Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) bloodstream infection (BSI) has become increasingly frequent threat recently, especially in the intensive care unit (ICU). High-dose tigecycline (TGC) regimen is proposed due to the limitation of treatment options. We investigated the efficacy and safety of high-dose TGC combination regimens for treating CRKP BSI. Furthermore, the risk factors for mortality were also determined.
This was a single center retrospective cohort study conducted from 2014 to 2016. A total of 40 patients with nosocomial CRKP BSI admitted to the ICU were included; they were classified into two groups according to the treatment regimens with high-dose TGC (HD group) or not (non-HD group). In-hospital mortality rates and microbiologic responses from both groups were reviewed and compared. Besides, the survival and non-survival groups were compared to identify the risk factors of mortality.
Twenty-three patients constituted the HD group (high-dosage TGC regimen was administered as 200 mg loading dose followed by 100 mg every 12 h) and 17 patients constituted the non-HD group (standard dose TGC therapy as 100 mg loading dose followed by 50 mg every 12 h and other antibiotics). The in-hospital mortality was 52.2% in the HD group and 76.5% in the non-HD group (P = .117). The Kaplan–Meier test showed significantly longer survival times in the HD group (mean: 83 days vs 28 days; P = .027). Microbiological eradication was observed in 13 patients (56.5%) in the HD group and 6 patients (36.3%) in the non-HD group (P = .184). A smaller fraction of patients in the HD group were subjected to vasoactive therapy (52.2% vs 88.2%; P = .016) compared to the non-HD group. There was no significant difference in the manifestation of adverse effects between the two groups. In the multivariate analysis, multiple organ dysfunction syndrome (MODS), vasoactive therapy, and exposure to carbapenems were regarded as the independent predictors of mortality.
A therapeutic regimen consisting of a high dose of TGC was associated with significantly longer survival time and numerically lower mortality in CRKP BSI. Adverse events were not increased with the double dose therapy.
Keywords: bloodstream infection, carbapenem resistant, high-dose tigecycline, Klebsiella pneumoniae
1. Introduction
In recent times, carbapenem-resistant Klebsiella pneumoniae (CRKP) associated bacterial infections have begun to emerge as a significantly important threat due to the widespread use of carbapenems in treatment.[1] In China, CHINET reported that the rate of carbapenem resistance among K pneumoniae isolates increased from 2.4% in 2005 to 13.4% in 2014.[2] CRKP are capable of producing carbapenemases, which have the ability to hydrolyze all the known cephalosporins, monobactams, carbapenems, and beta-lactamase inhibitors, thereby leading to fewer numbers of effective antibiotics at our disposal, longer lengths of stay, and higher mortality rates.[3,4] An infection of the bloodstream in the host is one of the most common types of infection associated with CRKP, which approximately increases the mortality rate three-fold compared to CRKP infections occurring at other sites.[5] Treatment options are limited, and these usually involve the use of colistin, tigecycline (TGC), carbapenems, and some aminoglycosides; but, an optimal treatment strategy is yet to be identified and clearly defined.[3,6] Moreover, no listing of colistin in mainland China until now (only available from abroad or Taiwan), making TGC the primary choice of treatment.
TGC is a glycylcycline antibiotic with a broad spectrum antibacterial activity. It was approved in 2005 by the US Food and Drug Administration (FDA) for the treatment of complicated skin and soft-tissue infections, complicated intra-abdominal infections, and community-acquired pneumonia, with the standard dosage being 50 mg twice daily after a 100 mg loading dose.[7] TGC has been used as a last-resort treatment option against severe infections due to the presence of multidrug-resistant variants of Gram-negative bacteria, such as carbapenemase-producing Enterobacteriaceae.[8] However, the efficacy of the standard dose of TGC in bloodstream infections (BSI) is still a matter of controversy mainly because of the low serum concentrations that can be achieved with TGC.[9] Increasing the dosage of TGC may be a reasonable clinical strategy, but few studies have evaluated its effectiveness and safety when dealing with CRKP BSI.
The purpose of this study was to evaluate the efficacy and safety of high-dose TGC combination regimens for the treatment of CRKP BSI in the ICUs of our hospital. The risk factors associated with mortality were also determined.
