Supplemental Digital Content is available in the text
Keywords: apoptosis, peripheral blood mononuclear cells, pyroptosis, sepsis, trauma
Abstract
Pyroptosis plays a pivotal role in sepsis and septic shock in animal studies. However, its clinical significance in pathological conditions has not been well elucidated. This study aimed to evaluate the correlation between the percentage of pyroptotic peripheral blood mononuclear cells (PBMCs) and the clinical index and to investigate the relationship between PBMCs pyroptosis and the development of sepsis in trauma patients.
This prospective study was conducted from October 2016 to May 2017 in a comprehensive trauma center. Sixty trauma patients and 10 healthy controls were enrolled. Peripheral blood samples were collected from the patients within 24 hours after injury. The percentages of pyroptotic and apoptotic PBMCs were measured using flow cytometry, and plasma levels of cytokines were evaluated using flow cytometric analysis with a human inflammation 13-plex panel.
Trauma patients who developed sepsis had higher percentages of pyroptotic and apoptotic PBMCs at admission. Patients who developed sepsis (n = 33) had higher interleukin (IL)-6, IL-18, and monocyte chemotactic protein-1 (MCP-1) concentrations at admission than patients (n = 27) who did not develop sepsis. The percentage of PBMCs pyroptosis was significantly correlated with injury severity score (ISS), acute physiology and chronic health evaluation (APACHE) II score, IL-10, IL-18, and MCP-1 levels in trauma patients. PBMCs pyroptosis is a better biomarker in predicting the development of sepsis after trauma.
This study indicates that the percentage of pyroptotic PBMCs increases during the early phase of trauma and that this increase is significantly correlated with the severity and state of inflammation in trauma patients. PBMCs pyroptosis is a potential marker for predicting the development of sepsis after trauma.
1. Introduction
Trauma is the leading cause of death in population under 45 years old.[1] Sepsis is a major contributing factor to trauma-related mortality.[2,3] The prognosis of patients with trauma-induced sepsis remains poor despite enormous progress in critical care during the past decades. Timely diagnosis and prompt intervention help to improve prognosis of these patients. However, there is still lack of biomarkers to predict the development of sepsis after trauma with high sensitivity and specificity.
The development of sepsis is attributed to dysfunction of the immune system and excessive inflammatory response.[4] Significant death of immune cells is the hallmark of sepsis-induced immune dysfunction.[5,6] Apoptosis, a well-delineated form of programmed cell death (PCD), plays a pivotal role in sepsis-induced immune dysfunction.[7] Studies have shown that it is associated with the severity of the systemic response in critically ill patients, including those with acute respiratory distress syndrome (ARDS), trauma, and sepsis.[8–10] Recent studies have reported that pyroptosis, another important form of PCD, contributes to development of sepsis and septic shock.[11,12] Pyroptosis is phenotypically similar to apoptosis, and both are positive in TdT mediated dUTP Nick End Labeling (TUNEL) and propidium iodide (PI) stains. The major difference between pyroptosis and apoptosis lies in 2 aspects. First, pyroptosis is mediated by inflammatory caspases (caspase-1/11/4/5), whereas apoptosis is mediated by apoptotic caspases (caspase-3/8/9). Secondly, apoptosis is a relatively “clean” form of PCD, whereas pyroptosis is a highly inflammatory form of PCD and induces extensive inflammatory response.[5,13]
Pyroptosis is involved in multiple pathological conditions, including identification of infection,[14,15] hereditary auto-inflammatory syndromes,[16] and inflammatory bowel disease.[17] In a mouse model of septic shock, pyroptosis, rather than cytokine secretion, is probably the crucial determinant of mouse lethality caused by excessive lipopolysaccharide.[18]
But to date, the significance of pyroptosis in clinical context has not been well delineated. As pyroptosis is related to both immune cell death and onset of excessive inflammation in sepsis, in the present, we hypothesize pyroptosis of PBMCs is increased in trauma patients, and can serve as a marker to predict development of sepsis.
2. Methods
2.1. Research setting and study participants
This prospective study of trauma patients was conducted over an 8-month period (from October 2016 to May 2017). Sixty severe trauma patients at Tongji Hospital were enrolled in this study. This study was approved by the medical ethics committee of Tongji Hospital of the Tongji Medical College of Huazhong University of Science and Technology. Written informed consent was obtained from all subjects or their surrogates.
The enrolment criteria consisted of a history of trauma (injury severity score [ISS] ≥16) and age between 18 and 75 years. Patients with known pre-existing immunological disorders, concomitant acute myocardial infarction, burns, thromboembolic events, and those receiving immune-suppressive or anti-coagulant medication were excluded. For comparison, 10 age-matched and sex-matched healthy volunteers who received an annual physical examination and had no clinical evidence of infection were recruited as controls.
2.2. Data collection
The medical records were prospectively recorded using pre-existing standardized evaluation forms that included demographic data, ISS, APACHE II score and biochemical parameters, which were obtained during the first 24 hours of admission.
