Abstract
Familial Mediterranean fever (FMF) has been associated with hematological malignancies but has not been reported in association with Hodgkin lymphoma (HL). We hereby describe the first pediatric patient with FMF and stage IIA nodular sclerosis HL. She was treated with prednisone, doxorubicin, vincristine and etoposide (OEPA) being on therapy with colchicine. However, she suffered more than expected treatment-related toxicity attributed either to chemotherapy (severe neutropenia) or colchicine (Abdominal pains and diarrhoea). Colchicine had to be discontinued. In the absence of colchicine, she tolerated very well the second cycle of chemotherapy. Currently, she is in remission at 17 months after her HL diagnosis, and her FMF is under control with colchicine without any signs of toxicity.
Keywords: Hodgkin lymphoma, Familial Mediterranean fever, Colchicine toxicity, Drugs interaction
Introduction
Hodgkin lymphoma (HL) is a lymphoid malignancy that accounts for approximately 7% of childhood cancers in the United States.1 Five-year survival rates are 98% and 97% for the <15 years and 15–19 years age cohorts, respectively. Several risk factors have been associated with developing HL, such as EBV infection,2 primary immune deficiencies3 and autoimmune lymphoproliferative syndrome (ALPS).4
Familial Mediterranean fever (FMF) (OMIM 249100) is an autoinflammatory syndrome that can be adequately treated in the majority of cases with colchicine.5 Clinically, FMF is characterized by recurrent episodes of fever, serositis, arthritis, dermal manifestations and long-term renal complications.6 The goals of FMF treatment are to prevent acute attacks, decrease the subclinical inflammation between the attacks and to prevent the development of amyloidosis. Colchicine suppresses pyrin oligomerization and interferes with neutrophil migration. It also prevents cytoskeletal changes that lead to pyrin inflammasome assembly. Alternative treatment options, particularly for colchicine-resistant or intolerant patients include interleukin-1 inhibitors such as anakinra and canakinumab.7 FMF has been reported in association with adult hematological malignancies such as multiple myeloma, acute lymphoblastic and myeloid leukemias and myelodysplastic syndromes.7–12 However, this entity has not been reported in association with HL in adults or children. We describe the first case of HL in a pediatric patient with FMF, who developed myelotoxicity due to the combination of colchicine with chemotherapy.
Case Presentation
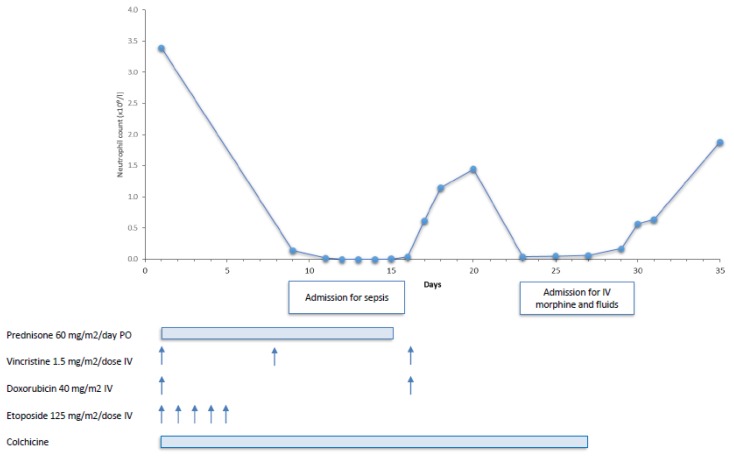
A 6-year-old girl, of Turkish descent, presented with a 6-week history of right posterior cervical lymphadenopathy. She had no recent history of fever, night sweats or weight loss. Past medical history was significant for FMF with a known M694V mutation in the MEFV gene (and an r40w variant of unknown significance in the MVK gene), diagnosed at age one year and well managed with colchicine 0.3 mg orally twice/day. Complete blood count (CBC) showed white blood cell count 8.3 × 103/μl, hemoglobin 130 g/L, and platelet count 298,000/μl. Lactate dehydrogenase was 850 IU/L, uric acid 174 umol/L and ESR 2 mm/h. An excisional biopsy of a cervical lymph node showed effacement of the normal nodal architecture with a smaller population of large atypical cells with large prominent eosinophilic nucleoli and occasional classical diagnostic Reed-Sternberg cells. Immunohistochemical stains revealed positivity for CD30, CD15 and PAX5. The tumour cells were focally positive for CD3 and negative for ALK-1, CD45 and CD20. In situ hybridization for EBV-EBER (where EBER is Epstein–Barr encoding region) was positive in the majority of large atypical cells. Staging included PET computerized tomography which demonstrated localized hypermetabolic right cervical lymph nodes with the absence of bulk disease. Therefore, the final diagnosis was stage IIA classical Hodgkin lymphoma, nodular sclerosis subtype. The patient (Figure 1) was treated according to the local standard of care for low risk HL using 2 cycles of OEPA (vincristine 1.5 mg/m2/dose IV days 1, 8 and 15, etoposide 125 mg/m2/dose IV days 1–5, prednisone 60 mg/m2/day PO days 1–15, and doxorubicin 40 mg/m2 IV days 1 and 15) chemotherapy only.13
Figure 1.