2. Materials and methods
2.1. Study design and population
This single center retrospective observational study was performed in the general intensive care unit (40 beds) of the Second Affiliated Hospital, Zhejiang University School of Medicine, a 2000-bed tertiary care teaching hospital in the People's Republic of China, during a 3-year period (January 2014–December 2016). Patients whose data were included in this study were those with a nosocomial CRKP BSI. CRKP BSI is defined as an infection where at least one positive blood culture for CRKP can be obtained along with clear clinical signs of infection (which forms the basis of suspicion source of CRKP infection). The terms CRKP BSI and the diagnostic criteria set above are in accordance with the definitions of the Centers for Disease Control and Prevention.[10] The collection date of the CRKP samples was considered as the BSI onset time in each case. Adequate empirical antibiotic therapy refers to the regimen that involves the administration of in vitro active antimicrobials for CRKP before the microbiological tests are performed. The term definitive treatment used in this report refers to the antibiotic therapy initiated within 24 hours of obtaining the microbiological results. A high-dose TGC regimen involves the administration of a 200-mg loading dose followed by a 100-mg dose after 12 hours. BSI or intracranial infections caused by multidrug-resistant bacteria are routinely treated with high-dose TGC in our ICU. Sepsis was defined as an infection that has led to a systemic inflammatory response syndrome in a patient. Septic shock was defined as sepsis associated with organ dysfunction and persistent hypotension despite volume replacement.[11] Multiple organ dysfunction syndrome (MODS) was defined as the simultaneous dysfunction of at least two organ systems in an acutely ill patient. Exclusion criteria were, patient <18 years old, those with BSI onset <48 hours after ICU admission, and those with duration of definitive treatment <72 hours. The primary outcome was in-hospital mortality and survival time. The secondary outcomes were bacterial clearance based on a negative blood culture result and the length of stay.
2.2. Data collection
Patients’ information were collected from the electronic medical records of the hospital. Data obtained included demographic characteristics, underlying diseases and conditions associated with comorbidity, recent (≤30 days) surgical procedures, the use of immunosuppressant and steroids, length of hospital stay, total hospitalization expenses, carbapenem exposure (exposure to carbapenems during the 30 days preceding admission to the ICU), definitive treatment, and the duration of each course of treatment. The severity of illness [calculated by the acute physiology and chronic health evaluation (APACHE II) score[12] and the sequential organ failure assessment (SOFA) score[13]] was calculated for each patient admitted to the ICU, on admission. Data on septic shock, inflammation markers [including white blood cell count (WBC), procalcitonin (PCT), C-reactive protein (CRP)], clinical outcomes, in-hospital bacterial clearance rates, time from hospitalization to the onset of BSI, and the minimum inhibitory concentration (MIC) were also collected. Possible adverse effects of TGC were continuously evaluated from the onset of TGC treatment (including empirical treatment). Serum creatinine, total bilirubin, ALT, and AST increase means increased more than 1.5 times baseline; fibrinogen reduce referred to fibrinogen lower than 1.5 g/L. In this study, blinding was used during the data collection and analysis. Furthermore, to ensure that selection bias and information bias associated with the study were controlled, research experts were consulted. The study was approved by an ethics committee (The Second Affiliated Hospital of Zhejiang University School of Medicine Institutional Review Board) prior to the commencement of the study.
2.3. Microbiological tests
Microbial identification was done using the VITEK system (bioMérieux, Marcy l’Etoile, France). The MIC used in this study, the determination of antimicrobial susceptibilities associated with the VITEK system, and the disk diffusion method, all complied with the 2016 Clinical Laboratory Standards Institute recommendations.
2.4. Statistical analysis
Continuous variables were expressed as mean or median while categorical variables were expressed as absolute numbers or their relative frequencies. Variable distributions were evaluated using the Kolmogorov–Smirnov test. Continuous variables were analyzed using the Student t-test (normally distributed variables) or the Mann–Whitney U-test (non-normally distributed variables), while categorical variables were analyzed using the χ2 test or the Fisher's test. Variables significant in the univariate analyses (P < .1) were added to a stepwise multiple logistic regression model to identify independent risk factors of mortality. Event-free survival curves were constructed using the Kaplan–Meier methods and compared using a log-rank test. Odds ratios and 95% confidence intervals were also computed. All statistical tests conducted in the study were reported as two-tailed tests and the P-values less than .05 were considered statistically significant. All statistical analyses were conducted using SPSS version 23.0 (IBM Corp., Armonk, NY).
