Abstract
Background
Dengue is a major health issue with seasonal rise in dengue fever cases imposing an additional burden on hospitals, necessitating bolstering of services in the emergency department, laboratory with creation of additional dengue fever wards.
Objectives
To study the clinical and hematological profile of dengue fever cases presenting to a hospital.
Methods
Patients with fever and other signs of dengue with either positive NS1 antigen test or IgM or IgG antibody were included. Age, gender, clinical presentation, platelet count and hematocrit were noted and patients classified as dengue fever without warning signs (DF) or with warning signs (DFWS), and severe dengue (SD) with severe plasma leakage, severe bleeding or severe organ involvement. Duration of hospitalization, bleeding manifestations, requirement for platelet component support and mortality were recorded.
Results
There were 443 adults and 57 children between 6 months to 77 year age. NS1 was positive in 115 patients (23%). Fever (99.8%) and severe body ache (97.4%) were the commonest presentation. DF was seen in 429 (85.8 %), DFWS in 55 (11%), SD with severe bleeding in 10 (2%) and SD with severe plasma leakage in 6 cases (1.2%). Outpatient department (OPD) treatment was needed in 412 (82%) and hospitalization in 88 (18%). Intravenous fluid resuscitation was needed in 16 (3.2%) patients. Thrombocytopenia was seen in 335 (67%) patients at presentation. Platelet transfusion was needed in 46 (9.2%). Packed red blood cell (PRBC) transfusion was given in 3 patients with DFWS and 10 of SD with severe bleeding. Death occurred in 3 patients of SD with severe plasma leak and 2 patients with SD and severe bleeding.
Conclusions
Majority of DF cases can be managed on OPD basis. SD with severe bleeding or with severe plasma leakage carries high mortality. Hospitals can analyze annual data for resource allocation for capacity expansion.
Keywords: Dengue fever, Dengue hemorrhagic fever, Dengue shock syndrome, Thrombocytopenia
Introduction
Dengue is an acute self-limited systemic viral infection caused by the dengue virus belonging to the family flaviviridae.1 Incidence of dengue fever (DF) has been increasing from past few years and dengue has become a global problem in recent times.2 Dengue fever with warning signs (DFWS) and severe dengue (SD) with severe plasma leakage, severe bleeding or severe organ involvement have emerged as important public health threat in urban areas. This is attributable to population migration to cities resulting in urban overcrowding and infrastructure construction in these areas providing unhindered opportunities for breeding of the vector.3 There is a seasonal rise in the number of cases especially during the months of May to September presenting to the emergency and outpatient departments which imposes an additional load to an already overburdened system especially for staffing, laboratory and acute ward admission. The clinical presentation of DF is triphasic with the febrile phase typically characterized by high fever, headache, myalgia, body ache, vomiting, joint pain, transient rash and mild bleeding manifestations such as petichiae, ecchymosis at pressure sites and bleeding from venipunctures.4 In the next critical phase there is a heightened risk of progression of the patient to severe dengue which is defined by presence of plasma leakage that may lead to shock and/or fluid accumulation such as ascites or pleural effusion with or without respiratory distress, severe bleeding, and/or severe organ impairment.5 The risk of severe bleeding in dengue is much higher with a secondary infection and is seen in about 2–4% of cases having secondary infection.6,7,8,9 Atypical presentations are also encountered with acute liver failure, encephalopathy with seizures, renal dysfunction, lower gastrointestinal bleeding.10 Several studies have previously analyzed the clinico-epidemiologic profile of dengue infection.11,12,13 In this study we evaluated patients with dengue presenting to the outpatient or emergency departments of a tertiary care hospital in an urban setting for their clinical and hematological profile, management and outcomes.
