR reinfection rate of this study (0.48/100 PY) is one of the lowest reported to date, suggesting low rates of HCV reinfection overall. Higher HCV reinfection rates were reported among PWID (1.14/100 PY) and persons with HIV infection (2.56/100PY). Sensitivity analysis, using less stringent criteria (one negative HCV test) to define clearance resulted in higher reinfection rates (3.34/100PY in PWID and 4.17/100PY in HIV infected individuals). These reported rates of HCV reinfection are similar to those of 1.21-4.9/100 PY reported in other retrospective and prospective studies of PWID treated for chronic HCV infection (12, 13, 17–19). The Islam study which represents one of the largest studies reported to date with the longest follow-up after clearance (35,672 person years), has the additional advantage of minimal loss to follow-up due to use of government health records and limited confounding due to a relatively long follow up period likely providing more valid estimates of reinfection rates among PWID with chronic HCV infection. This like other studies is however limited by its retrospective nature, lack of systematic testing of persons at risk for HCV reinfection at regular intervals and evaluation of post SVR HCV reinfection in individuals who received pegylated interferon based HCV treatment.
Table 1.
Overview of studies of hepatitis C reinfection
| Author, year | Years of Data Collection | Population | Location and Study Design | Recruitment | Number of subjects | No of reinfections | Reinfection Rate (per 100 PY (95% CI) or %) | Follow up Period (mean, median or total) |
|---|---|---|---|---|---|---|---|---|
| Systematic reviews and meta analyses | ||||||||
| Aspinall 2013 | PWID | Meta-analysis 5 studies |
131 | 7 | 2.4 (0.9–6.1) | |||
| Hagan 2015 | HIV infected MSM | Meta-analysis 2 studies |
170 | 38 | 11.4 (7.4–17.7) | |||
| Simmons 2016 | “Low risk” | Meta-analysis 31 studies |
7969 | 4 | 0.0 (0.0–0.0) | |||
| Simmons 2016 | High risk: PWID, incarcerated | Meta-analysis 14 studies |
771 | 36 | 1.9 (1.1–2.8) | |||
| Simmons 2016 | HIV/HCV coinfected | Meta-analysis 4 studies |
309 | 31 | 3.2 (0.0–12.3) | |||
| Interferon era studies | ||||||||
| Predominantly MSM studies | ||||||||
| Ingiliz 2014 | 2001 – 2013 | HIV infected MSM Acute HCV |
Retrospective Germany |
HIV and hepatitis care centers | 302 | 48 | 16% | Total: 12 Y |
| Ingiliz 2017 | 2002 – 2014 | HIV infected MSM Acute HCV |
Retrospective Europe |
Clinical centers within the NEAT network (European AIDS Treatment Network) | Total 606 SVC 111 SVR 494 |
149 | 7.3 7.3 7.8 |
Mean: 3.9 Y IQR 1.6–4.9 Y |
| Martin 2015 | 2002 – 2014 | HIV infected MSM | Retrospective United Kingdom |
Hospital based | 191 | 2002–2008 22 2008–2014 30 |
13.2 (8.7–20.1) 5.3 (3.7–7.6) |
Total: 589 PY |
| Vanhommerig 2014 | 2009 – 2014 | HIV infected MSM Acute HCV |
Retrospective Netherlands |
HIV outpatient clinic | 48 | 18 | 29% | Median: 4.0 Y IQR 2.5–5.7 Y |
| Predominantly PWID studies | ||||||||
| Weir 2016 | 1999 – 2012 | PWID | Retrospective Scotland |
Scottish HCV clinical database | 277 | 7 | 1.71 | Median 4.5 Y 410 PY |
| Islam 2017 | 1992 – 2013 | Retrospective British Columbia |
British Columbia Center for Disease Control PublicHealth database. |
5915 3690 (SVC) 2225 (SVR) |
452 402 50 |
1.27 1.59 0.48 |
Mean: 5.4 Y IQR: 2.9–8.7 Y |
|
| Pineda 2015 | 2001 – 2013 | HIV infectedPWID (93%) | Retrospective Spain |
Hospital based | 84 | 4 (total) 3 (PWID) 1 (MSM) |
1.21 Inhalational drug users 8.72 |
Mean: 2.8 Y Range: 1–12 Y |
| Midgard 2016 | 2004 – 2014 | PWID | Prospective Norway and Sweden |
RCT of short duration pegylated interferon and ribavirin in genotype 2 and 3 HCV | 94 | 12 | 1.7 Drug use relapsers 4.9 |
Median: 7.1 Y |
| Aitken 2016 | 2008 – 2014 | PWID, urban Acute |
