Abstract
In chronic glomerulopathies, renal fibrosis (RF) results from extracellular matrix remodeling processes regulated by matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP). We assessed urinary (u-) and serum (s-) MMP-1, -2, -9, TIMP-1, -2 concentrations and MMP-1, -2, -9/TIMP-1, -2 ratios in children with nephrotic syndrome. Steroid-dependent and steroid-resistant nephrotic patients (SDNS-Ps and SRNS-Ps, respectively) were compared with respect to measured parameters. The correlations of measured parameters with magnitude of proteinuria and histopathological diagnosis were determined.
The study comprised of 39 children with nephrotic syndrome and 20 healthy controls. Twenty-three patients had SDNS and 16 ones—SRNS. The concentrations MMPs and TIMPs were measured using enzyme-linked immunosorbent assay.
In nephrotic patients, higher u-MMP-1, -2, -9/creatinine ratios and u-TIMP-1, -2/creatinine ratios were observed as compared with controls. Nephrotic children were also characterized by lower MMP-1, -2, -9/TIMP-1 ratios. In SRNS-Ps, u-MMP-2/creatinine ratio and u-TIMP-1/creatinine ratio were higher as compared with SDNS-Ps. Magnitude of proteinuria correlated positively with u-MMP-2/creatinine ratio and negatively with u-MMP-2/TIMP-1. In minimal change disease (MCD) patients as compared with those with other glomerulopathies, there was higher u-MMP-2/TIMP-1 ratio. No significant differences in s-MMPs, s-TIMPs, and s-MMPs/TIMPs ratios between nephrotic patients and controls were observed.
Children with nephrotic syndrome are characterized by increased u-fibrotic biomarkers excretions. U-MMP-1, -2, -9 excretions and u-MMP-2/TIMP-1 ratio may become potential early biomarkers for RF. SRNS-Ps, those with heavier proteinuria and other than MCD glomerulopathies, seem to be more susceptible to early RF.
Keywords: children, metalloproteinases, nephrotic syndrome, renal fibrosis, tissue inhibitors of metalloproteinases
1. Introduction
Progressive renal fibrosis (RF) associated with chronic glomerulopathies results from imbalance between extracellular matrix (ECM) formation and degradation. Identification of early biomarkers for kidney fibrosis is of great importance to patients with chronic glomerulopathies because early institution of nephroprotective treatment may protect them from chronic kidney disease (CKD) occurrence and its progression.
Matrix metalloproteinases (MMPs) are a large family of zinc-containing enzymes that are involved in ECM remodeling processes.[1–3] However, recent studies revealed that MMPs may be implicated in initiation and progression of kidney fibrosis and CKD development.[2,4–6] Tissue inhibitors of metalloproteinases (TIMP) are endogenous, specific inhibitors of MMPs.
As yet the mode of action of only a few renal MMPs and TIMPs was unraveled. The most important role in ECM remodeling processes seems to play MMP-1, -2, -9 and their inhibitors TIMP-1 and -2.[7,8]
MMP-1 is interstitial colagenase, which degrades native collagen and is hypothesized to be antifibrotic enzyme.[9,10] MMP-2 and -9 are gelatinases, which cleave denatured collagen, type IV collagen, and laminin.[11] MMP-2 is expressed in glomeruli, proximal tubules,[11–13] and collecting ducts.[14] The expression of MMP-9 was detected mainly in glomeruli,[11,12,15] although there are reports of its expression in proximal and distal tubules[16] and in collecting ducts.[14] MMP-2 and -9 were also showed to be pivotal to recruitment and chemotaxis of inflammatory cells.[17] MMP-2 has also the potential to activate MMP-1 and -9 by cleaving their prodomains.[18] Tan et al[19] disclosed that MMP-9 contributes to the pathogenesis of RF via macrophage recruitment through osteopontin cleavage. It was proved that MMP-2 and -9 induced tubular cell epithelial–mesenchymal transition.[19,20] MMP-2 can also promote ECM production and accumulation.[21] Recent studies showed positive correlation between urinary TGF-beta excretion and urinary excretions of MMP-2 and -9 that confirm their profibrotic action.[22] Experimental data and human studies demonstrated that increased MMPs glomerular expression correlates positively with severity of glomerular damage and progression of kidney disease.[23–26] Recent studies in patients with chronic kidney disease (CKD), diabetic nephropathy, and after kidney transplantation demonstrated that higher serum levels and/or urinary excretions of MMPs and TIMPs might be biomarkers for an early stage of RF or its progression. Therefore, the assessment of utility of selected MMPs and MMPs/TIMPs ratios as potential early biomarkers for kidney fibrosis in children with chronic glomerulopathies is justified and may be clinically relevant.