Material and Methods
This was an observational prospective study conducted at a tertiary care hospital over a period of 05 months during the dengue fever season between May 2013 and Sept 2013. Patients presenting to the emergency department, outpatient department (OPD) or pediatric OPD with complaints of fever and clinical features of dengue with positive NS1 antigen test or dengue antibody serology IgM or IgG or both were included in the study. Age, gender, clinical presentation, duration of fever, dehydration, hemodynamic status, urine output, hepatomegaly, ascites, pleural effusion, presence of petechiae, positive tourniquet test, other bleeding manifestations, hematocrit and platelet count were recorded at presentation. Increased hematocrit was taken as a value > 45% while thrombocytopenia was defined a platelet count < 1 lac/cu.mm. Patients were categorized as dengue fever without warning signs (DF), dengue fever with warning signs (DFWS), or severe dengue (SD) based on presence of abdominal pain, vomiting, pleural effusion, ascites, lethargy and restlessness, hepatomegaly, severe bleeding, respiratory distress, and other organ involvement as per the World Health Organization (WHO) classification.5 Diagnosis of dengue was made on the basis of NS1 antigen positivity and/or detection of IgM and IgG antibodies using a commercially available one-step immunochromatographic assay (SD Bioline Dengue Duo, Alere, Germany). NS1 antigen test was done in all patients with clinical features suggestive of dengue infection presenting within 5 days of onset of the symptoms. In patients with clinical features suggestive of dengue infection who presented beyond 5 days of onset of symptoms IgM and IgG antibody test was done. All patients with bleeding manifestations, thrombocytopenia with platelet count < 30,000 cu/mm were admitted. All pregnant patients and infants irrespective of their platelet counts were admitted. The mainstay of therapy was maintenance of hydration status and early recognition of plasma leakage and shock. Management of cases was done strictly as per the guidelines for clinical management of dengue.5 Paracetamol was given for fever and pain relief with complete avoidance of any other non-steroidal analgesic (NSAID). Patients were treated with oral rehydration therapy, intravenous (IV) fluid therapy, packed red blood cell (PRBC) transfusion, platelet concentrates depending upon the clinical condition. Patients with DF were managed with oral rehydration salt (ORS) solution, oral paracetamol and advised review every 3 days. Patients with warning signs including a rising hematocrit (>20% increase over baseline) and falling platelet count were managed with 0.9% Normal Saline (NS) infusion started at 5–7 ml/kg/hour for 1–2 hours, 3–5 ml/kg/hour for the next 2–4 hours and finally 2–3 ml/kg/hour maintaining the urine output at 0.5–1 ml/kg/hour and monitoring the hematocrit for rise. In spite of this if the hematocrit continued to show a rising trend, a NS bolus of 10 ml/kg was given followed by another round of NS infusion as described. IV fluids were continued till patients clinical condition was stable and oral intake adequate. Patients with SD with severe plasma leakage or severe bleeding were given fluid resuscitation with IV NS bolus of 20 ml/kg over 1–2 hours, repeated under close supervision. The intake-output charting was done meticulously realizing fully that the input to output ratio was not adequate for judging fluid requirement during this period. Fluid resuscitation was considered adequate with decreasing tachycardia, improving blood pressure, pulse volume, warm extremities, capillary refill time (CRT) < 2 seconds, urine output ≥ 0.5 ml/kg/hour, decreasing metabolic acidosis and normal sensorium. Patients were discharged once there were no signs of dehydration, adequate urine output and platelet count > 50,000 cu/mm. Oxygen therapy by face mask was given wherever it was necessary. PRBC transfusion was given in patients with clinical bleeding with significant blood loss (6–8 ml/kg) or evidence of hemolysis. Institutional ethical committee clearance was obtained and written informed consent was taken from all patients. Demographic and clinical characteristics were described as proportions. Data was analyzed using SPSS 17.