Prospective Australia |
Community based PWID | 139 | 39 | 12.4 | 314.5 PY |
| Machouf 2015 | PWID (82%) | Prospective Canada |
Clinic based | 338 | 22 | Former PWID 1.7 Current PWID 3.6 |
Median:2.7 Y IQR 1.7–4.8 Y 1175 PY |
|
| Martinello 2016 | 2004–2015 | MSM (53%) PWID (70%) |
Prospective Australia and New Zealand |
Three prospective open-label studies of HCV treatment in recent (< 18 months) HCV infection | 120 | 10 | 7.4 | 135 PY |
| Young* 2017 | 2003 –2016 | HIV infected MSM PWID (74%) MSM (33%) |
Retrospective Canada |
HIV clinics across 6 Canadian provinces | 257 | 18 | 3.1 | Median 1.5 Y IQR 2.9–8.7 Y 589 PY |
| Oral DAA-era studies | ||||||||
| Dore 2016 | 2014–2016 | PWID on OST | Prospective International |
RCT of elbasvir/grazoprevir in patients on OST (80% adherent to OST visits) | 301 | 6 | 4.6 | 24 weeks post EOT |
| Dore 2017 (A subset of Dore 2016) |
2014–2017 | PWID on OST | Prospective International |
RCT of elbasvir/grazoprevir in patients on OST | 199 | 10 | 2.3 | 24 months post EOT |
51 of 257 patients received oral-DAA therapies
PWID-People who inject drugs
MSM-Men who have sex with men
EOT-End of treatment
OST-Opioid substitution therapy
Y-Years
PY-Person years
Since the populations of patients currently being treated with oral DAA therapy are somewhat different from the selected group of patients who received interferon (22), the generalizability of these interferon era reinfection rates to the oral DAA era may be limited. However, it is reassuring to note, that in the clinical trials conducted by Dore and colleagues among 301 PWID receiving opioid substitution therapy (OST) who achieved SVR after receipt of the oral DAA combination of elbasvir/grazoprevir and who were followed prospectively for up to 24 weeks after SVR, rates of HCV reinfection were only 4.6/100PY(17). A subgroup of 199 of these PWID; 37% of whom reported injection drug use after HCV cure, were enrolled in ongoing follow up, through to 3 years after the end of treatment. In the first 24 months of follow up, there were only 4 additional reinfections reported for a total of 10 reinfections and a reinfection rate of 2.3/100PY. Of note, 3 of the 10 individuals reinfected went on to clear their HCV reinfections spontaneously, leading to an HCV persistence reinfection rate of 1.6/100PY. Consistent with interferon era studies, PWID reporting ongoing injection drug use after cure had higher HCV reinfection rates than those not reporting injection drug use after cure (HCV reinfection rate 4.2/100PY vs. 0.4/100PYs)(21). While these data are encouraging, they are limited by small sample size and short follow-up. For example, in the study by Midgard et al., HCV reinfections were noted up to 7 years after initial HCV cure. Moreover, data are needed from persons treated outside of the clinical trial setting who may be at heightened risk for reinfection.
Nevertheless, these studies also provide some information on risk factors for reinfection. It is not surprising to note that current injection drug use, especially high frequency drug use with cocaine and methamphetamines was associated with higher rates of reinfection (13). In one study of HIV-infected PWID which also included MSM, ongoing drug use including use of drugs by inhalation, which may be associated with high-risk sexual practices, was associated with an 8-fold increase in risk of HCV reinfection (12). It thus makes intuitive sense that, in the British Columbia Testers Cohort, interventions that reduced drug use, as measured by proxies such as utilization of opiate agonist therapy and mental health services, were associated with a reduction in HCV reinfection risk (20). However, other factors such as younger age, male sex, and HIV coinfection have been associated with an increased risk of HCV reinfection (20).