3. Results
During the course of the study, a total of 40 patients with CRKP BSI met the inclusion criteria and the incidence of CRKP BSI in our ICU was 6.62%. All patients received mechanical ventilation, central venous catheters, arterial catheters, nasogastric tubes, and urinary catheters on ICU admission. The most common sources of infection were primary BSIs (13 cases; 32.5%) and lung infections (10 cases; 25%) (Table 1). The crude in-hospital mortality was 62.5% while 42.5% died of septic shock.
Table 1.
The probable source of blood stream infection.
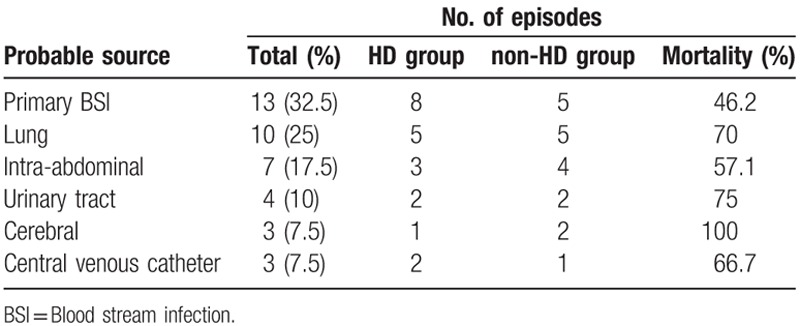
The majority of the patients were males (35 cases; 87.5%). The mean age was 63.7 years (SD ± 14.07 years). The median time from admission to infection was 15 days (IQR, 9.25–20.75 days). The total in-hospital bacterial clearance rate was 47.5%, and the median time to bacterial clearance was 13 days (IQR, 7–21 days). All patients had two or more comorbidities. There were no cases involving solid organ transplantation or receiving chemotherapy. Overall, 62.5% of patients underwent steroid therapy. Two (5%) patients received immunosuppressive therapy (cyclosporine or leflunomide) to treat systemic lupus erythematosus. The mean APACHE II score on ICU admission was 20.5 (SD ± 8) and the mean SOFA score was 5.0 (SD ± 3.12). The median duration of mechanical ventilation was 24 days (IQR, 13–39.5 days). The median hospital and ICU length of stay were 37.5 (IQR, 29–62.25) and 30 (IQR,17.25–43.5) days, respectively. Twelve patients were transferred from the ICU to the rehabilitation wards or the medical wards while eight patients stayed in a common ward prior to the ICU admission.
Over the duration of the study, 23 patients received the high-dose TGC therapy (HD group) and the rest (17 patients) were in the non-HD group. All patients received concomitant treatment with carbapenems or beta-lactamase inhibitors or aminoglycosides; however, no significant differences occurred between the two groups. There were no significant statistical differences between the two groups with respect to demographic parameters and disease severity (Table 2). The in-hospital mortality was observed as 52.2% and 76.5% in the HD and non-HD groups, respectively (P = .117). Microbiological eradication was observed in 13 (56.5%) patients and 6 (36.3%) patients in the HD and non-HD groups, respectively (P = .184). As shown in Figure 1, survival was significantly longer in the HD group (mean: 83 days vs. 28 days; P = .027). The proportion of patients who required vasoactive therapy (52.2% vs 88.2%; P = .016) was significantly lower in the HD group after the onset of CRKP BSI. Although not statistically significant, the occurrence of MODS (34.8% vs 58.8%; P = .131) was lower in the HD group after the onset of CRKP BSI. Furthermore, the median length of mechanical ventilation was 22 days (IQR,13–37 days) for patients in the HD group compared to 26 days (IQR,11–42 days) in the non-HD group. The mean hospital expenses were lower in the HD group (mean ± SD: 290552 ± 156308 yuan vs 434403 ± 297200 yuan; P = .082). With respect to adverse effects, no differences were observed between the two groups in terms of glucose abnormalities (specifically hypoglycemia), serum creatinine, total bilirubin, fibrinogen, alanine transaminase (ALT), aspartate transaminase (AST), and gastrointestinal symptoms (nausea, vomiting, or diarrhea). Besides, we did not find other adverse effects, for example, allergic reaction, acute pancreatitis, jaundice, drowsiness, itchy, and pain, etc. The majority (79.5%) of isolates were susceptible in vitro to TGC (MIC ≤ 2 mg/L) with one isolate missing, the distribution of non-susceptible isolation was not significantly different between the two groups. All isolates were resistant to meropenem, but all the MICs were found to be ≤ 32 mg/L. In vitro susceptibility was found in only 10% of the isolates for amikacin and levofloxacin. All isolates were resistant to cefoperazone sodium, sulbactam sodium, imipenem, piperacillin, tazobactam, and cephalosporins.