Results
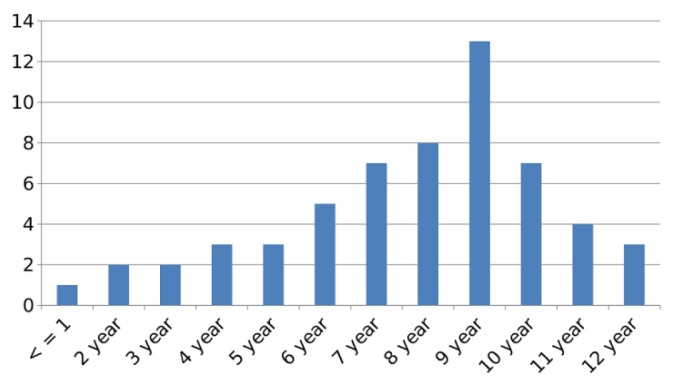
There were 443 adult patients and 57 children who were diagnosed to have the various dengue syndromes over the 5 month period of observation. Of the 443 adult patients, 223 were males and 220 were females. Amongst the females 4 patients were pregnant. There were 36 boys and 21 girls in the pediatric population. Patients’ age varied from 6 months to 77 years. Age wise distribution of children with DF is as shown (Figure 1). NS1 was positive in 115 patients (115/500; 23%), IgM antibody test was positive in 337 patients (337/500; 67.4%) while in 37 patients (37/500; 7.4%) the IgM antibody was negative but they showed positivity for IgG antibody. In 11 patients (11/500; 2.2%) initial sample was negative for NS1 antigen test but IgM antibody test done two to five days later was positive. The commonest presenting complaint was fever (99.8%) with severe arthralgia and myalgia (97.4%). Other symptoms were loose motions (12.6%), rashes (45.8%), vomiting (10.2%), breathlessness (1.6%), headache (47%), retro-orbital pain (65%) and abdominal pain (11.8%). Breathlessness was seen in 8 patients (1.6%) all of whom had serositis. DF was diagnosed in 429 cases (429/500; 85.8 %), DFWS in 55 cases (55/500; 11%), SD with severe bleeding in 10 (10/500; 2%) and SD with severe plasma leakage in 6 cases (6/500; 1.2%) (Table 1). Four hundred and twelve cases (412/500; 82%) were treated as outpatients while 88 patients (88/500; 18%) required admission. All 55 cases of DFWS, 10 patients of SD with severe bleeding, 6 patients of SD with severe plasma leak, all 4 pregnant patients and 13 children were hospitalized. Mild dehydration was noted in 179 patients of DF (179/484; 36.9%) who were treated with oral rehydration therapy, while 45 cases of DFWS (45/484; 9.3%) required intravenous fluid therapy. Sixteen patients (16/490; 3.2%) had severe dehydration requiring IV fluid resuscitation of which 10 cases were of DFWS and 6 were of SD with severe plasma leak (Table 2). Thrombocytopenia was seen in 335 (335/500; 67%) patients while increased hematocrit was seen in 66 (66/500; 13.2%) patients at the time of presentation (Table 1). Bleeding manifestations were seen in 36 patients of DFWS (36/55; 65.4%). Out of these 27 patients had petechiae, 4 patients had epistaxis, 3 had hematemesis and 2 had melena. Amongst these, 10 patients had platelet count < 10,000/cu.mm, 23 patients had platelet count was between 11–20,000/cu.mm while in 3 patients the platelet count was between 21–30,000/cu.mm (Table 3). Platelet transfusions were given in all 36 cases. PRBC transfusion was required in 3 patients with DFWS with hemoglobin < 8.5 gm/dl and 10 patients with SD with severe bleeding. All 10 patients with SD with severe bleeding required platelet transfusions. Three patients of SD with severe plasma leak and 2 patients of SD with severe bleeding died. There was no mortality in the pediatric cases or the pregnant women.
Figure 1.
Age distribution of Pediatric case.
Table 1.
Demographic and clinical characteristics of patients enrolled in the study.