2.3. Outcome measurements
The patients were reviewed daily until death or discharge. The primary outcome measures were sepsis and 28-day mortality. Sepsis patients were defined as having a sepsis-3 classification.[19] According to sepsis-3, sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction was identified as an acute change in total sequential (sepsis-related) organ failure assessment (SOFA) score ≥2 points consequent to the infection. The infections were diagnosed according to the isolation of a predominant organism from blood or body fluids cultures obtained under sterile conditions. Shock was defined as the need for vasoactive drugs on days 1–3.
2.4. Blood sampling and isolation of PBMCs
Blood samples were collected from the trauma patients within 24 hours after injury and from the sepsis patients within 24 hours of diagnosis. All blood samples from both the study group and control group were collected by venipuncture of the forearm veins. The samples were collected in an EDTA vacutainer and centrifuged at 370 × g for 5 minutes, and the plasma samples were collected and stored at −80 °C until assayed. PBMCs were isolated from the blood samples using density gradient centrifugation with Ficoll–Hypaque (TBD science; Tianjin, China). All flow cytometry assays were performed within 1 hour after blood extraction to ensure that the results were as similar as possible to in vivo conditions. The subsequent analyses were performed after the collection of all samples.
2.5. Flow cytometry
Apoptosis and pyroptosis of PBMCs were measured by flow cytometry (BD FACS Canto II; BD Biosciences, San Jose, CA). Fluorescent-labeled inhibitors of caspases probe assays (FLICA) (Immunochemistry Technologies, Minneapolis, MN) were performed to determine the intracellular activation of specific types of cell death. Each FLICA probe contained a 3- or 4-amino acid sequence targeted by a specific activated caspase. Interference from pro-caspases or the inactive form of the enzymes did not occur. FLICA probes were directly added to 100 μL of the cell suspension (approximately 1 × 106 cells/mL), incubated for 1 hour at 37 °C under 5% CO2 in the dark, and washed 3 times with PBS supplemented with 2 mM EDTA and 2% foetal bovine serum. FLICA probes were cell-permeable and covalently bound to the active forms of specific caspases. After washing, the FLICA fluorescent signal was specifically retained within cells containing the appropriate active form of the caspase, while the reagent was washed away in cells lacking the appropriate active caspase. Then, the sample was stained with PI within 30 minutes before flow cytometric analysis. Ten thousand events were counted per sample.
2.6. Analyses of cytokine plasma levels
The levels of IL-1β, interferon gamma (IFN)-α, IFN-γ, tumor necrosis factor (TNF)-α, MCP-1, IL-6, IL-8, IL-10, IL-12p70, IL-17-A, IL-18, IL-23, and IL-33 were determined by flow cytometric analysis using a human inflammation 13-plex panel (Enabling Legendary DiscoveryTM, CA) according to the manufacturer's instructions.
2.7. Statistical analysis
The data are expressed as the mean ± SD or the media (95% confidence interval [CI]) and analyzed using Student t test or analysis of variance (ANOVA), unless otherwise stated. Categorical variables were compared using the chi-squared test or Fisher exact test, as appropriate. The cytokine concentrations were transformed into their natural logarithm and analyzed with parametric tests. The flow cytometry data were analyzed using Flow Jo Software version 10.6 (Treestar, Inc., Ashland, OR). The statistical analyses were performed using GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA) or IBM SPSS version 23 (IBM Crop., Armonk, NY). A P value of <0.05 was considered statistically significant.
Correlations between pyroptotic or apoptotic PBMCs and other markers were evaluated using scatterplots and Spearman rank-correlation coefficients. Binary logistic regression was performed to identify variables that were independently associated with sepsis development using SPSS. Univariate analysis was conducted initially and variables identified as significant with a P < .1 were then added into a forwards likelihood-ratio stepwise regression with significance set at P < .05 for inclusion and P > .1 for removal. A receiver operating characteristic (ROC) curve was used to evaluate the value of pyroptotic PBMCs as a diagnostic biomarker in sepsis patients. A value of P < .05 was considered statistically significant.
3. Results
Clinical data from a total of 67 patients were collected. Seven patients were excluded, finally, a total of 60 patients were thus enrolled in the present study. The grouping method expressed as Fig. 1.
Figure 1.
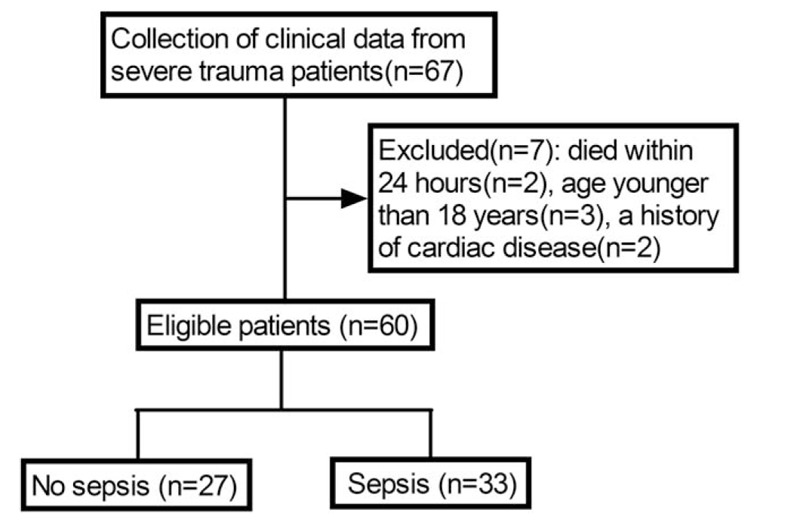
Screening flowchart.