Table 2.
Analysis of the patients in the HD group compared with non-HD group.
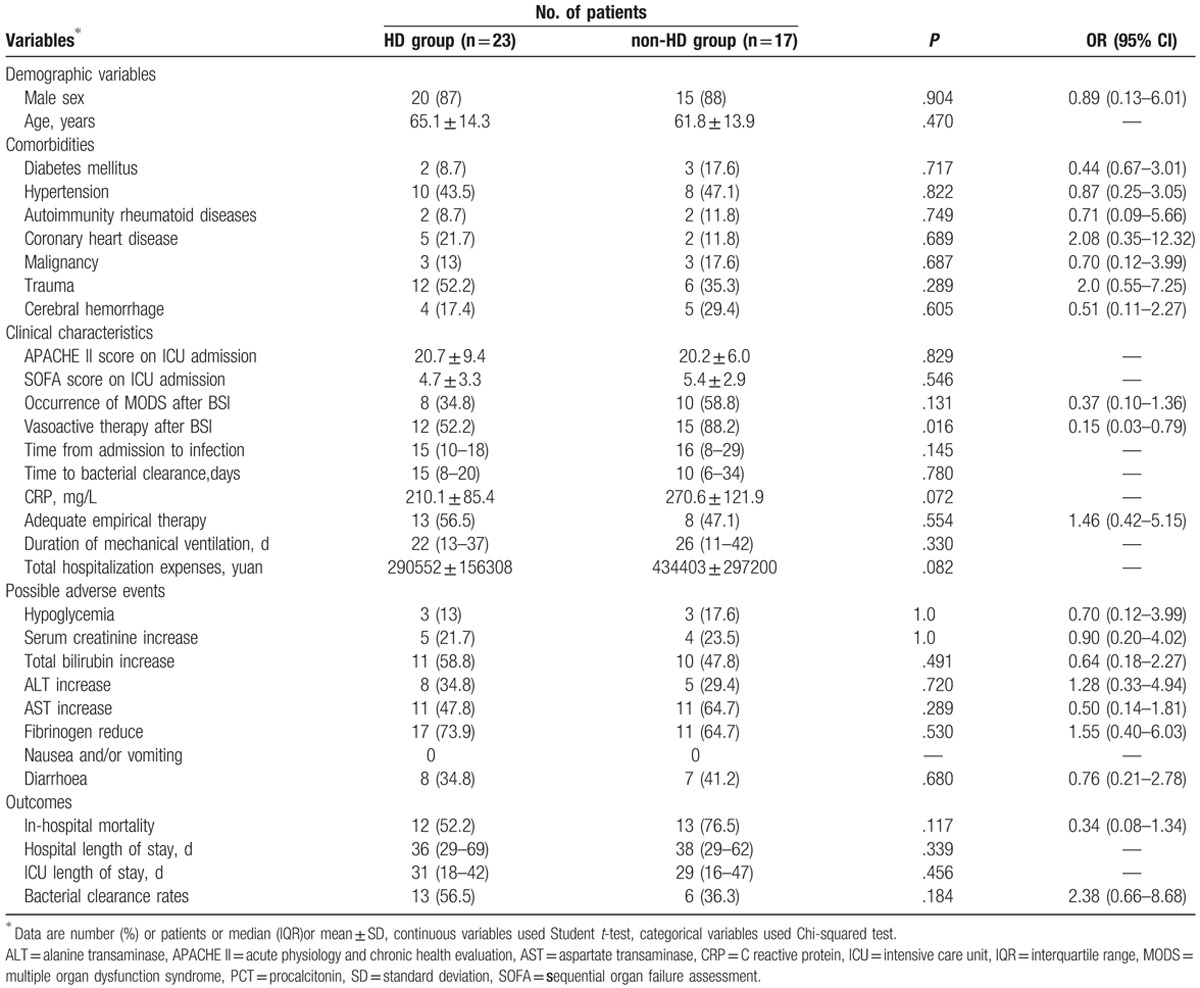
Figure 1.