Multiple studies including a recent meta-analysis have reported higher rates of HCV reinfection among HIV-infected persons (7, 20). The role of HIV infection in increasing the risk of HCV reinfection is not completely clear. HIV infection is associated with an approximately 3-fold reduction in rates of spontaneous recovery from an acute HCV infection (23, 24). As such, one may postulate that HIV-infected persons have similar rates of HCV reinfection to HIV uninfected persons but may be less likely to spontaneously clear these reinfections and are thus more likely to have reinfections detected. Alternatively, it is also possible that HIV-infected persons studied in these predominantly male cohorts may represent HIV-infected MSM with high risk sexual practices which led to both initial HCV infection and reinfection. The rates of HCV reinfection in HIV-infected MSM in the interferon era range from 5.3-13.2/100PY (10, 14, 25). These HCV reinfection data among MSM also suggest that a subgroup of these individuals will go on to have multiple HCV reinfections (10). An additional concern is the risk of transmission of HCV virus with resistant variants (26).
A recent study by Martinello and colleagues among individuals treated for recent HCV infection (<18 months HCV infection duration), evaluated reinfection in four prospective studies of interferon based HCV treated individuals in Australia and New Zealand (16). Ten HCV reinfection cases were identified among 120 individuals; 64 (53%) of whom where HIV-infected MSM and 84 (69%) who had a history of injection drug use. Seven of the 10 reinfections occurred in HIV-infected MSM of whom 5 reported injection drug use (IDU) during follow up. The other 3 reinfections occurred in HIV-uninfected PWID who also reported IDU during follow up. The overall reinfection incidence was 7.4/100 PYs (95% CI 4.0–13.8) which resulted in a projected cumulative reinfection incidence of 7.2% at 1 year and 14.5% at 2 years from end of treatment. Consistent with previous studies the incidence of reinfection was higher in HIV-coinfected individuals 10.3/100 PY (95% CI 4.9–21.7) than HIV uninfected (4.5/100 PY (95% CI 1.4–13.9); though not statistically significantly different (P=0.23). Conversely, injection drug use at end of, or post treatment was associated with a significantly higher risk of reinfection (incidence rate (IR) 15.5/100 PY [95% CI 7.8–31.1] versus IR 2.6/100PY [95% CI 0.6–10.3]; adjusted incidence rate ratio 7.86, p-value 0.0008). It is important to note that that median duration of injection drug use at enrollment was shorter among those with reinfection compared to those without reinfection (2.8 years versus 6.2 years). This is consistent with our prior understanding and knowledge of incident HCV infection; that is, if PWID remain HCV uninfected after 5 years of injecting drugs, they are unlikely to acquire HCV infection. Experience with injection drug use and engaging in harm reduction practices may thus reduce both the risk of HCV infection and reinfection.
The other issue that the Martinello study highlights is the overlap of sexual and injection drug use risk factors in increasing the risk of HCV reinfection. Traditionally, individuals at risk of reinfection have been grouped as HIV-infected MSM and PWID; however, there is clearly a subset of HIV-infected men who use injection drugs and have sex with men. Although the exact mode of HCV transmission among HIV-infected MSM is unknown, it usually occurs in the setting of high risk practices such as unprotected, traumatic sex with increased chances of blood contact. These high risk practices however also frequently occur in the setting of recreational drug use, including injection drugs in the setting of chemsex (the use of drugs to increase sexual disinhibition and arousal)(27). As such interventions targeted at both safer sexual practices and safer drug use practices are indicated among HIV-infected MSM.