3.1. Patient characteristics
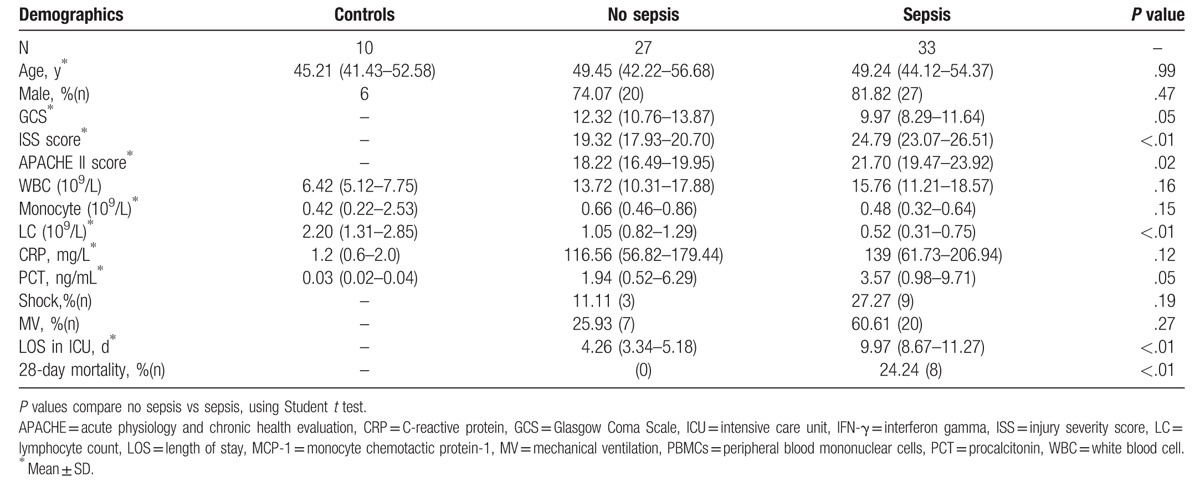
The study cohort included a control group (n = 10) and an injury group (n = 60). The trauma patients included 47 men and 13 women. A cohort of blunt polytrauma patients with an ISS ≥16 was classified by outcome (sepsis, n = 33, and no sepsis, n = 27). Patients who later developed sepsis had higher ISS and APACHE II scores (sepsis vs no sepsis: 24.79 [23.07–26.51] vs 19.32 [17.93–20.70], P < .01; 23.00 [20.91–25.09] vs 11.63 [10.34–12.92], P < .01, respectively) (Table 1). Mean SOFA scores were gradually increased and peaked on day 8 in sepsis patients (Supplemental Fig. 1). The causes of trauma were motor vehicle collision (n = 22), fall (n = 8), and others (n = 3). The locations of infection were the chest (n = 18), abdomen (n = 6), soft tissue (n = 4), urinary (n = 3), and others (n = 2). The sepsis patients comprised 27 men and 6 women. The cause of sepsis was bacterial infection in all cases. Nineteen Gram-positive and 14 Gram-negative bacteria were isolated, including 8 mixed bacterial infections. Six positive blood cultures and 27 other site cultures (sputum, pleural fluid, ascites, urine, pus) were identified (Supplemental Table 1). No significant differences regarding age and sex were observed among the sepsis patients, no sepsis patients, and healthy controls.
Table 1.
Demographics.
3.2. Percentage of PBMCs pyroptosis and apoptosis were higher in severe trauma patients who developed sepsis
After the exclusion of dead cells, the PBMCs were gated. The pyroptosis (FAM-FLICA-Caspase-1+ PI+) and apoptosis (FAM-FLICA-Caspase-3+ PI+) of PBMCs were examined (Fig. 2). Within 24 hours of injury, patients who later developed sepsis had a higher proportion of pyroptotic PBMCs in their peripheral blood (sepsis vs no sepsis: 14.23% vs 8.70%, P < .01) (Fig. 2A). The percentage of apoptotic PBMCs was also significantly higher in patients who subsequently developed sepsis (sepsis vs no sepsis: 13.33% vs 5.93%, P < .01) (Fig. 2B).
Figure 2.