The purpose of the study was to assess urinary and serum MMP-1, -2, -9 and TIMP-1, -2 concentrations in children with nephrotic syndrome (NS). Steroid-dependent and steroid-resistant patients were compared with respect to measured parameters. The correlations of measured parameters with magnitude of proteinuria, histopathological diagnosis, duration of the disease, and number of relapses were determined. Similar comparison was made between children treated and those nontreated with cyclosporine A.
2. Patients, materials and methods
2.1. Patients
Baseline characteristics of patients and controls are presented in Table 1. The study comprised of 39 children (33 boys and 6 girls) aged 4 to 18 years (median 13.7 years) with NS treated in the Department of Pediatric Nephrology, Children's University Hospital in Lublin, Poland. According to KIDIGO guidelines,[27] 23 patients were affected with steroid-dependent nephrotic syndrome (2 or more relapses of proteinuria during corticosteroid therapy, or within 14 days of ceasing therapy, SDNS) and 16 ones—with steroid-resistant nephrotic syndrome (failure to achieve complete remission of proteinuria after 8 weeks of corticosteroid therapy, SRNS). In the majority of patients, a histopathological examination revealed minimal change disease (MCD)—25 (64%). Focal segmental glomerulosclerosis (FSGS), mesangioproliferative glomerulonephritis, membranoproliferative glomerulonephritis (MPGN), and membranous glomerulonephritis were diagnosed in 4 (10.3%), 6 (15.5%), 2 (5.1%), and 2 (5.1%) children, respectively. Before enrolment into the study, all patients were treated with steroids according to KIDIGO 2012 guidelines.[27] Treatment of onset of NS was started with prednisone in dose of 60 mg/m2 per d or 2 mg/kg per d (maximum, 60 mg/d) for 4 to 6 weeks, then switched to 40 mg/m2 or 1.5 mg/kg (maximum, 40 mg) on alternate days for 4 weeks and tapered for 2 to 5 months. In relapse of NS, the initial dose of prednisone was used until urinary protein was negative in 3 consecutive days. Steroid-free time interval before enrolment to the study was from 1 day to 6 months.
Table 1.
Characteristics of study and control groups.
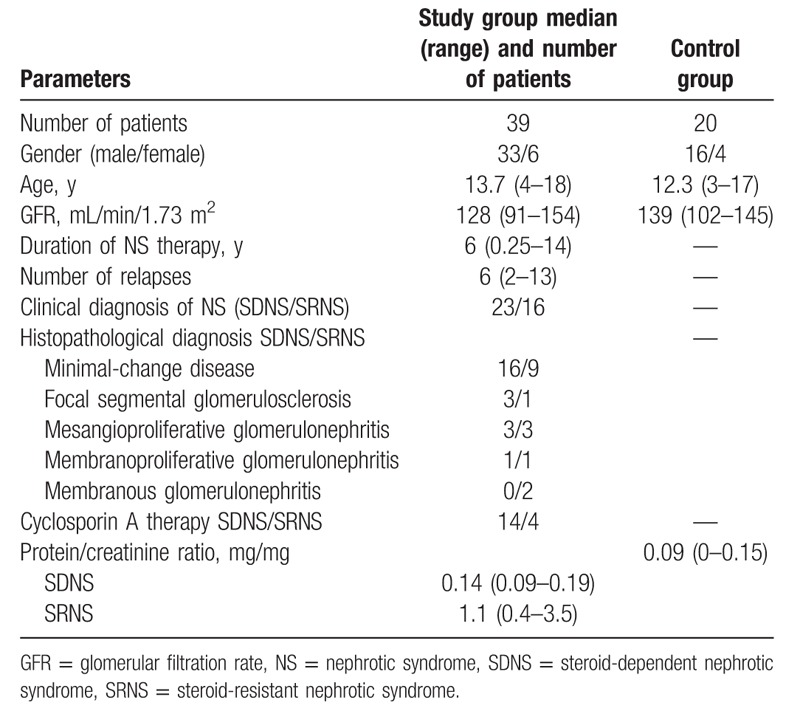
Eighteen patients were treated with cyclosporine A in dose of 2 to 5 mg/kg per d (C0 cyclosporine plasma level of 85–162 ng/mL) for 2 to 4 years. The median duration of NS and the median number of relapses were 6 years (0.25–14 years) and 6 (2–13), respectively. All children had normal estimated glomerular filtration rate calculated by the Schwartz formula: 0.55 × body height (cm)/serum creatinine (Cr) level (mg/dL). None of the patients showed clinical evidence of infection, had diabetes, malignancies or peripheral vascular disease, smoked. In 20 patients, hypertension was diagnosed. In all, blood pressure was well controlled with the use of angiotensin-converting enzyme (ACE) inhibitors (14 patients) and calcium channel blockers (6 patients). Nineteen patients had nephroprotective treatment with ACE inhibitors or angiotensin II receptor antagonists.