| Patients’ characteristic | N | % |
|---|---|---|
|
| ||
| Patients enrolled, n = 500 | ||
| Adults | 443 | 88.6 |
| Children | 57 | 11.4 |
|
| ||
| Adult gender distribution, n = 443 | ||
| Males | 223 | 44.6 |
| Females | 220 | 44 |
|
| ||
| Pediatric gender distribution, n = 57 | ||
| Males | 36 | 63.2 |
| Females | 21 | 36.8 |
|
| ||
| Age group, n = 500 | ||
| < 1 year | 01 | 0.2 |
| 1–5 years | 10 | 2 |
| 6–12 years | 46 | 9.2 |
| 13–18 years | 63 | 12.6 |
| 19–45 years | 280 | 56 |
| 46–75 years | 53 | 10.6 |
| > 75 years | 47 | 9.4 |
|
| ||
| Dengue fever, n = 500 | ||
| Primary | 463 | 92.6 |
| Secondary | 37 | 7.4 |
|
| ||
| Clinical features | ||
| Fever | 492 | 99.8 |
| Bodyache | 487 | 97.4 |
| Headache | 235 | 47 |
| Retro-orbital pain | 325 | 65 |
| Abdominal pain | 59 | 11.8 |
| Loose stools | 63 | 12.6 |
| Vomiting | 51 | 10.2 |
| Skin rash | 229 | 45.8 |
| Breathlessness | 8 | 1.6 |
| Bleeding manifestations | 46 | 9.2 |
|
| ||
| Dehydration at presentation, n = 490 | ||
| No dehydration | 250 | 51.7 |
| Mild | 179 | 37 |
| Moderate | 45 | 9.3 |
| Severe | 10 | 2 |
| Shock | 6 | 1.2 |
|
| ||
| Clinical syndrome, n = 500 | ||
| DF without warning signs | 429 | 85.8 |
| DF with warning signs | 55 | 11 |
| SD with severe plasma leak | 6 | 1.2 |
| SD with severe bleeding | 10 | 2 |
|
| ||
| Diagnosis, n = 500 | ||
| NS1 antigen test positive | 115 | 23 |
| IgM positive ± IgG positive | 337 | 67.4 |
| IgG positive only | 37 | 7.4 |
| NS1 negative & IgM positive | 11 | 2.2 |
|
| ||
| Hematological findings, n = 500 | ||
| Thrombocytopenia | 335 | 67 |
| Increased hematocrit | 66 | 13.2 |
DF = Dengue fever; SD = Severe dengue
Table 2.
Treatment and outcome details of the admitted patients.
| Treatment and outcome | N | % |
|---|---|---|
|
| ||
| Treatment, n = 500 | ||
| OPD | 412 | 82 |
| IP | 88 | 18 |
|
| ||
| Duration of hospitalization, n = 88 | ||
| < 7 days | 12 | 14 |
| 7 – 14 days | 49 | 55 |
| > 14 days | 27 | 31 |
|
| ||
| Fluid therapy, n = 490 | ||
| Oral rehydration | 179 | 36.5 |
| IV fluid therapy | 45 | 9.2 |
| IV fluid resuscitation | 16 | 3.3 |
| Not required | 250 | 51 |
|
| ||
| Blood component, n = 500 | ||
| Platelet concentrate | 46 | 9.2 |
| Packed RBC | 13 | 2.6 |
| Fresh whole blood | 0 | 0 |
|
| ||
| Mortality, n = 500 | ||
| SD with severe bleeding | 2 | 0.4 |
| SD with severe plasma leak | 3 | 0.6 |
| DF with/without warning signs | 0 | 0 |
DF = Dengue fever; IP = In-patient; OPD = Outpatient department; SD = Severe dengue; RBC = Red blood cell.
Table 3.
Correlation of thrombocytopenia with bleeding manifestation and number of cases in Dengue fever patients.
| Platelet count (per cu.mm) | |||||||
|---|---|---|---|---|---|---|---|
| Bleeding manifestation | < 10,000 | 11–20,000 | 21–30,000 | 31–40,000 | 41–50,000 | 51–1 lac | >1 lac |
| Epistaxis | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| Melena | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Hematemesis | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petechiae | 4 | 20 | 3 | 0 | 0 | 0 | 0 |
| Total number with platelet count, n (%) | 10 (28) | 23 (64) | 3 (8) | 0 | 0 | 0 | 0 |
Discussion
Our institute located in South-West Delhi where this study was carried out serves as the nodal center for management of dengue fever for the clientele we serve. Our study aimed at a descriptive clinico-hematologic profile of the dengue morbidity during the seasonal spike. We evaluated 500 cases of serologically confirmed dengue cases over 5 months, of which 88 required admission using strict admission criteria (88/500; 18%). The age spectrum was wide including infants, children, pregnant women, young adults and elderly. Young children of school going age were particularly susceptible within the pediatric age group (Figure 1). The commonest clinical presentation was severe arthralgia, myalgia, headache and retro-orbital pain. In children high fever with vomiting, pain abdomen and an erythematous macular rash was the commonest clinical presentation. High fever in infants and young children (below 5 years) predisposes them to febrile seizures and was vigorously addressed. Majority of the patients who required hospitalization had warning signs at presentation (55 cases of DFWS and 2 cases of SD with severe bleeding) (57/88; 62.5%). Tachypnea at presentation was associated with serositis in all cases (8/500; 1.6%) and served as a simple sign prompting admission. Majority of patients with some dehydration could be managed with oral rehydration. About 9% cases required intravenous fluid therapy and only 3% who progressed to SD with severe plasma leak or severe bleeding required aggressive IV fluid resuscitation. Thrombocytopenia was seen in the majority applying the standard definition (335/500; 67%). However, platelet transfusion was required in only 36 patients of DFWS and 10 cases of SD. The average requirement of platelet concentrate for DFWS patients was 3 while for SD patients was 12. None of the patients with platelet count > 30,000/ cu. mm received platelet transfusions. Patients required hospitalization for a period of 7–14 days due to DFWS with clinical bleeding and other warning signs (55/88; 62.5%). Only cases with SD with severe bleeding or severe plasma leakage required hospitalization for > 14 days.