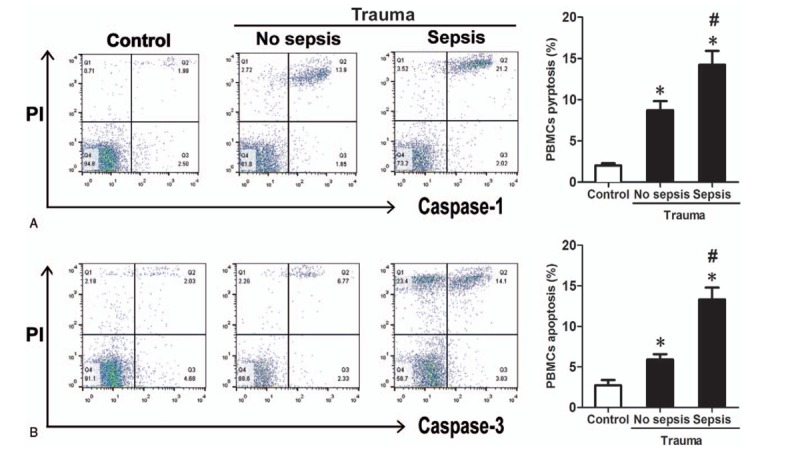
PBMCs pyroptosis and apoptosis in healthy control subjects and trauma patients (no sepsis and sepsis groups). Pyroptotic PBMCs are distinctly immunostained positive for FAM-FLICA-caspase-1 and PI, as shown in area Q2 (A). Apoptotic PBMCs are distinctly immunostained positive for FAM-FLICA-caspase-3 and PI, as shown in area Q2 (B). The proportions of pyroptotic and apoptotic PBMCs in the blood are higher in patients with and without sepsis than in the healthy control subjects (A, B). Patients who developed sepsis (n = 33) had higher percentages of PBMCs pyroptosis and apoptosis when compared with patients who did not develop sepsis (n = 27) (12.76 ± 7.55% vs 6.13 ± 4.22, P < .01; 11.51 ± 8.31 vs 7.13 ± 5.20, P < .01; respectively). ∗ Denotes P < .05 using Student t test comparing with control. # denotes P < .05 using Student t test comparing no sepsis and sepsis. PBMCs = peripheral blood mononuclear cells, PI = propidium iodide.
3.3. Both PBMCs apoptosis and pyroptosis were associate with the severity of trauma
We assessed the correlation between the percentage of pyroptotic PBMCs and the disease severity scoring systems (ISS and APACHE II score). We found that both the percentages of pyroptotic and apoptotic PBMCs were significantly correlated with the ISS (r = 0.60, P < .01 and r = 0.34, P < .01, respectively) and the APACHE II score (r = 0.48, P < .01 and r = 0.55, P < .01, respectively) (Fig. 3A, B).
Figure 3.
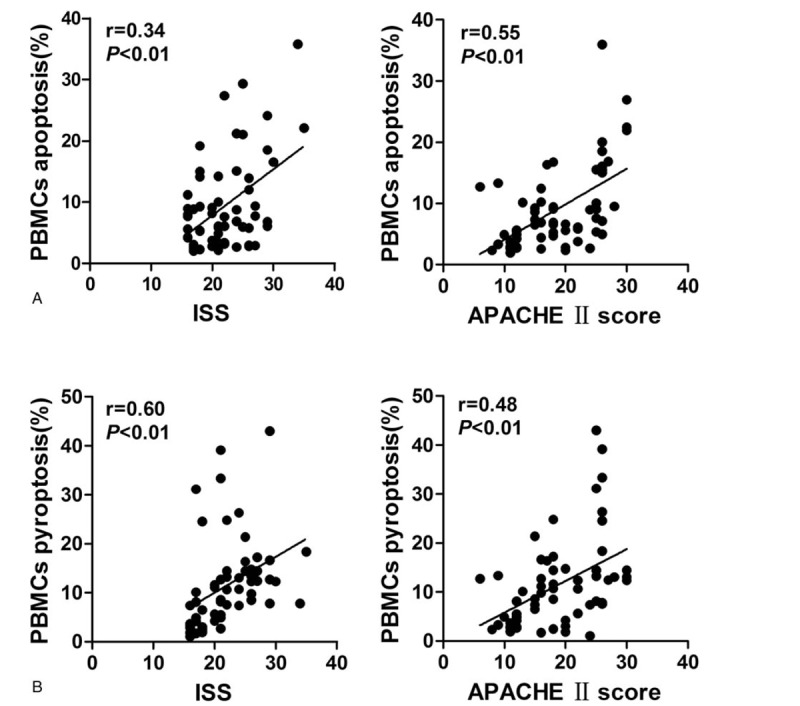
Both PBMCs apoptosis and pyroptosis are associate with the severity of trauma. PBMCs = peripheral blood mononuclear cells.
3.4. Correlations of PBMCs apoptosis and pyroptosis with biochemical parameters in trauma patients
Cytokine quantification was performed on stored plasma from these patients. The levels of IL-6, IL-8, IL-10, and IL-18 were significantly higher in the no sepsis patients than in the control group; and the levels of IL-6, IL-8, IL-10, IL-18, MCP-1, and IFN-γ were significantly higher in the sepsis patients than in the control group (Fig. 4). This result confirms the systemic activation of inflammation in both the sepsis and no sepsis groups. A comparison between the 2 groups demonstrated higher concentrations of MCP-1, IL-6, and IL-10 in patients who developed sepsis (Fig. 4). Compared with the control group, no differences were observed in the levels of IL-1β, IFN-α, TNF-α, IL-23, IL-33, IL-12p70, and IL-17-A in the no sepsis and sepsis patients (not shown).