The age- and sex-matched 20 apparently healthy volunteers (median age 12.3 years) were controls.
2.2. Methods
The mid-stream first-morning urine specimen was collected from each study participant on the same day.
Routine laboratory techniques were used to measure proteinuria. Concentrations of serum and urinary Cr were determined by Jaffe test.
Urinary MMP-1, -2, -9 and TIMP-1, -2 concentrations were measured using commercially available enzyme-linked immunosorbent assay test kits (R&D Systems) after prior preparation of urine samples following the manufacturer's instructions. Monoclonal antibody specific for MMP-1, -2, -9 and TIMP-1, -2 was used to detect MMPs and TIMPs in the urine samples. Horseradish peroxidase-conjugated avidin was added, followed by a color-forming peroxidase substrate containing tetramethylbenzidine. The color was then measured at 450 nm by a microtiter plate reader. The results were calculated by reference to standard curves.
The urinary enzyme excretions and urinary inhibitor excretions were expressed as enzyme/Cr and inhibitor/Cr ratios in nanograms per milligram of Cr (ng/mg). Similarly, the urinary protein excretion was expressed as protein/Cr ratio in milligrams per milligram of Cr (mg/mg).
The statistical analysis was performed using STATISTICA 7.1.
Nonparametric statistic methods were used as the data were not normally distributed in Shapiro–Wilk test and the patients groups were relatively small.
Differences between groups were assessed using nonparametric Mann–Whitney U test and correlation coefficients were calculated using Spearman test. P ≤ .05 was considered significant.
3. Results
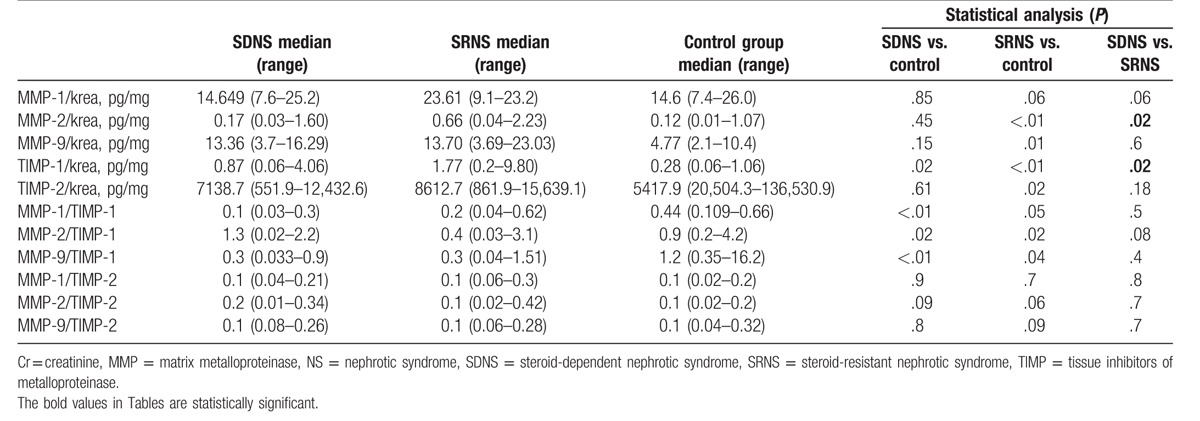
In children with NS significantly higher median values of urinary MMP-1, -2, -9/Cr ratios and TIMP-1, -2/Cr ratios were observed as compared with controls (P < .05). In comparison with controls, patients with NS were also characterized by significantly lower median values of MMP-1, -2, -9/TIMP-1 ratios (Table 2). In children with SRNS, the median values of urinary MMP-2/Cr ratio (P = .01) and urinary TIMP-1/Cr ratio (P = .02) were significantly higher as compared with children with SDNS (Table 3).
Table 2.
The results of urinary excretion of MMPs and TIMPs in study and control groups.