Understanding and preventing HCV reinfection
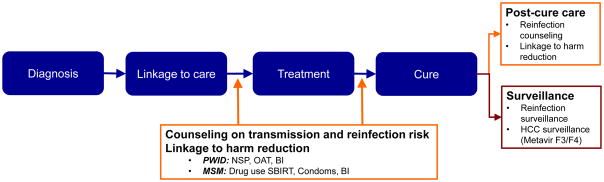
Concerted efforts to eliminate HCV through treatment will require treatment of individuals who are actively transmitting HCV to others and thereby replenishing or sustaining the population of HCV-infected persons; successful and sustained HCV cure of such persons is critical to the reduction of HCV prevalence. Although counterintuitive to some, a key marker that the population of persons at risk for HCV transmission to others has been successfully targeted for treatment is the occurrence of reinfection in the social network. While strategies must be incorporated to minimize reinfections, the detection of reinfection in a treated population should not be interpreted to be a programmatic failure but rather should be considered in the larger context of the specific elimination effort. An individual’s risk of HCV reinfection after treatment and cure should be considered as part of the initial evaluation of every person for whom curative HCV treatment is considered. As with other diseases such as sexually transmitted infections, this risk assessment should not deter HCV treatment of these individuals. Rather, it should be used to tailor an appropriate strategy of education, counselling and linkage to services which will help the individual to reduce his or her risk of subsequent reinfection (Figure 1). Multipronged interventions combining harm reduction and behavioral interventions will be most effective in reducing rates of HCV reinfection (28). These interventions will vary based on specific behaviors and contributing conditions (e.g., depression or alcohol use disorders) but at a minimum must include education on the risk of reinfection including the practices which will increase risk, brief evidence-based behavioral interventions and linkage to harm reduction services. Among actively injecting PWID this will clearly require linkage to opioid substitution therapy (OST) which has been shown to reduce injection frequency significantly (29, 30) and needle and syringe programs (NSP) which aid adoption of safer injection practices through provision of needles, syringes and other drug use paraphernalia (28). In all persons, depression or untreated mental illness may also be an important contributor to risky behaviors (20); concurrent screening for and treatment of mental illness should be strongly considered at the time of HCV care engagement. Similarly, social conditions such as homelessness may also enhance reinfection risk and should be assessed and addressed (29). (Table 2).
Figure 1.
HCV care continuum+: Preserving the benefits of HCV cure
*PWID-People who inject drugs; NSP-Needle/syringe services program; OAT-Opioid agonist treatment; BI-behavioral intervention; MSM-Men who have sex with men; SBIRT-Screening, brief intervention, referral for treatment as needed
Table 2.
Risk factors for HCV reinfection and proposed reinfection prevention interventions
| Population | Risk Factor for HCV infection or reinfection | HCV reinfection prevention Intervention |
|---|---|---|
| PWID | Relapse into IDU (18) High frequency injection drug use including use of cocaine and methamphetamines(13) Hospitalization for opiate and IDU related causes(19) Sharing injection use paraphernalia |
|
| Shorter duration of injection(39) Younger age (Age <30 years) Low levels of education Poor social functioning |
|
|
| MSM | Receptive anal intercourse without a condom(40) Rectal trauma with bleeding(41) Inconsistent condom use(42) Sex while using illicit drugs (chemsex)(40) |
|
| Contextual factors | Continued injection drug use and sharing of drug use paraphernalia after HCV cure Continued high risk sexual practices after HCV cure |
|
|
||
|
Risk factors for initial HCV infection likely also predispose to HCV reinfection and have been included in this table
Aside from individual factors, contextual factors should also be taken into account and addressed. Drug use and high risk sexual practices and the attendant risk of HCV infection and reinfection do not occur in isolation. Clusters of HCV infection have been identified among networks of high risk HIV-infected MSM (31). Similarly, HCV clusters have been shown to overlap with reported injection relationships among PWID (32). Injection partnerships and sharing of drug use paraphernalia have also been associated with HCV reinfection (32). Collectively, these data support the notion that treatment of a group or network of PWID concurrently may be the most effective strategy to reduce HCV disease burden through cure and prevention of reinfection by reducing the reservoir of persons with active hepatitis C viremia (community viral load) in the group. This notion is further supported by recent mathematical modeling data based on observed HCV transmission patterns among PWID in Australia, suggesting that a “treat your friends strategy” performed better at reducing overall HCV burden than a random treatment strategy among PWID (33). While awaiting empirical proof, this is an exciting concept and suggests that treatment of PWID and their drug use partners simultaneously may reduce HCV reinfection in networks of PWID.
Additionally the availability of an effective HCV vaccine will be critical for elimination efforts not only to prevent initial infections but also reinfections (34). Efforts should continue to develop such a vaccine because even if only partially effective, the combination of a vaccine with available harm reduction measures may be enough to significantly reduce the risk of HCV reinfection in higher risk individuals.