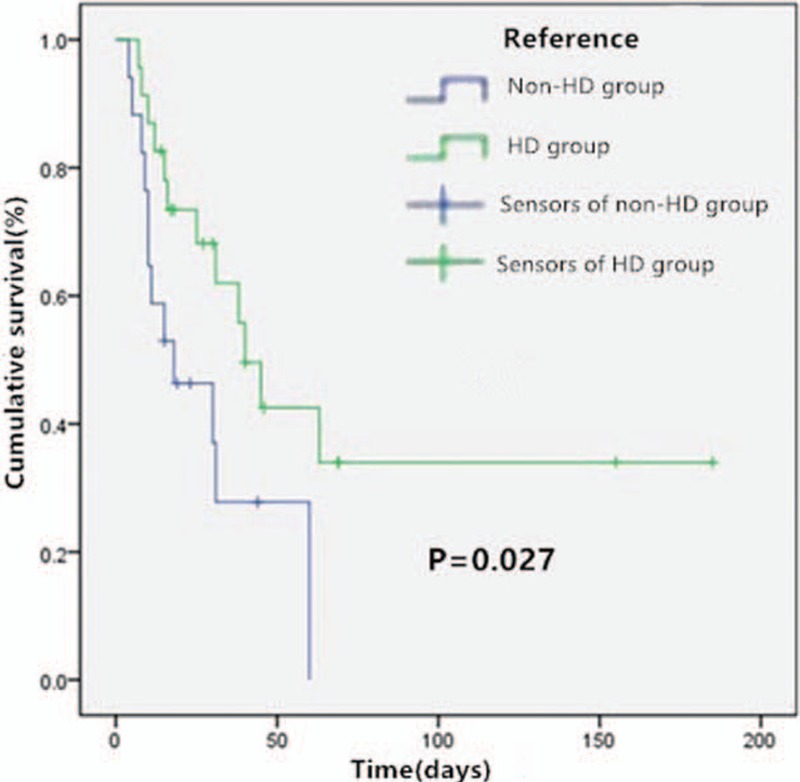
Kaplan–Meier curves for cumulative survival analysis.
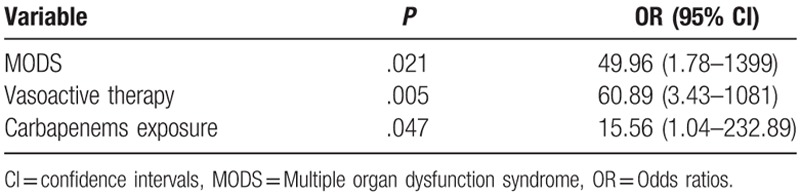
Characteristics of the survivor and non-survivor sub-groups are depicted in Table 3. Significant differences were observed between survivors and non-survivors with respect to SOFA scores on ICU admission (P = .014), presentation with septic shock (P = .001), occurrence of MODS (P < .001), vasoactive therapy (P < .001), steroid therapy (P = .023), carbapenems exposure (P = .046), time from admission to infection (P = .047), white blood cell count (P = .031), and bacterial clearance (P = .011). Moreover, compared to the survivor group, the non-survivor group showed significant differences in terms of the following characteristics, older average age, higher APACHE II score, predominantly males, shorter hospital stay, mechanical ventilation time, and higher hospitalization costs. In the multivariate logistic regression analyses, MODS (P = .021), the requirement and duration of vasoactive therapy (P = .005), and carbapenems exposure (P = .047) were independent predictors of in-hospital mortality (Table 4).
Table 3.
Univariate analysis of factors associated with death.