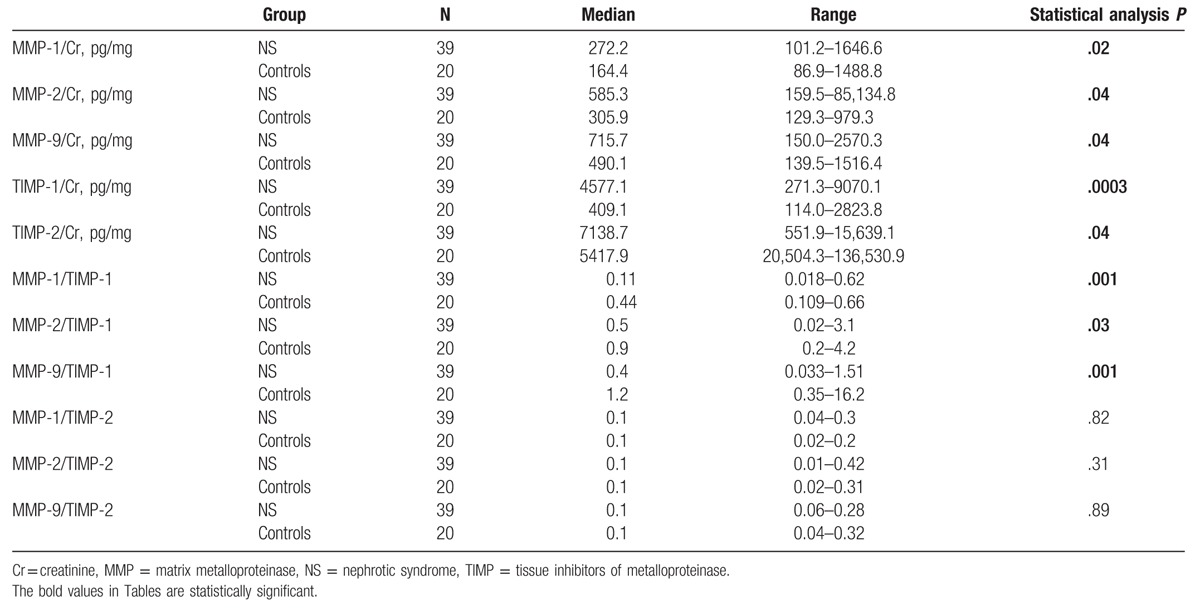
Table 3.
The results of concentrations of urinary MMPs and TIMPs in SDNS, SRNS, and controls.
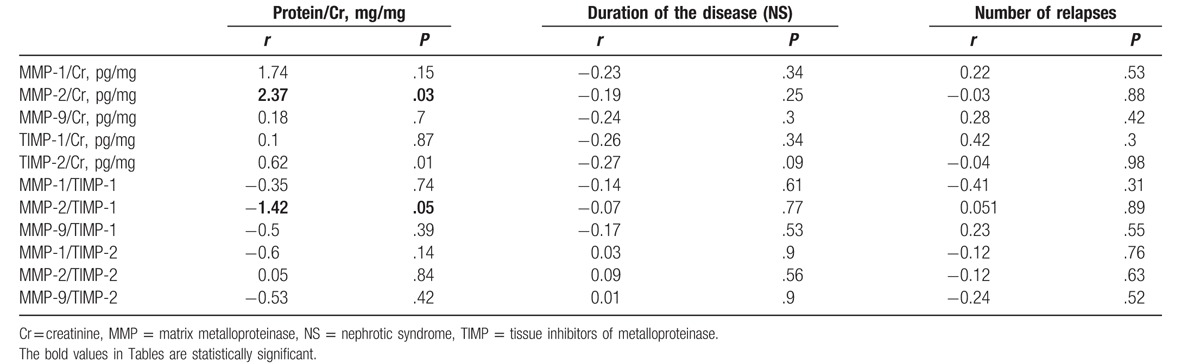
We disclosed significant positive correlation between the median value of urinary MMP-2/Cr ratio and magnitude of proteinuria (r = 2.37, P = .03) whereas correlation between the median value of urinary MMP-2/TIMP-1 ratio (r = −1.42, P = .05) and magnitude of proteinuria was significantly negative. In children with MCD as compared to those with other glomerulopathies, there was significantly higher median value of urinary MMP-2/TIMP-1 ratio (P < .05). The correlations between measured biomarkers and duration of the disease/number of relapses did not show any significant differences (Table 4).
Table 4.
The correlations between concentrations of urinary MMPs and TIMPs and magnitude of proteinuria, duration of the disease, and number of relapses in children with NS.
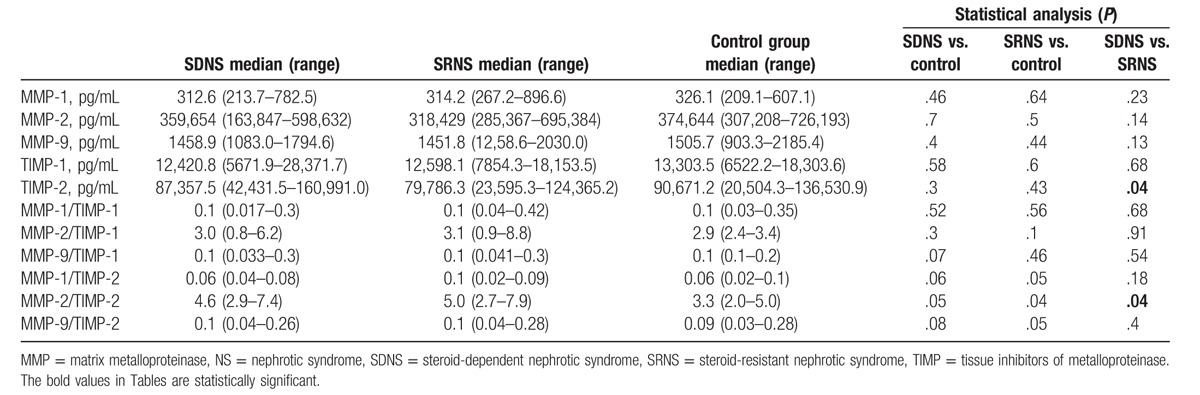
No significant differences in median values of serum MMPs, TIMPs, and MMPs/TIMPs ratios between nephrotic patients and controls were observed (Tables 5 and 6).