Figure 4.
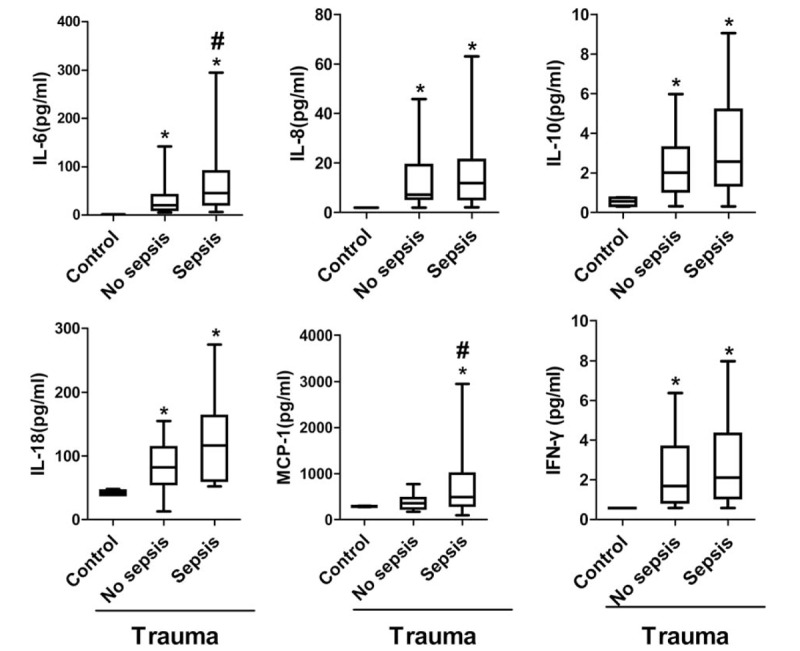
The plasma levels of cytokines in control, no sepsis, and sepsis patients. Cytokines were quantified in plasma obtained <24 hours following injury in trauma patients (n = 60) with critical injury severity (ISS ≥16) and control patients (n = 10). Patients who developed sepsis (n = 33) had higher concentrations of IL-6, IL-18, and MCP-1 when compared with patients who did not develop sepsis (n = 27). IL-6: 1.36 (1.35–1.37), 32.06 (12.91–51.21), 76.21 (41.02–111.40), P = .03. IL-8: 1.93 (1.92–1.94), 14.47 (6.66–22.28), 32.00 (10.31–53.69), P = .13. IL-10: 0.55 (0.20–0.90), 4.38 (1.05–7.71), 6.31 (2.30–10.32), P = .45. IL-18: 40.26 (36.89–43.64), 84.42 (64.83–104.00), 121.10 (92.38–149.80), P = .03. MCP-1: 285.60 (266.00–305.30), 365.00 (273.50–456.40), 673.80 (399.60–947.90), P = .04. IFN-γ: 0.57 (0.56–0.59), 2.15 (1.20–3.10), 2.27 (1.26–3.27), P = .86. Data are presented as control, no sepsis, sepsis, P value in pg/mL as geometric mean (95% CI). ∗ Denotes P < .05 using Student t test comparing with control. # denotes P < .05 using Student t test comparing no sepsis and sepsis. CI = confidence interval, IFN-γ = interferon gamma, IL = interleukin, ISS = injury severity score, MCP-1 = monocyte chemotactic protein-1.
We also found significant correlations between the percentage of pyroptotic PBMCs and the levels of IL-10, IL-18, and MCP-1 (r = −0.43, P < .01; r = 0.34, P < .01; and r = −0.29, P = .03, respectively) (Fig. 5B). However, no significant correlations were observed between the percentage of apoptotic PBMCs and these cytokines (Fig. 5A).
Figure 5.