Appropriate timing of NS1 antigen test is important. We performed NS1 antigen testing in patients presenting within 5 days of onset of symptoms in order to reliably identify cases of primary dengue infection as well as secondary dengue infection also in which the NS1 antigen test remains positive for a shorter time frame.14 There were 11 (2.2%) patients in whom the NS1 antigen test turned out to be negative but were later confirmed to be dengue IgM antibody test positive. We used the one-step immunochromatographic assay for IgM and IgG antibody testing which identifies acute as well as past dengue infections with excellent sensitivity and specificity.15 There were 37 (7.4%) patients in whom the IgM antibody was negative but IgG antibody was positive. These patients were cases of secondary dengue infection which were confirmed by a ≥ 4 fold elevation in the IgG antibody titres by enzyme-linked immunosorbent assay (IgG-ELISA) in the convalescent serum sample at follow-up done at a reference laboratory. Out of these 37 patients, 4 had DFWS requiring hospitalization but none had SD. The 11 (2.2%) patients in whom the NS1 antigen test was negative were cases of acute infection confirmed by IgM antibody testing who presented more than five days since onset of fever which explains the initial negative NS1 antigen test.
The use of NS1 antigen test, IgM and IgG antibody testing for diagnosis of dengue infection can show false positivity due to cross reaction with other flaviviral infections.16 We did not use real-time polymerase chain reaction (RT-PCR) for viral RNA detection for diagnosis due to feasibility issues and these are the limitations of our study. The strength of this study is the inclusion of serologically confirmed cases, inclusion of patients of all age groups and threadbare clinical and hematological profile of the enrolled cases, which can help guide local health authorities on resource allocation for capacity expansion. This is a single center experience and since our hospital serves a specific clientele (armed forces personnel, in active service or retired and their dependents), this is a limitation of this study. However this should not affect its external validity and the generalizability of its findings.
Several outbreaks of DF have been reported over the past 2 decades17,18,19,20,21,22,23 and a seasonal trend during the monsoon period has been noted24 due to the warm environment and high relative humidity25 favoring vector growth. The reported case fatality rate shows a declining trend26 from 6–9%17,18,27 to 0%23 attributable to increased awareness and better case diagnosis and management.5 There has been increasing atypical and rare presentations of DF resulting in the expanded dengue definition.28,29 Some studies similar to ours from other parts of the country have reported significant differences in the incidence of atypical presentation like neurological signs or the incidence of serositis11,12 while others have reported similar findings.23 These differences may be due to co-infection with other pathogens28 or secondary heterotypic dengue virus infections.30 Dengue is grossly underreported in our country.31 The WHO estimates that nearly 5 lac people are admitted with dengue in our country annually and that India accounts for nearly 20% of all cases in the south-east Asian region (SEAR).32
Conclusions
We have presented the profile of dengue cases seen during the seasonal surge of cases handled in the emergency, OPD and laboratory of a hospital serving as a nodal hospital for management of DF in South-West Delhi. Our findings show that majority of DF cases can be managed on OPD basis, NS1 antigen test maybe false negative if done too early in the course of the illness, patients with DFWS require admission of up to 7–14 days, thrombocytopenia is common but very few patients will require platelet transfusion and the average requirement of platelet concentrates in DFWS and SD is 3 and 12. SD with plasma leak and bleeding carry high mortality.
Table 4.