Table 5.
The results of serum concentrations of MMPs and TIMPs in study and control groups.
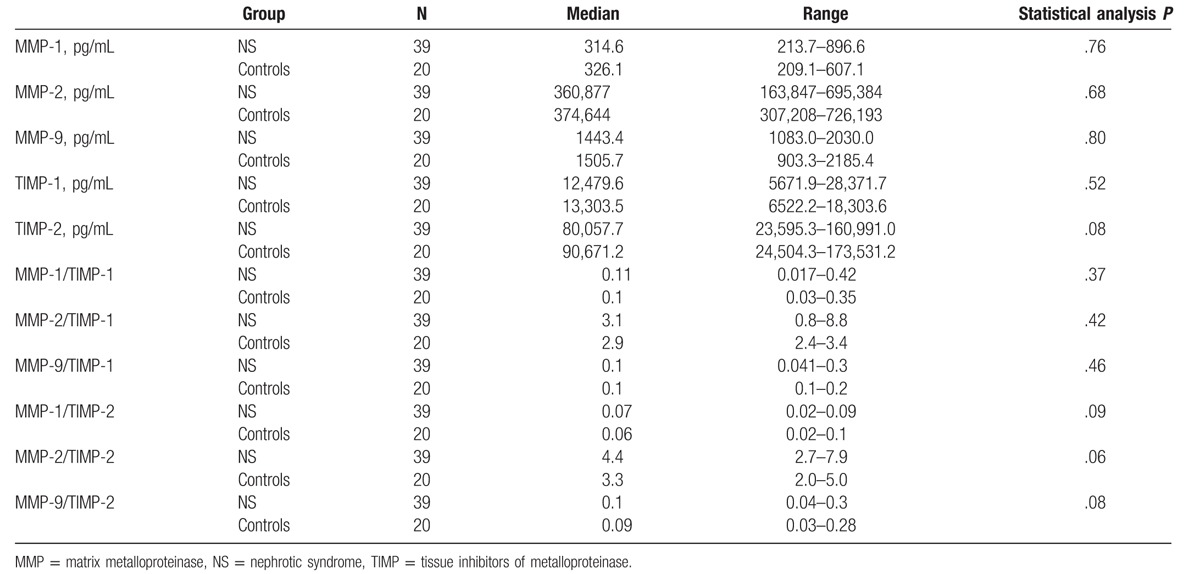
Table 6.
The results of serum concentrations of MMPs and TIMPs in SDNS, SRNS, and controls.
There were no significant correlations between measured biomarkers (urinary MMPs and TIMPs) and dose of prednisone. Similarly, no correlations between measured biomarkers and steroid-free time interval before enrolment to the study were observed.
There were no significant differences in median values of urinary MMPs and TIMPs excretions between children treated and those nontreated with cyclosporine A.
4. Ethical aspects
The study was approved by Ethics Committee of the Medical University of Lublin.
Informed consent was obtained from all individual participants included in the study, either the patients or the parents or legal guardians.
5. Discussion
In chronic glomerulopathies, persistent or recurrent proteinuria may lead to progressive kidney fibrosis and CKD occurrence. There is a small number of reports on serum levels and/or urinary excretions of MMPs and TIMPs in patients with kidney diseases and none of them regard children with glomerulopathies.
“In vitro” and animal studies showed higher activity of MMPs and TIMPs in kidney during fibrosis process.[4,26–30] Experimental model of kidney fibrosis showed elevated MMP-1 activity in glomeruli and suggested its significant role in initiation of glomerular remodeling processes.[28] Hirt-Minkowski et al[31] revealed significant positive correlation between serum level and/or urinary excretion of MMP-1 and TIMP-1 and existing or early developing interstitial fibrosis in renal allograft. Elevated serum MMP-1 level was also observed in lupus nephritis.[23] Morillas et al[32] demonstrated higher serum MMP-1 and TIMP- 1 levels in hypertensive patients with target organ damage.
In our patients with SDNS and SRNS, significant increase in urinary MMP-1 and TIMP-1 excretions was observed. Those patients were also characterized by significant decrease in urinary MMP-1/TIMP-1 ratio. These findings may suggest activation of ECM remodeling processes in children with more severe forms of NS.