Lastly, a recognition that the HCV care continuum does not end with viral cure is critical; surveillance for HCV reinfection in the oral DAA era needs to be expanded and become a routine part of clinical care (Figure 1). In this regard, a simple clinical definition of HCV reinfection would greatly ease the process for reinfection surveillance since comparison of HCV variants by sequencing before and at the time of recurrent viremia (the gold standard for detecting HCV reinfection) is often not feasible outside of very specialized research settings. Defining reinfection in real world clinical practice is essential to monitoring for reinfection; further, a uniform definition that can be applied across clinical studies of HCV reinfection in the oral DAA era is essential to compare incidence rates and to evaluate the effectiveness of risk reduction interventions.
Defining reinfection in the oral DAA era
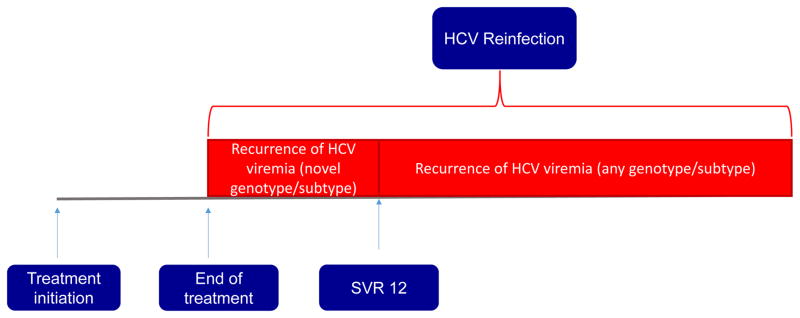
HCV reinfection after treatment induced clearance of the virus is suspected when an individual has reoccurrence of viremia after HCV clearance. HCV clearance is believed to have occurred when hepatitis C virus (most commonly through HCV RNA testing) is not detectable at the lowest limit of detection after HCV treatment is completed. Evidence of HCV clearance is often evaluated at the end of an HCV treatment course and then 12 weeks after end of treatment. This testing conducted 12 weeks after end of treatment seeks to assess for sustained virologic response (SVR 12) which by definition is achieved if blood testing is negative for HCV RNA at this time point. On the basis of extensive follow-up studies, SVR 12 is widely considered evidence of HCV viral eradication and considered to be equivalent to virologic cure. However, some individuals will have reoccurrence of viremia either between the end of treatment and the time point for assessment of SVR 12 or after the time point for SVR 12. This may occur either due to viral relapse (in which the HCV persisted in the individual and remerged) or due to reinfection due to transmission of HCV from another individual. Traditionally, the ascertainment of reinfection has depended on a switch in HCV genotype from the pretreatment to the post treatment virus or phylogenetic analysis of the individuals HCV pretreatment and post- treatment virus to assess for similarities in these viruses. In the oral DAA era, rates of virologic relapse after the achievement of SVR12 are exceeding low. When virologic relapse occurs, it usually occurs within the first 4 weeks after end of treatment (35). Most patients who achieve SVR 12 will go on to achieve SVR 24 and remain HCV RNA not detected for years (36). These data provide the basis for a clinical diagnosis of probable HCV reinfection in the absence of definitive virologic evidence which is difficult, if not impossible, to obtain in routine practice. Probable reinfection can be defined as the detection of HCV viremia in persons who achieved SVR12 following completion of treatment with an effective oral DAA combination regimen with proven efficacy of 95% or greater who also have probable or definite risk factors for reinfection. If available, a genotype switch in pretreatment and post- treatment virus may also be considered clinical evidence of HCV reinfection (Figure 2).
Figure 2.
Algorithm for defining HCV reinfection in clinical practice
A proposed clinical definition for HCV reinfection when the gold standard of phylogenetic analysis of pre- and post-treatment HCV sequences are not available
Summary
Currently available data suggest low rates of HCV reinfection in the interferon era. While data from small samples support low rates of HCV reinfection in the DAA era, there is a need for data from larger real-world samples in the DAA era which describes both the rates of reinfection and associated factors. This information should inform the development of targeted interventions to reduce this risk of reinfection. HCV reinfection should be minimized but not feared as we work towards HCV elimination. Rather reinfection should be viewed as an indication of uptake of hepatitis C treatment in the populations most likely to sustain ongoing HCV epidemics. HCV reinfections should be minimized by coupling HCV treatment to harm reduction and well-designed targeted behavioral interventions.