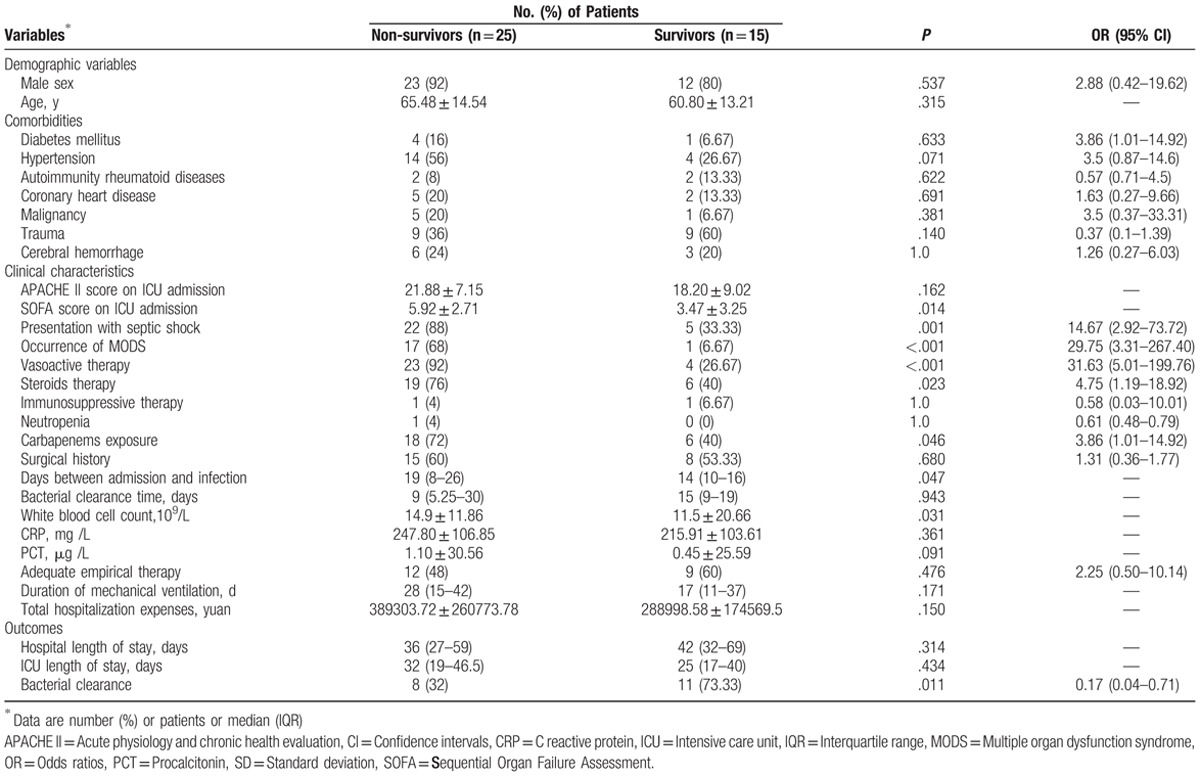
Table 4.
Multivariate analysis of risk factors for mortality.
4. Discussion
In this study, 40 CRKP BSI cases were studied. The incidence of CRKP BSI in the ICU was 6.62%, which is higher than previously reported.[14,15] This discrepancy could be because Zhejiang Province has one of the highest prevalences of CRKP infections in China.[2] This depicts the seriousness of the condition of patients included in this study. All the patients who participated in this study required variety invasive procedures. Moreover, the APACHE II scores of the patients were quite high indicating a more or less severe form of the disease. All patients in this study also underwent treatment using multiple antibiotics prior to their participation in this study, which increased the possibility of CRKP infection.[16,17] ICU stay was reported as an independent risk factor of CRKP acquisition.[18,19] Since patients have a high risk of CRKP infection in ICU, empirical therapy plays an important role in their prognosis. In our study, 52.5% of the patients received adequate empirical therapy.
The best treatment regimen for CRKP infections is yet to be determined. Combination therapy with two or more active drug agents under in vitro conditions is widely accepted.[20] The use of TGC combination therapy for serious infections is somewhat controversial and still under debate. The efficacy of TGC in BSI has been questioned because of its low-serum concentrations. Recent meta-analyses and retrospective studies show that TGC is not associated with higher survival and may even cause more deaths and other serious side effects in severe forms of infectious disease.[21–23] The FDA in particular, warned against the use of TGC for serious infections in 2010. In contrast, other studies have shown that TGC therapy, when administered as a constituent of combination therapy, (especially high-dose TGC-based therapy), can be effective and can reduce the mortality associated with the severe forms of infectious disease.[24,25] Another retrospective study involving 15 patients receiving different doses of TGC for severe CRKP infections has been reported. The favorable clinical response reported in the above-mentioned study was 87.5%. The overall 30-day mortality rate was 33.3% and 20% for daily doses of 100 mg and 200 mg, respectively.[26] Besides, De Pascale et al.[7] reported that high doses of TGC can be administered without undue toxicity for the treatment of serious infections in critically ill patients. In the current study, the mortality was 24.3% lower in HD patients compared to the non-HD group. Moreover, the group where a high dosage of TGC was administered displayed significantly longer survival times (P = .027) and less requirement of vasoactive therapy (P = .016). We think requirement for vasoactive therapy is partly an outcome indicator for septic shock, but there are many factors to influence the outcome and our sample size was small, so our results need further large simple study to be confirmed.