The profibrotic role of MMP-2 was reported in experimental studies.[8,17,18] In a few studies, increased urinary MMP-2 excretion was observed at an early stage of diabetic nephropathy. In addition, positive correlation between urinary MMP-2 excretion and risk factors for diabetic nephropathy was demonstrated.[33–36] Higher serum MMP-2 level was observed also in patient with interstitial fibrosis/tubular atrophy of transplanted kidney.[37] Other authors showed increased serum MMP-2 level and increased urinary MMP-2 excretion in patients with CKD associated with progressive kidney fibrosis.[20,38–41] Higher serum MMP-2 and MMP-9 levels were found in patients with CKD,[42,43] diabetic nephropathy,[33,34,43] membranous nephropathy,[24] FSGS, MPGN, lupus nephritis,[13,44,45] and acute kidney injury.[46–47] Our studies showed increased urinary MMP-2 excretion and decreased urinary MMP-2/TIMP-1 ratio. In children with SRNS, we observed higher urinary MMP-2/Cr ratio as compared with children with SDNS. These findings may suggest activation of ECM remodeling processes both in children with SDNS and in those with SRNS but steroid-resistant nephrotic children seem to be at higher risk for RF and CKD.
In our patients, positive correlation between magnitude of proteinuria and urinary MMP-2 excretion was observed whereas correlation between magnitude of proteinuria and urinary MMP-2/TIMP-1 ratio was negative. These results may suggest that patients with proteinuria are at higher risk for RF due to predominance of ECM degradation inhibition processes. In addition, our results may indicate an important role of urinary MMP-2 and MMP-2/TIMP-1 ratio as an early biomarkers for RF.
Taking into account histopathological diagnosis, children with MCD showed higher urinary MMP-2/TIMP-1 ratio in comparison with those with other glomerulopathies. These results may indicate that nephrotic children with other than MCD glomerulopathy are more susceptible to RF due to more intense activation of ECM remodeling processes.
Previously published studies with use of experimental models of FSGS, lupus nephritis, MPGN showed increased glomerular expression and/or activity of MMP-9.[13,44–45] Similarly, increased glomerular MMP-9 activity was found in patients with lupus nephritis, IgA nephropathy, Henoch–Schonlein purpura (HSP), and postinfectious acute glomerulonephritis.[48] Other authors reported higher urinary MMP-9 activity in patients with CKD[38–40] and HSP with renal involvement.[49] Our study showed increased urinary MMP-9 excretions and decreased urinary MMP-9/TIMP-1 ratio. These results may suggest activation of ECM remodeling processes in children with SDNS and SRNS.
Experimental studies conducted in glomerulosclerosis and interstitial fibrosis found higher expression and activity of TIMPs, particularly TIMP-1, in glomeruli.[30,50] Urinary TIMP-1, -2 excretions were reported to be increased in patients with CKD.[22,51] In our study, all patients showed elevated urinary TIMP-1, -2 excretions. In children with SRNS, urinary TIMP-1/Cr ratio was significantly higher than that in children with SDNS. This may suggest an increase in activity of ECM remodeling inhibition processes in SRNS. Furthermore, in our patients decreased urinary MMP-1, -2, -9/TIMP-1 ratios were disclosed. This may suggest predominance of ECM degradation inhibition processes and fibrosis development in SDNS and SRNS. No significant differences in serum MMPs and TIMPs between nephrotic patients and controls observed in our study may support the hypothesis that their local production and tubular excretion into urine is the most relevant to tubulointerstitial injury and development of kidney fibrosis.
The main limitation of this study is relatively small group of patients. Further studies are required to confirm our findings.
6. Conclusions
Children with SDNS and SRNS are characterized by increased urinary excretion of fibrotic biomarkers. Urinary MMP-1, -2, -9 excretions and urinary MMP-1, -2, -9/TIMP-1 ratios, particularly urinary MMP-2 excretion and urinary MMP-2/TIMP-1 ratio, may become useful early biomarkers for kidney fibrosis. Children with SRNS, those with proteinuria and other than MCD glomerulopathies seem to be more susceptible to an early kidney fibrosis.