Clinical course and neutrophils count in relationship with drugs administration.
Her first treatment course was complicated by prolonged myelosuppression including CTCAE grade 4 neutropenia, fever and severe abdominal pain. She needed to be admitted on day 8 for clinical sepsis, requiring three intravenous fluid boluses and antibiotics. Furthermore the second infusion of Vincristine and Doxorubicin was postponed to Day 16. No other signs of colchicine toxicity, such as bloody stool, renal failure or disseminated intravascular coagulation were noted. Blood cultures remained negative. She was discharged home after twelve days but needed readmission on day 22 due to severe abdominal pain and loose stools, treated with intravenous hydration and morphine for nine days. After consultation with the Rheumatology service, colchicine was discontinued from day 27 of cycle 1 onwards, to prevent possible interactions with chemotherapy. Cycle 2 was started 36 days after start of cycle 1. She tolerated this much better, without any complications and count recovery within the expected timeframe. Clinical and radiographic evaluations showed no evidence of disease at the end of therapy. The patient currently remains in remission 17 months after completion of therapy. Subsequently, she developed similar fever attacks and colchicine was resumed at the same dose 3 months after completion of chemotherapy. Her FMF has been well controlled since, without any signs of colchicine toxicity.
Discussion
Familial Mediterranean fever (FMF) is an autosomal recessive disorder, although autosomal dominant and heterozygous patients have been reported as well. It is characterized by recurrent self-limiting acute painful attacks of fever and inflammation in the peritoneum or pleura, arthritis, splenomegaly and skin rashes, lasting 12–72 hours. Amyloidosis with renal failure is a complication and may develop without overt crises.6 Several sets of criteria have been developed in order to classify the disease, such as the Tel Hashomer criteria in adults and the modified criteria in childhood.14–15 The disease is associated with mutations in the MEFV gene, localized on chromosome 16p13, encoding for the protein pyrin, which is involved in the regulation of inflammation and apoptosis.16 It has an important role in the innate immune system and interacts with caspase-1 and other inflammasome components to regulate interleukin IL-1β production. Different genotypes lead to distinct phenotypes. One of the most common mutations, including among the Turkish population, is the 2080A-G transition in the pyrin gene, resulting in a met694-to-val (M694V) substitution.17 In adults, a high frequency of carriers of the MEFV gene in patients with hematological neoplasms has been reported, none of whom had a diagnosis of Hodgkin lymphoma. However, it remains unclear how inherited variants in the MEFV gene are associated with tumor susceptibility or promotion in hematologic neoplasms.10
Management of FMF with colchicine has benefitted patients by reducing painful attacks and preventing amyloidosis.18 Therefore, this drug was continued during initiation of anti-cancer treatment. However, our patient did not tolerate the combination with the low-intensity chemotherapy regimen.
Colchicine has a narrow therapeutic index. It is readily absorbed after oral administration, but has a variable bioavailability (ranging from 25% to 88%) and undergoes extensive first-pass metabolism. It has a wide volume of distribution (Vd) and binds to intracellular elements. Colchicine is primarily metabolized by the liver, undergoes significant enterohepatic re-circulation, and is also excreted by the kidneys. CYP3A4 and P-glycoprotein inhibitors can increase serum colchicine concentrations. Etoposide, prednisone, vincristine and doxorubicin are substrates of CYP3A4, and therefore the clearance of these drugs may decrease with concurrent administration of colchicine, and their side effects may increase. Since colchicine is also a substrate of CYP3A4, its clearance may be decreased by these drugs due to competitive inhibition. In addition, the target for both colchicine and vincristine is tubulin beta chain which may add to toxicity when administered together.19 Thus, our patient may have experienced gastrointestinal adverse effects and prolonged bone marrow suppression, as described in patients with colchicine toxicity, due to CYP3A4-mediated impairment of colchicine or antineoplastic agent metabolism or an additive effect on tubulin inhibition.20
Colchicine was discontinued in our patient and the second cycle of chemotherapy was well tolerated, without fever or abdominal pain. She is currently disease-free 17 months after completing therapy.