The safety and tolerability of the high dosage TGC therapy are of great concern. Nausea and vomiting are the most often reported manifestations of intolerance.[21] It was reported that these adverse effects of the gastrointestinal system, were dose-dependent and increased in frequency with increasing TGC dosage.[27] A randomized phase-2 trial showed that the incidence of gastrointestinal adverse effects was higher in patients treated with the high TGC dosage.[28] As for the safety, renal, hepatic, and hematological toxicity associated with TGC should be evaluated.[26] However, in some articles, no adverse effects were found in patients receiving high-dose TGC therapy compared with standard dose.[7,29] Similarly, our study reported no case of nausea or vomiting; this was probably due to the fact that all patients included in this study were breathing with the aid of mechanical ventilation, and were also under sedation. No other adverse effects were observed in HD patients. Therefore, we believe that the treatment regimen consisting of the double dose TGC is not associated with more adverse events.
The mortality rates associated with CRKP BSI have been widely reported and they range from 40% to 70%.[5,30,31] These rates were reported to be significantly higher compared to carbapenem susceptible K pneumoniae (CSKP) BSI.[32,33] Additionally, the duration of hospitalization was also found to be significantly longer with CRKP BSI.[34] In a retrospective study, the in-hospital mortality was 42.4% for CRKP BSI, compared to 19.8% in patients with CSKP BSI and the median length of hospitalization for patients with the CRKP BSI and CSKP BSI were 50 and 24 days, respectively. All of these results were statistically significant.[35] In this study, the in-hospital mortality rate was 62.5%, which is consistent with the findings of previously conducted studies.
Earlier studies have evaluated the benefits of inadequate empirical therapy, immunosuppression, corticosteroid use, septic shock, the effect of age, high SOFA, APACHE II scores, poor functional status, and the duration of ICU stay as predictors of mortality in CRKP infections.[16,36,37] The results obtained from our analysis on the risk factors for mortality were similar to those obtained from previous studies. Carbapenem exposure has been reported as an independent risk factor for CRKP infections.[31,38] Analogously, we find that carbapenem exposure (exposure to carbapenems during the 30 days preceding admission to the ICU) is an independent predictor of mortality. However, we did not find a relationship between immunosuppression and mortality, which could probably be due to the small sample size in the current study.
There were several limitations associated with this study. First, the current study was a retrospective, single center study with a limited sample size. Second, we did not exclude polymicrobial infection cases. Finally, carbapenemase types were not assessed, thereby leading to the loss of some data associated with the microbial sensitivity test.
5. Conclusion
In the present study, the incidence and mortality rates of CRKP BSI were high in critically ill patients. The high dosage TGC therapy was associated with significantly longer survival time and numerically lower mortality. Moreover, adverse events were not increased with the double dose therapy. The independent factors of mortality were MODS, vasoactive therapy, and exposure to carbapenems. Further prospective studies are needed to confirm the results put forth by this study.
Acknowledgments
We thank Dr Zhijiang Xu from the Microbiology Department for providing the microbiological data and Dr Pengfei Wang for his assistance with the statistical analyses.
Footnotes
Abbreviations: ALT = alanine transaminase, APACHE II = acute physiology and chronic health evaluation, AST = aspartate transaminase, BSI = bloodstream infection, CI = confidence intervals, CRKP = carbapenem-resistant Klebsiella pneumoniae, CSKP = carbapenem-susceptible Klebsiella pneumoniae, ICU = intensive care unit, MIC = minimum inhibitory concentration, MODS = Multiple organ dysfunction syndrome, OR = Odds ratios, SD = standard deviation, SOFA = sequential organ failure assessment, TGC = tigecycline, WBC = white blood cell count.
This study was supported by the National Natural Science Foundation of China (Grant No. 419000-523501172/161). The funding sources had no involvement in the study design, collection, analysis, and interpretation of data, or in the writing of the report or in the decision to submit the article for publication.
The authors report no conflicts of interest.