Footnotes
Abbreviations: ACE = angiotensin-converting enzyme, CKD = chronic kidney disease, Cr = creatinine, ECM = extracellular matrix, FSGS = focal segmental glomerulosclerosis, HSP = Henoch–Schonlein purpura, MCD = minimal change disease, MMP = matrix metalloproteinase, MPGN = membranoproliferative glomerulonephritis, NS = nephrotic syndrome, RF = renal fibrosis, s = serum, SDNS = steroid-dependent nephrotic syndrome, SRNS = steroid-resistant nephrotic syndrome, TIMP = tissue inhibitors of metalloproteinase, u = urinary.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res 2003;92:827–39. [DOI] [PubMed] [Google Scholar]
- [2].Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Ren Physiol 2007;292:F905–11. [DOI] [PubMed] [Google Scholar]
- [3].Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006;69:562–73. [DOI] [PubMed] [Google Scholar]
- [4].Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 2000;15:290–301. [DOI] [PubMed] [Google Scholar]
- [5].Tan TK, Zheng G, Hsu TT, et al. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol 2010;176:1256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aresu L, Benali S, Garbisa S, et al. Matrix metalloproteinases and their role in the renal epithelial mesenchymal transition. Histol Histopathol 2011;26:307–13. [DOI] [PubMed] [Google Scholar]
- [7].Okada Y, Gonoji Y, Naka K, et al. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells: purification and activation of the precursor and enzymic properties. J Biol Chem 1992;267:21712–9. [PubMed] [Google Scholar]
- [8].Olson MW, Toth M, Gervasi DC, et al. High affinity binding of latent matrix metalloproteinase-9 to the alpha2(IV) chain of collagen IV. J Biol Chem 1998;273:10672–81. [DOI] [PubMed] [Google Scholar]
- [9].Morrison CJ, Butler GS, Rodriguez D, et al. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol 2009;21:645–53. [DOI] [PubMed] [Google Scholar]
- [10].Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol 2002;22:51–86. [DOI] [PubMed] [Google Scholar]
- [11].Schaefer L, Han X, Gretz N, et al. Tubular gelatinase A (MMP-2) and its tissue inhibitors in polycystic kidney disease in the Han: SPRD rat. Kidney Int 1996;49:75–81. [DOI] [PubMed] [Google Scholar]
- [12].Inkinen KA, Soots AP, Krogerus LA, et al. Fibrosis and matrix metalloproteinases in rat renal allografts. Transpl Int 2005;18:506–12. [DOI] [PubMed] [Google Scholar]
- [13].Tomita M, Koike H, Han GD, et al. Decreased collagen-degrading activity could be a marker of prolonged mesangial matrix expansion. Clin Exp Nephrol 2004;8:17–26. [DOI] [PubMed] [Google Scholar]
- [14].Piedagnel R, Murphy G, Ronco PM, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Biol Chem 1999;274:1614–20. [DOI] [PubMed] [Google Scholar]
- [15].Kuroda T, Yoshida Y, Kamiie J, et al. Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis in WKY rats. Clin Exp Nephrol 2004;8:206–15. [DOI] [PubMed] [Google Scholar]
- [16].Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int 2005;68:155–66. [DOI] [PubMed] [Google Scholar]
- [17].Li Q, Park PW, Wilson CL, et al. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–46. [DOI] [PubMed] [Google Scholar]
- [18].Toth M, Chvyrkova I, Bernardo MM, et al. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun 2003;308:386–95. [DOI] [PubMed] [Google Scholar]
- [19].Tan TK, Zheng G, Hsu TT, et al. Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 2013;93:434–49. [DOI] [PubMed] [Google Scholar]
- [20].Pawlak K, Mysliwiec M, Pawlak D. Peripheral blood level alterations of MMP-2 and MMP-9 in patients with chronic kidney disease on conservative treatment and on hemodialysis. Clin Biochem 2011;44:838–43. [DOI] [PubMed] [Google Scholar]
- [21].Marti HP. Role of matrix metalloproteinases in the progression of renal lesions. Presse Med 2000;29:811–7. [PubMed] [Google Scholar]
- [22].Musiał K, Bargenda A, Zwolińska D. Urine survivin, E-cadherin and matrix metalloproteinases as novel biomarkers in children with chronic kidney disease. Biomarkers 2015;20:177–82. [DOI] [PubMed] [Google Scholar]
- [23].Nakamura T, Ebihara I, Tomino Y, et al. Effect of a specific endothelin A receptor antagonist on murine lupus nephritis. Kidney Int 1995;47:481–9. [DOI] [PubMed] [Google Scholar]
- [24].McMillan JI, Riordan JW, Couser WG, et al. Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J Clin Invest 1996;97:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koide H, Nakamura T, Ebihara I, et al. Increased mRNA expression of metalloproteinase-9 in peripheral blood monocytes from patients with immunoglobulin A nephropathy. Am J Kidney Dis 1996;28:32–9. [DOI] [PubMed] [Google Scholar]
- [26].Qing-Hua G, Ju-Ming L, Chang-Yu P, et al. The kidney expression of matrix metalloproteinase-9 in the diabetic nephropathy of Kkay mice. J Diabetes Complications 2008;22:408–12. [DOI] [PubMed] [Google Scholar]