In summary, to the best of our knowledge, we describe the first case of Hodgkin lymphoma in a patient with familial Mediterranean fever. Colchicine should be given with caution in patients treated with drugs substrate of CYP3A4 and/ or effecting on tubulin polymerization, like vincristine. To this end, a strict collaboration between oncology and rheumatology is warranted when managing these patients.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014 Mar-Apr;64(2):83–103. doi: 10.3322/caac.21219. https://doi.org/10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Kim Y, Choi JW, Kim YS. Prevalence and prognostic significance of Epstein-Barr virus infection in classical Hodgkin’s lymphoma: a meta-analysis. Arch Med Res. 2014 Jul;45(5):417–31. doi: 10.1016/j.arcmed.2014.06.001. https://doi.org/10.1016/j.arcmed.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Tran H, Nourse J, Hall S, Green M, Griffiths L, Gandhi MK. Immunodeficiency-associated lymphomas. Blood Rev. 2008 Sep;22(5):261–81. doi: 10.1016/j.blre.2008.03.009. https://doi.org/10.1016/j.blre.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rösen-Wolff A, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001 Jul 1;98(1):194–200. doi: 10.1182/blood.v98.1.194. https://doi.org/10.1182/blood.V98.1.194. [DOI] [PubMed] [Google Scholar]
- 5.Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med. 1972 Dec 21;287(25):1302. doi: 10.1056/NEJM197212212872514. [DOI] [PubMed] [Google Scholar]
- 6.Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol. 2017 Aug;36(8):1707–1713. doi: 10.1007/s10067-017-3715-5. https://doi.org/10.1007/s10067-017-3715-5. [DOI] [PubMed] [Google Scholar]
- 7.Soriano A, Verecchia E, Afeltra A, Landolfi R, Manna R. (2013) IL-1β biological treatment of familial Mediterranean fever. Clin Rev Allergy Immunol. 2013 Aug;45(1):117–30. doi: 10.1007/s12016-013-8358-y. https://doi.org/10.1007/s12016-013-8358-y. [DOI] [PubMed] [Google Scholar]
- 8.Celik S, Tangi F, Oktenli C. Increased frequency of Mediterranean fever gene variants in multiple myeloma. Oncol Lett. 2014 Oct;8(4):1735–1738. doi: 10.3892/ol.2014.2407. Epub 2014 Aug 4. https://doi.org/10.3892/ol.2014.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayan O, Kilicaslan E, Celik S, Tangi F, Erikci AA, Ipcioglu O, et al. High Frequency of Inherited Variants in the MEFV Gene in Acute Lymphocytic Leukemia. Indian J Hematol Blood Transfus. 2011 Sep;27(3):164–8. doi: 10.1007/s12288-011-0095-x. https://doi.org/10.1007/s12288-011-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oktenli C, Celik S. High frequency of inherited variants in the MEFV gene in patients with hematologic neoplasms: a genetic susceptibility? Int J Hematol. 2012 Apr;95(4):380–5. doi: 10.1007/s12185-012-1061-6. https://doi.org/10.1007/s12185-012-1061-6. [DOI] [PubMed] [Google Scholar]
- 11.Celik S, Oktenli C, Kilicaslan E, Tangi F, Sayan O, Ozari HO, et al. Frequency of inherited variants in the MEFV gene in myelodysplastic syndrome and acute myeloid leukemia. Int J Hematol. 2012 Mar;95(3):285–90. doi: 10.1007/s12185-012-1022-0. https://doi.org/10.1007/s12185-012-1022-0. [DOI] [PubMed] [Google Scholar]
- 12.Celik S, Erikci AA, Tunca Y, Sayan O, Terekeci HM, Umur EE, et al. The rate of MEFV gene mutations in hematolymphoid neoplasms. Int J Immunogenet. 2010 Oct;37(5):387–91. doi: 10.1111/j.1744-313X.2010.00938.x. https://doi.org/10.1111/j.1744-313X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorffel W, Luders H, Ruhl U. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin’s disease in children and adolescents: Analysis and outlook. Klin Padiatr. 2003;215:139–145. doi: 10.1055/s-2003-39372. https://doi.org/10.1055/s-2003-39372. [DOI] [PubMed] [Google Scholar]
- 14.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997 Oct;40(10):1879–85. doi: 10.1002/art.1780401023. https://doi.org/10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 15.Yalçinkaya F, Ozen S, Ozçakar ZB, Aktay N, Cakar N, Düzova A, et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology (Oxford) 2009 Apr;48(4):395–8. doi: 10.1093/rheumatology/ken509. https://doi.org/10.1093/rheumatology/ken509. [DOI] [PubMed] [Google Scholar]
- 16.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. https://doi.org/10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz E, Ozen S, Balci B, Duzova A, Topaloglu R, Besbas, et al. Mutation frequency of familial Mediterranean fever and evidence for a high carrier rate in the Turkish population. Europ J Hum Genet. 2001;9:553–555. doi: 10.1038/sj.ejhg.5200674. https://doi.org/10.1038/sj.ejhg.5200674. [DOI] [PubMed] [Google Scholar]
- 18.Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986 Apr 17;314(16):1001–5. doi: 10.1056/NEJM198604173141601. https://doi.org/10.1056/NEJM198604173141601. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012 Nov;29(11):2943–71. doi: 10.1007/s11095-012-0828-z. https://doi.org/10.1007/s11095-012-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila) 2010 Jun;48(5):407–14. doi: 10.3109/15563650.2010.495348. https://doi.org/10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]