Acknowledgments
Financial Support
This work was supported by NIH/NIDA grants K23DA041294 (to OFN), K24DA034621 (to MS) and P30 AI094189
Footnotes
Conflicts of interest
Dr. Sulkowski reports grants and personal fees from AbbVie, grants from BMS, personal fees from Cocrystal, grants and personal fees from Gilead, grants and personal fees from Janssen, grants and personal fees from Merck, personal fees from Trek, outside the submitted work.
References
- 1.Global Hepatitis C Report. Vol. 2017 World Health Organization; 2017. May 13, http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1. [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–8. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Draft global health sector strategy on viral hepatitis, 2016–2021- The first of it’s kind. 2015 [Google Scholar]
- 4.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166(9):637–48. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackard JT, Sherman KE. Hepatitis C virus coinfection and superinfection. J Infect Dis. 2007;195(4):519–24. doi: 10.1086/510858. [DOI] [PubMed] [Google Scholar]
- 6.Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Subst Use Misuse. 2016;51(9):1218–23. doi: 10.3109/10826084.2016.1161054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62(6):683–94. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(Suppl 2):S80–9. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 9.Ingiliz P, Krznaric I, Stellbrink HJ, Knecht G, Lutz T, Noah C, et al. Multiple hepatitis C virus (HCV) reinfections in HIV-positive men who have sex with men: no influence of HCV genotype switch or interleukin-28B genotype on spontaneous clearance. HIV Med. 2014;15(6):355–61. doi: 10.1111/hiv.12127. [DOI] [PubMed] [Google Scholar]
- 10.Ingiliz P, Martin TC, Rodger A, Stellbrink HJ, Mauss S, Boesecke C, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66(2):282–7. doi: 10.1016/j.jhep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Vanhommerig JW, Thomas XV, van der Meer JT, Geskus RB, Bruisten SM, Molenkamp R, et al. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis. 2014;59(12):1678–85. doi: 10.1093/cid/ciu695. [DOI] [PubMed] [Google Scholar]
- 12.Pineda JA, Nunez-Torres R, Tellez F, Mancebo M, Garcia F, Merchante N, et al. Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J Infect. 2015;71(5):571–7. doi: 10.1016/j.jinf.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Young J, Rossi C, Gill J, Walmsley S, Cooper C, Cox J, et al. Risk Factors for Hepatitis C Virus Reinfection After Sustained Virologic Response in Patients Coinfected With HIV. Clin Infect Dis. 2017;64(9):1154–62. doi: 10.1093/cid/cix126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin TC, Singh GJ, McClure M, Nelson M. HCV reinfection among HIV-positive men who have sex with men: a pragmatic approach. Hepatology. 2015;61(4):1437. doi: 10.1002/hep.27391. [DOI] [PubMed] [Google Scholar]
- 15.Aitken CK, Agius PA, Higgs PG, Stoove MA, Bowden DS, Dietze PM. The effects of needle-sharing and opioid substitution therapy on incidence of hepatitis C virus infection and reinfection in people who inject drugs. Epidemiol Infect. 2017;145(4):796–801. doi: 10.1017/S0950268816002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinello M, Grebely J, Petoumenos K, Gane E, Hellard M, Shaw D, et al. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat. 2017;24(5):359–70. doi: 10.1111/jvh.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165(9):625–34. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 18.Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020–6. doi: 10.1016/j.jhep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Weir A, McLeod A, Innes H, Valerio H, Aspinall EJ, Goldberg DJ, et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug Alcohol Depend. 2016;165:53–60. doi: 10.1016/j.drugalcdep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Islam N, Krajden M, Shoveller J, Gustafson P, Gilbert M, Buxton JA, et al. Incidence, risk factors, and prevention of hepatitis C reinfection: a population-based cohort study. Lancet Gastroenterol Hepatol. 2017;2(3):200–10. doi: 10.1016/S2468-1253(16)30182-0. [DOI] [PubMed] [Google Scholar]
- 21.Dore GGJ, Altice F, Litwin A, Dalgard O, Gane E, editors. American Association for the Study of Liver Diseases. Washington DC: 2017. Hepatitis C virus reinfection and injection risk behavior following Elbasvir/Grazoprevir treatment in participants on opiate agonist therapy: C-edge costar part B. [Google Scholar]