Duration of hospitalization with different indications.
| Duration of hospitalization | ||||
|---|---|---|---|---|
| Indication for admission | Number of cases, n = 88 | < 7 days | 7 – 14 days | > 14 days |
| SD with severe bleeding | 10 | 2* | 0 | 8 |
| SD with severe plasma leak | 6 | 3# | 0 | 3 |
| DF with bleeding | 36 | 0 | 27 | 9 |
| DF with other warning signs | 19 | 2 | 11 | 6 |
| Pregnancy | 4 | 0 | 3 | 1 |
| Pediatric age group | 13 | 5 | 8 | 0 |
DF = Dengue fever; SD = Severe dengue;
= SD with severe bleeding mortality;
= SD with severe plasma leak mortality.
Acknowledgements
The authors gratefully acknowledge the support of the patients and the staff in the Accident and Emergency Department Base Hospital and Pediatric OPD of Army Hospital (Referral and Research) Delhi.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33(4):330–42. doi: 10.1016/s0188-4409(02)00378-8. https://doi.org/10.1016/S0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. https://doi.org/10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhuri M. What can India do about dengue fever? BMJ. 2013;346:f643. doi: 10.1136/bmj.f643. https://doi.org/10.1136/bmj.f643. [DOI] [PubMed] [Google Scholar]
- 4.Simmons CP, Farrar JJ, Chau NV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. https://doi.org/10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. dengue guidelines for diagnosis, treatment, prevention and control. 2009. [accessed 15 Nov 2017]. http://whqlibdoc.who.int/publications/2009/9789241547871eng.pdf. [PubMed]
- 6.Amin P, Acicbe O, Hidalgo J, Jimenez JIS, Baker T, Richards GA. Dengue fever: report from the task force on tropical diseases by the world federation of societies of intensive and critical care medicine. J Crit Care. 2017 doi: 10.1016/j.jcrc.2017.11.003. https://doi.org/10.1016/j.jcrc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2(1):33–42. doi: 10.1016/s1473-3099(01)00171-2. https://doi.org/10.1016/S1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 8.Kouri GP, Guzman MG, Bravo JR. Why dengue haemorrhagic fever in Cuba? 2: an integral analysis. Trans R Soc Trop Med Hyg. 1987;81:821–23. doi: 10.1016/0035-9203(87)90042-3. https://doi.org/10.1016/0035-9203(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 9.Basheer A, Iqbal N, Mookkappan S, Anitha P, Nair S, Kanungo R, Kandasamy R. Clinical and laboratory characteristics of dengue-orientia tsutsugamushi co-infection from a tertiary care center in south india. Mediterr J Hematol Infect Dis. 2016;8(1):e2016028. doi: 10.4084/MJHID.2016.028. https://doi.org/10.4084/mjhid.2016.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Karoli R, Fatima J, Siddiqi Z, Kazmi KI, Sultania AR. Clinical profile of dengue infection at a teaching hospital in North India. J Infect Dev Ctries. 2012;6(7):551–54. doi: 10.3855/jidc.2010. https://doi.org/10.3855/jidc.2010. [DOI] [PubMed] [Google Scholar]
- 12.Mandal SK, Ganguly J, Sil K, Chatterjee S, Chatterjee K, Sarkar P, et al. Clinical profiles of dengue fever in a teaching hospital of eastern India. Nat J Med Res. 2013;3(2):173–76. [Google Scholar]
- 13.Roy MP, Gupta R, Chopra N, Meena SK, Aggarwal KC. Seasonal Variation and Dengue Burden in Paediatric Patients in New Delhi. J Trop Pediatr. 2017 doi: 10.1093/tropej/fmx077. https://doi.org/10.1093/tropej/fmx077. [DOI] [PubMed] [Google Scholar]