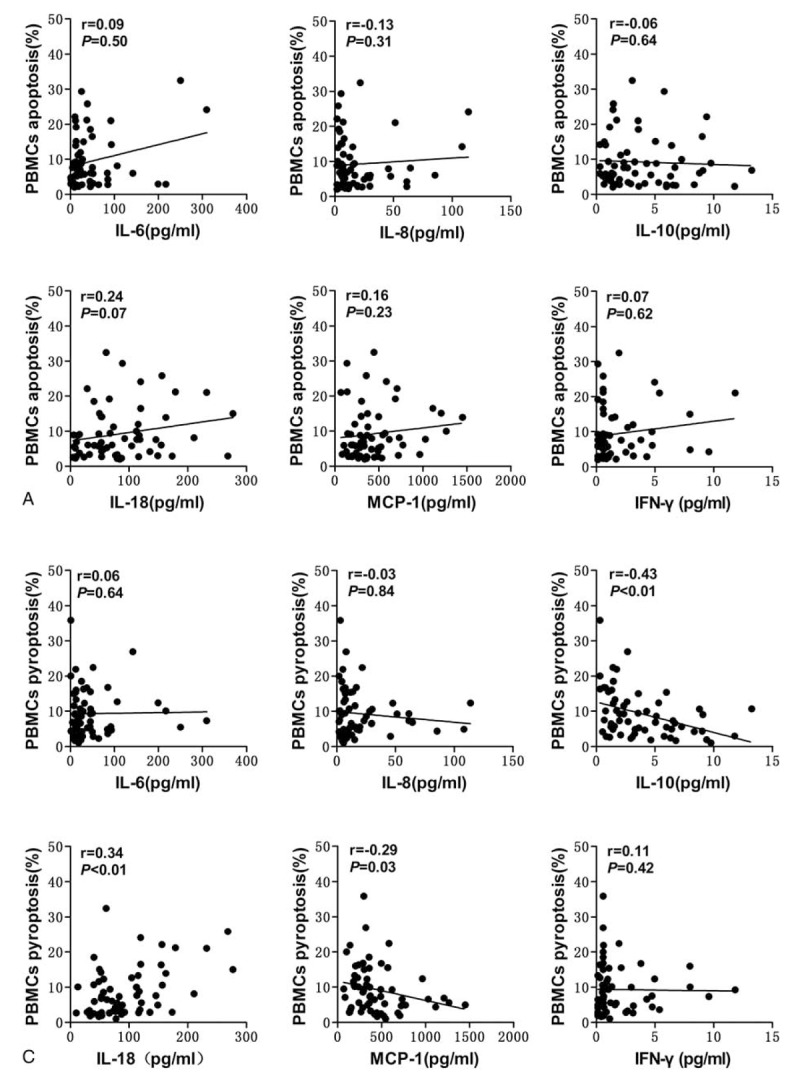
Correlations of PBMCs apoptosis and pyroptosis with biochemical parameters in trauma patients. Significant correlations were found between the percentage of pyroptotic PBMCs and the levels of IL-6, IL-8, IL-10 and MCP-1 in trauma patients. IL = interleukin, MCP-1 = monocyte chemotactic protein-1, PBMCs = peripheral blood mononuclear cells.
3.5. PBMCs pyroptosis is associated with the development of sepsis
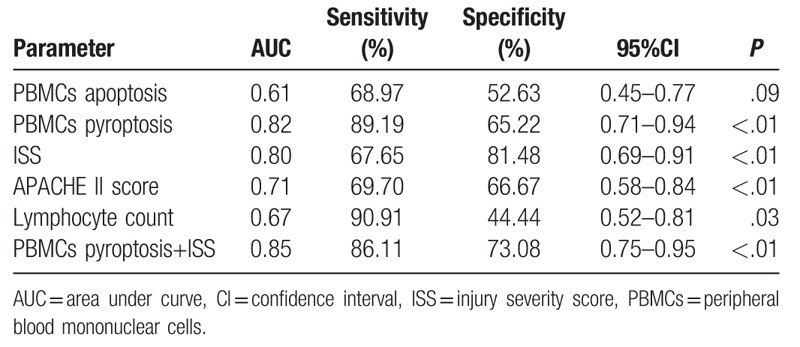
Binary logistic regression analysis showed that ISS, PBMCs pyroptosis, and Lymphocyte count were independent risk factors for sepsis patients, PBMCs pyroptosis being the strongest (Table 2). ROC curves for the PBMCs pyroptosis, PBMCs apoptosis, ISS, APACH II score, and lymphocyte count for the 2 groups were constructed based on statistically significant differences (Fig. 6), and areas under the ROC curves (AUCs) were calculated (Table 3). In terms of predicting sepsis, AUCs of PBMCs apoptosis, PBMCs pyroptosis, ISS, APACH II score, and lymphocyte count were 0.61, 0.82, 0.80, 0.71, and 0.67, respectively. PBMCs pyroptosis was better than any other indicator, with an AUC of 0.82 (sensitivity = 89.19%, specificity = 65.22%). Combined use of PBMCs pyroptosis and ISS was better than any single indicator alone, with an AUC of 0.85 (sensitivity = 86.11%, specificity = 73.08%) (Table 3).
Table 2.
Binary logistic regression analysis of variables independently associated with the development of sepsis.
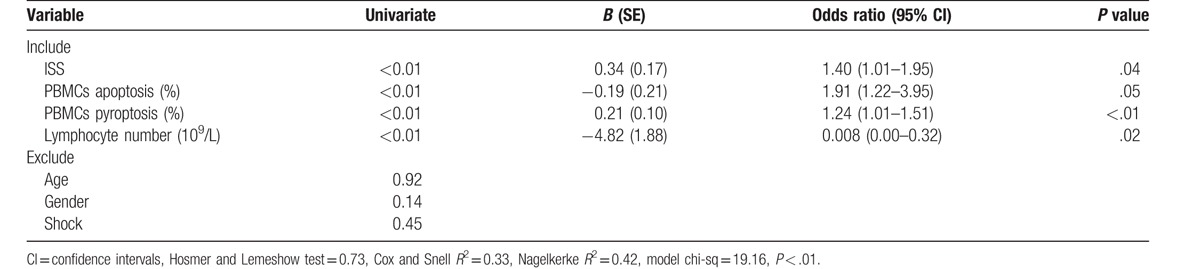
Figure 6.