- [27].Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012;2:163–76. [Google Scholar]
- [28].Ahmed AK, Haylor JL, El Nahas AM, et al. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 2007;71:755–63. [DOI] [PubMed] [Google Scholar]
- [29].Ishidoya S, Morrissey J, McCracken R, et al. Delayed treatment with enalapril halts tubulointerstitial fibrosis in rats with obstructive nephropathy. Kidney Int 1996;49:1110–9. [DOI] [PubMed] [Google Scholar]
- [30].Sharma AK, Mauer SM, Kim Y, et al. Altered expression of matrix metalloproteinase-2, TIMP, and TIMP-2 in obstructive nephropathy. J Lab Clin Med 1995;125:754–61. [PubMed] [Google Scholar]
- [31].Hirt-Minkowski P, Marti HP, Hönger G, et al. Correlation of serum and urinary matrix metalloproteases/tissue inhibitors of metalloproteases with subclinical allograft fibrosis in renal transplantation. Transpl Immunol 2014;30:1–6. [DOI] [PubMed] [Google Scholar]
- [32].Morillas P, Quiles J, de Andrade H, et al. Circulating biomarkers of collagen metabolism in arterial hypertension: relevance of target organ damage. J Hypertens 2013;31:1611–7. [DOI] [PubMed] [Google Scholar]
- [33].Altemtam N, Nahas ME, Johnson T. Urinary matrix metalloproteinase activity in diabetic kidney disease: a potential marker of disease progression. Nephron Extra 2012;2:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van der Zijl NJ, Hanemaaijer R, Tushuizen ME, et al. Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: a marker of incipient diabetic nephropathy? Clin Biochem 2010;43:635–9. [DOI] [PubMed] [Google Scholar]
- [35].Thrailkill KM, Bunn RC, Moreau CS. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care 2007;30:2321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McKittrick IB, Bogaert Y, Nadeau K, et al. Urinary matrix metalloproteinase activities: biomarkers for plaque angiogenesis and nephropathy in diabetes. Am J Renal Physiol 2011;301:F1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rödder S, Scherer A, Raulf F, et al. Renal allografts with IF/TA display distinct expression profiles of metzincins and related genes. Am J Transplant 2009;9:517–26. [DOI] [PubMed] [Google Scholar]
- [38].Musiał K, Zwolińska D. Novel indicators of fibrosis-related complications in children with chronic kidney disease. Clin Chim Acta 2014;430:15–9. [DOI] [PubMed] [Google Scholar]
- [39].Musiał K, Zwolińska D. New markers of apoptosis in children on chronic dialysis. Apoptosis 2013;18:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Musiał K, Zwolińska D. Matrix metalloproteinases and soluble Fas/FasL system as novel regulators of apoptosis in children and young adults on chronic dialysis. Apoptosis 2011;16:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chang HR, Yang SF, Li ML, et al. Relationships between circulating matrix metalloproteinase-2 and -9 and renal function in patients with chronic kidney disease. Clin Chim Acta 2006;366:243–8. [DOI] [PubMed] [Google Scholar]
- [42].Cheng S, Pollock AS, Mahimkar R, et al. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 2006;20:1898–900. [DOI] [PubMed] [Google Scholar]
- [43].Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine 2009;35:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu S, Li Y, Zhao H, et al. Increase in extracellular cross-linking by tissue transglutaminase and reduction in expression of MMP-9 contribute differentially to focal segmental glomerulosclerosis in rats. Mol Cell Biochem 2006;284:9–17. [DOI] [PubMed] [Google Scholar]
- [45].Tveita A, Rekvig OP, Zykova SN. Glomerular matrix metalloproteinases and their regulators in the pathogenesis of lupus nephritis. Arthritis Res Ther 2008;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 2008;73:863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Novak KB, Le HD, Christison-Lagay ER, et al. Effects of metalloproteinase inhibition in a murine model of renal ischemia-reperfusion injury. Pediatr Res 2010;67:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Urushihara M, Kagami S, Kuhara T, et al. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol Dial Transplant 2002;17:1189–96. [DOI] [PubMed] [Google Scholar]
- [49].Erol M, Yigit O, Tasdemir M, et al. Potential of serum and urinary matrix metalloproteinase-9 levels for the early detection of renal involvement in children with Henoch-Schonlein purpura. Iran J Pediatr 2016;26:e6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Duymelinck C, Dauwe SE, de Greef KE, et al. TIMP-1 gene expression and PAI-1 antigen after unilateral ureteral obstruction in the adult male rat. Kidney Int 2000;58:1186–201. [DOI] [PubMed] [Google Scholar]
- [51].Horstrup JH, Gehrmann M, Schneider B, et al. Elevation of serum and urine levels of TIMP-1 and tenascin in patients with renal disease. Nephrol Dial Transplant 2002;17:1005–13. [DOI] [PubMed] [Google Scholar]