References
- [1].Kim D, Ahn JY, Lee CH, et al. Increasing Resistance to Extended-Spectrum Cephalosporins, Fluoroquinolone, and Carbapenem in Gram-Negative Bacilli and the Emergence of Carbapenem Non-Susceptibility in Klebsiella pneumoniae: Analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) Data From 2013 to 2015. Ann Lab Med 2017;37:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect 2016;22suppl 1:S9–14. [DOI] [PubMed] [Google Scholar]
- [3].Daikos GL, Markogiannakis A, Souli M, et al. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae: a clinical perspective. Expert Rev Anti Infect Ther 2012;10:1393–404. [DOI] [PubMed] [Google Scholar]
- [4].Cristina ML, Sartini M, Ottria G, et al. Epidemiology and biomolecular characterization of carbapenem-resistant klebsiella pneumoniae in an Italian hospital. J Prev Med Hyg 2016;57:E149–e156. [PMC free article] [PubMed] [Google Scholar]
- [5].Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009;30:972–6. [DOI] [PubMed] [Google Scholar]
- [6].Girometti N, Lewis RE, Giannella M, et al. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 2014;93:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Pascale G, Montini L, Pennisi M, et al. High-dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014;18:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Falagas ME, Karageorgopoulos DE, Nordmann P. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol 2011;6:653–66. [DOI] [PubMed] [Google Scholar]
- [9].Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis 2013;75:331–6. [DOI] [PubMed] [Google Scholar]
- [10].Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128–40. [DOI] [PubMed] [Google Scholar]
- [11].Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [13].Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- [14].Alicino C, Giacobbe DR, Orsi A, et al. Trends in the annual incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a 8-year retrospective study in a large teaching hospital in northern Italy. BMC Infect Dis 2015;15:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao F, Zhang J, Fu Y, et al. Dissemination of extensively drug-resistant and KPC-2 producing Klebsiella pneumoniae isolated from bloodstream infections. J Infect Dev Ctries 2015;9:1016–21. [DOI] [PubMed] [Google Scholar]
- [16].Papadimitriou-Olivgeris M, Marangos M, Christofidou M, et al. Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand J Infect Dis 2014;46:642–8. [DOI] [PubMed] [Google Scholar]
- [17].Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011;69:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gomez Rueda V, Zuleta Tobon JJ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colomb Med (Cali) 2014;45:54–60. [PMC free article] [PubMed] [Google Scholar]
- [19].Kritsotakis EI, Tsioutis C, Roumbelaki M, et al. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-{beta}-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case-control study. J Antimicrob Chemother 2011;66:1383–91. [DOI] [PubMed] [Google Scholar]
- [20].Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012;55:943–50. [DOI] [PubMed] [Google Scholar]
- [21].Shen F, Han Q, Xie D, et al. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis 2015;39:25–33. [DOI] [PubMed] [Google Scholar]
- [22].Prasad P, Sun J, Danner RL, et al. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012;54:1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He H, Zheng Y, Sun B, et al. Tigecycline combination for ventilator-associated pneumonia caused by extensive drug-resistant Acinetobacter baumannii. J Thorac Dis 2016;8:2784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Falagas ME, Vardakas KZ, Tsiveriotis KP, et al. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 2014;44:1–7. [DOI] [PubMed] [Google Scholar]
- [25].Ni W, Han Y, Liu J, et al. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Balandin Moreno B, Fernandez Simon I, Pintado Garcia V, et al. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand J Infect Dis 2014;46:175–80. [DOI] [PubMed] [Google Scholar]
- [27].Muralidharan G, Micalizzi M, Speth J, et al. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 2005;49:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ramirez J, Dartois N, Gandjini H, et al. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013;57:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Humphries RM, Kelesidis T, Dien Bard J, et al. Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose tigecycline and colistin. J Med Microbiol 2010;59:1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 2012;18:54–60. [DOI] [PubMed] [Google Scholar]
- [31].Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008;29:1099–106. [DOI] [PubMed] [Google Scholar]
- [32].Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 2017;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hussein K, Raz-Pasteur A, Finkelstein R, et al. Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 2013;83:307–13. [DOI] [PubMed] [Google Scholar]
- [34].Gallagher JC, Kuriakose S, Haynes K, et al. Case-case-control study of patients with carbapenem-resistant and third-generation-cephalosporin-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2014;58:5732–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control 2016;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Papadimitriou-Olivgeris M, Marangos M, Fligou F, et al. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis 2013;77:169–73. [DOI] [PubMed] [Google Scholar]
- [37].Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis 2017;36:1125–31. [DOI] [PubMed] [Google Scholar]
- [38].Hu Y, Ping Y, Li L, et al. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries 2016;10:208–13. [DOI] [PubMed] [Google Scholar]