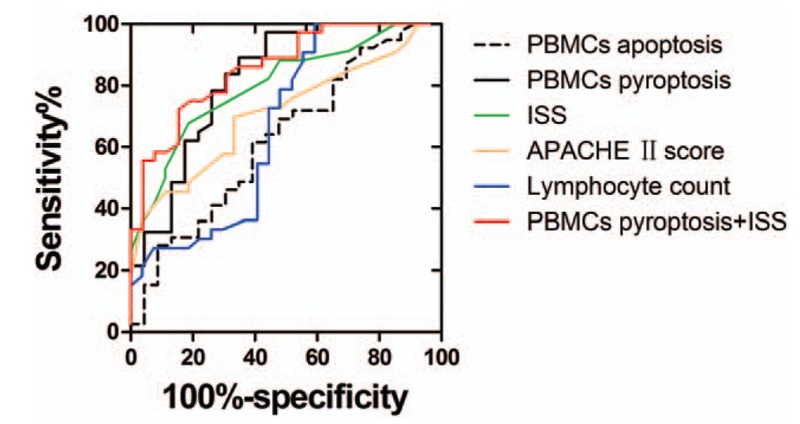
The ROC curves for predicting sepsis. ROC = receiver operating characteristic.
Table 3.
Area under the ROC curve for predicting sepsis in trauma patients.
4. Discussion
PBMCs include any peripheral blood cells that have a round nucleus. These cells consist of lymphocytes (T cells, B cells, and NK cells) and monocytes. In the present study, we found that the percentages of pyroptotic PBMCs was significantly higher in trauma patients than those in healthy controls and significantly correlated with the ISS and APACHE II score, 2 most widely applied scoring systems in trauma patients. Furthermore, the percentage of pyroptotic PBMCs is significantly correlated with IL-10, IL-18, and MCP-1, which are associated with immune disorder in trauma patients.[20,21] Lastly, PBMCs pyroptosis is a better predictor of the development of sepsis in trauma patients than ISS, PBMCs apoptosis, and lymphocyte count.
These results demonstrate the existence of PBMCs pyroptosis in trauma patients. Immune cell apoptosis is associated with poor outcomes in trauma patients,[22] but the occurrence of PBMCs pyroptosis in trauma patients is a new finding. Previous studies have indicated that the role of pyroptosis in injury should not be underestimated.[23,24] Pyroptosis is mainly mediated by inflammatory caspases (caspase-1/4/5/11). Inflammatory caspases cleaves and activates the pyroptotic substrate gasdermin D (GSDMD), which promotes the physical rupture of pyroptotic cells, causes the release of the pro-inflammatory cytokines IL-1β and IL-18, alarmins and endogenous danger-associated molecular proteins. IL-18 is associated with ARDS risk and indices of morbidity and mortality in critically ill patients.[25,26] In this study, plasma level of IL-18 is markedly elevated, and it is associated with PBMCs pyroptosis. This result confirmed the activation of PBMCs pyroptosis in the early stage of trauma, and it may be related to the poor prognosis of trauma.
Although pyroptosis plays an important role in development of sepsis, its effect remains complex and confusing. As pyroptosis of infected cells can enable innate immune effector cells to kill the pathogen by removing the protective, intracellular replicative niche of the bacteria, both pyroptosis and cytokine production can play a role in clearance of pathogens, but excessive or inappropriate activation of caspase-1 and pyroptosis may be detrimental to the host.[27,28] In model of trauma with hemorrhagic shock,[23,24] caspase-1 was activated independent of NLRP3 and induced the higher levels of IL-18. In another study,[24] caspase-1 was activated in myeloid and lymphoid cells in response to burn injury. Mice treated with caspase-1 peptide inhibitor Ac-YVAD-CMK (YVAD) also showed higher levels of pro-inflammatory cytokines IL-6 and IL-33 and lower levels of the anti-inflammatory cytokines IL-4 and IL-13. These findings suggest an anti-inflammatory and protective role for caspase-1 activation in host responses to injury. So, increase of caspase-1 level may represent a compensatory self-protection mechanism, while the marked increase of caspase-1 levels in severe trauma may represent excessive activation of this negative regulation mechanism, which results in adverse outcomes.
The results of this study should be interpreted with caution for several reasons. First, the number of trauma patients was relatively small and the data were collected at a single institution. Second, the percentage of pyroptotic PBMCs was only determined at a single time point, and the chronological changes of PBMCs pyroptosis was not delineated. In addition, further studies are needed to determine which subsets of pyroptotic immune cells involved in the pathogenesis of trauma and their clinical significance.
5. Conclusion
This study demonstrated that increased percentage of pyroptotic PBMCs is significantly correlated with the severity of trauma and is associated with the development of sepsis, suggesting that PBMCs pyroptosis plays an important role in the pathogenesis of inflammation following trauma.
Acknowledgments
The authors thank all the doctors, nurses, and patients for their dedication in the study. They also thank the medical ethics committee of Tongji Hospital at the Tongji Medical College of Huazhong University of Science and Technology.