- 14.Pal S, Dauner AL, Mitra I, Forshey BM, Garcia P, Morrison AC, et al. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE. 2014;9(11):e113411. doi: 10.1371/journal.pone.0113411. https://doi.org/10.1371/journal.pone.0113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SM, Sekaran SD. Early diagnosis of dengue infection using a commercial dengue duo rapid test kit for the detection of NS1, IGM and IGG. Am J Trop Med Hyg. 2010;83(3):690–5. doi: 10.4269/ajtmh.2010.10-0117. https://doi.org/10.4269/ajtmh.2010.10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zammarchi L, Spinicci M, Bartoloni A. Zika virus: a review from the virus basics to proposed management strategies. Mediterr J Hematol Infect Dis. 2016;8(1):e2016056. doi: 10.4084/MJHID.2016.056. doi: https://doi.org/10.4084/mjhid.2016.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anuradha S, Singh NP, Rizvi SN, Agarwal SK, Gur R, Mathur MD. The 1996 outbreak of dengue hemorrhagic fever in Delhi, India. Southeast Asian J Trop Med Public Health. 1998;29(3):503–6. [PubMed] [Google Scholar]
- 18.Kabra SK, Jain Y, Pandey RM, Madhulika, Singhal T, Tripathi P, et al. Dengue haemorrhagic fever in children in the 1996 Delhi epidemic. Trans R Soc Trop Med Hyg. 1999;93(3):294–8. doi: 10.1016/s0035-9203(99)90027-5. https://doi.org/10.1016/S0035-9203(99)90027-5. [DOI] [PubMed] [Google Scholar]
- 19.Singh NP, Jhamb R, Agarwal SK, Gaiha M, Dewan R, Daga MK, et al. The 2003 outbreak of Dengue fever in Delhi, India. Southeast Asian J Trop Med Public Health. 2005;36(5):1174–8. [PubMed] [Google Scholar]
- 20.Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, et al. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1. doi: 10.1186/1743-422X-5-1. https://doi.org/10.1186/1743-422X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jhamb R, Kumar A, Ranga GS, Rathi N. Unusual manifestations in dengue outbreak 2009, Delhi, India. J Commun Dis. 2010;42(4):255–61. https://doi.org/10.1016/S1201-9712(10)60301-3. [PubMed] [Google Scholar]
- 22.Jain S, Sharma SK. Challenges & options in dengue prevention & control: A perspective from the 2015 outbreak. Indian J Med Res. 2017;145:718–21. doi: 10.4103/ijmr.IJMR_1325_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laul A, Laul P, Merugumala V, Pathak R, Miglani U, Saxena P. Clinical Profiles of Dengue Infection during an Outbreak in Northern India. J Trop Med. 2016 doi: 10.1155/2016/5917934. Article ID 5917934. https://doi.org/10.1155/2016/5917934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dar L, Broor S, Sengupta S, Xess I, Seth P. The First Major Outbreak of Dengue Hemorrhagic Fever in Delhi, India. Emerg Inf Dis. 1999;5(4):589–90. doi: 10.3201/eid0504.990427. https://doi.org/10.3201/eid0504.990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy MP, Gupta R, Chopra N, Meena SK, Aggarwal KC. Seasonal Variation and Dengue Burden in Paediatric Patients in New Delhi. J Trop Pediatr. 2017 doi: 10.1093/tropej/fmx077. https://doi.org/10.1093/tropej/fmx077. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarti A, Arora R, Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg. 2012;106:273–82. doi: 10.1016/j.trstmh.2011.12.007. https://doi.org/10.1016/j.trstmh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal A, Chandra J, Aneja S, Patwari AK, Dutta AK. An epidemic of dengue hemorrhagic fever and dengue shock syndrome in children in Delhi. Indian Pediatr. 1998;35(8):727–32. [PubMed] [Google Scholar]
- 28.Kadam DB, Salvi S, Chandanwale A. Expanded dengue. J Assoc Physicians India. 2016;64(7):59–63. [PubMed] [Google Scholar]
- 29.Tansir G, Gupta C, Mehta S, Kumar P, Soneja M, Biswas A. Expanded dengue syndrome in secondary dengue infection: A case of biopsy proven rhabdomyolysis induced acute kidney injury with intracranial and intraorbital bleeds. Intractable Rare Dis Res. 2017;6(4):314–18. doi: 10.5582/irdr.2017.01071. https://doi.org/10.5582/irdr.2017.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. https://doi.org/10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 31.Kakkar M. Dengue fever is massively under-reported in India, hampering our response. BMJ. 2012;345:e8574. doi: 10.1136/bmj.e8574. https://doi.org/10.1136/bmj.e8574. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO) Dengue and severe dengue: Fact sheet No. 117. 2013. [accessed on 15 Nov 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/