- 22.Janjua NZ, Islam N, Wong J, Yoshida EM, Ramji A, Samji H, et al. Shift in disparities in hepatitis C treatment from interferon to DAA era: A population-based cohort study. J Viral Hepat. 2017;24(8):624–30. doi: 10.1111/jvh.12684. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 24.Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, et al. Protection against persistence of hepatitis C. Lancet. 2002;359(9316):1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 25.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS. 2015;29(17):2335–45. doi: 10.1097/QAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abravanel F, Metivier S, Chauveau M, Peron JM, Izopet J. Transmission of HCV NS5A Inhibitor-Resistant Variants Among HIV-Infected Men Who Have Sex With Men. Clin Infect Dis. 2016;63(9):1271–2. doi: 10.1093/cid/ciw554. [DOI] [PubMed] [Google Scholar]
- 27.Pufall Erica LMK, Shahmanesh Maryam, Nardone Anthony, Gilson Richard, Delpech Valerie, Ward Helen Postive Voices Study Group. Chemsex and High-Risk Sexual Behaviours in HIV-Positive Men Who Have Sex With Men. Conference on Retroviruses and Opportunistic Infections; Boston. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204(1):74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craine N, Hickman M, Parry JV, Smith J, Walker AM, Russell D, et al. Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect. 2009;137(9):1255–65. doi: 10.1017/S095026880900212X. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M, Amsterdam C. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–62. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136(5):1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacks-Davis R, Daraganova G, Aitken C, Higgs P, Tracy L, Bowden S, et al. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PLoS One. 2012;7(10):e47335. doi: 10.1371/journal.pone.0047335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellard M, Rolls DA, Sacks-Davis R, Robins G, Pattison P, Higgs P, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–70. doi: 10.1002/hep.27403. [DOI] [PubMed] [Google Scholar]
- 34.Cox AL. MEDICINE. Global control of hepatitis C virus. Science. 2015;349(6250):790–1. doi: 10.1126/science.aad1302. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida EM, Sulkowski MS, Gane EJ, Herring RW, Jr, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61(1):41–5. doi: 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 36.Sarrazin C, Isakov V, Svarovskaia ES, Hedskog C, Martin R, Chodavarapu K, et al. Late Relapse Versus Hepatitis C Virus Reinfection in Patients With Sustained Virologic Response After Sofosbuvir-Based Therapies. Clin Infect Dis. 2017;64(1):44–52. doi: 10.1093/cid/ciw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt L, Reed J, Minozzi S, Vickerman P, Hagan H, French C, et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev. 2016;2016(1) doi: 10.1002/14651858.CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021. doi: 10.1002/14651858.CD012021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacks-Davis R, Aitken CK, Higgs P, Spelman T, Pedrana AE, Bowden S, et al. High rates of hepatitis C virus reinfection and spontaneous clearance of reinfection in people who inject drugs: a prospective cohort study. PLoS One. 2013;8(11):e80216. doi: 10.1371/journal.pone.0080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease C, Prevention. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60(28):945–50. [PubMed] [Google Scholar]
- 41.Schmidt AJ, Rockstroh JK, Vogel M, An der Heiden M, Baillot A, Krznaric I, et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany--a case-control study. PLoS One. 2011;6(3):e17781. doi: 10.1371/journal.pone.0017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wandeler G, Gsponer T, Bregenzer A, Gunthard HF, Clerc O, Calmy A, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55(10):1408–16. doi: 10.1093/cid/cis694. [DOI] [PubMed] [Google Scholar]
- 43.Myers JJ, Shade SB, Rose CD, Koester K, Maiorana A, Malitz FE, et al. Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: results from the Health Resources and Services Administration (HRSA)’s Special Projects of National Significance initiative. AIDS Behav. 2010;14(3):483–92. doi: 10.1007/s10461-010-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson JL, Milam J, McCutchan A, Stoyanoff S, Bolan R, Weiss J, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: a multi-clinic assessment. AIDS. 2004;18(8):1179–86. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]