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, APACHE = acute physiology and chronic health evaluation, ARDS = acute respiratory distress syndrome, AUC = area under curve, CI = confidence interval, CRP = C-reactive protein, FLICA = fluorescent labeled inhibitors of caspase, GCS = Glasgow coma scale, GSDMD = gasdermin D, ICU = intensive care unit, IFN-γ = interferon gamma, IL = interleukin, ISS = injury severity score, LC = lymphocyte count, LOS = length of stay, LPS = lipopolysaccharide, MCP-1 = monocyte chemotactic protein-1, MV = mechanical ventilation, PBMCs = peripheral blood mononuclear cells, PCD = programmed cell death, PCT = Procalcitonin, PI = propidium iodide, ROC = receiver operating characteristic, TNF = tumor necrosis factor, TUNEL = TdT mediated dUTP Nick End Labeling, WBC = white blood cell.
Authors’ contributions: Z-fL and X-jB equally contributed to the design of the research and interpretation of the data. Y-cW, Q-xL, Xi-eX, and TL performed the research and collected data. Y-cW and WG analyzed the data and cytokines. Y-cW and Z-fL supervised the flow cytometry experiment. Z-fL and X-jB supervised the project. All authors read and approved the final manuscript.
Funding: Z-fL is currently receiving grants from National Natural Science Foundation of China (No. 81571891) and Technology Research Plan of Wuhan (No. 2015060101010035). X-jB is currently receiving grants from National Natural Science Foundation of China (No. 81772129), the 12th Five-Year Plan of China (No. 2012BAI11B00) and the Nature Science Foundation of Hubei Province (No. 2013CFA075).
Ethics statement: Written informed consent was obtained from all subjects or from their surrogates, and the study protocol was approved by the medical ethics committee of Tongji Hospital at the Tongji Medical College of Huazhong University of Science and Technology.
Consent statement: Not applicable. No individual personal data are included in the study. All patients provided necessary consent to participate in the present study.
Competing interests: The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Frohlich M, Lefering R, Probst C, et al. Epidemiology and risk factors of multiple-organ failure after multiple trauma: an analysis of 31,154 patients from the TraumaRegister DGU. J Trauma Acute Care Surg 2014;76:921–7. discussion 927–928. [DOI] [PubMed] [Google Scholar]
- [2].Aldrian S, Wernhart S, Negrin L, et al. Epidemiological and economic aspects of polytrauma management in Austria. Wien Klin Wochenschr 2012;124:78–84. [DOI] [PubMed] [Google Scholar]
- [3].Dewar D, Moore FA, Moore EE, et al. Postinjury multiple organ failure. Injury 2009;40:912–8. [DOI] [PubMed] [Google Scholar]
- [4].Napolitano LM, Ferrer T, McCarter RJ, et al. Systemic inflammatory response syndrome score at admission independently predicts mortality and length of stay in trauma patients. J Trauma 2000;49:647–52. [DOI] [PubMed] [Google Scholar]
- [5].Yang Y, Jiang G, Zhang P, et al. Programmed cell death and its role in inflammation. Mil Med Res 2015;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol 2017;17:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell 2011;147:742–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fialkow L, Fochesatto Filho L, Bozzetti MC, et al. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care 2006;10:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 2006;6:813–22. [DOI] [PubMed] [Google Scholar]
- [10].Tang PS, Mura M, Seth R, et al. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 2008;294:L632–41. [DOI] [PubMed] [Google Scholar]
- [11].Aziz M, Jacob A, Wang P. Revisiting caspases in sepsis. Cell Death Dis 2014;5:e1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol 2016;26:R568–72. [DOI] [PubMed] [Google Scholar]
- [13].Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017;277:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol 2007;9:2562–70. [DOI] [PubMed] [Google Scholar]
- [15].Shao W, Yeretssian G, Doiron K, et al. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 2007;282:36321–9. [DOI] [PubMed] [Google Scholar]
- [16].Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am J Physiol Regul Integr Comp Physiol 2007;292:R86–98. [DOI] [PubMed] [Google Scholar]
- [17].Siegmund B, Lehr HA, Fantuzzi G, et al. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA 2001;98:13249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 1995;80:401–11. [DOI] [PubMed] [Google Scholar]
- [19].Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li TS, Zhao B, Wang C, et al. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med 2008;233:1081–7. [DOI] [PubMed] [Google Scholar]
- [21].Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 2002;18:487–94. [DOI] [PubMed] [Google Scholar]
- [23].Menzel CL, Sun Q, Loughran PA, et al. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med 2011;17:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Osuka A, Hanschen M, Stoecklein V, et al. A protective role for inflammasome activation following injury. Shock 2012;37:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eidt MV, Nunes FB, Pedrazza L, et al. Biochemical and inflammatory aspects in patients with severe sepsis and septic shock: The predictive role of IL-18 in mortality. Clin Chim Acta 2016;453:100–6. [DOI] [PubMed] [Google Scholar]
- [26].Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012;185:1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015;265:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 2010;11:1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


