Abstract
Rationale:
Acute kidney injury (AKI) with hyperparathyroidism caused by nitrite was rare, and renal function and parathyroid hormone (PTH) decreased to normal range after therapy.
Patient concerns:
Acute kidney injury was diagnosed in a 40-year-old male with hyperparathyroidism and cyanosis of his hands and both forearms.
Diagnoses:
The patient ate some recently pickled vegetables, and he experienced nausea, vomiting and diarrhoea without oliguria or anuria; Additionally, his hands and both forearms had a typical blue ash appearance. After admission, the laboratory findings indicated theincreasing serum creatinine (Scr) and parathyroid hormone (PTH). He was diagnosed as acute kidney injury with hyperparathyroidism caused by nitrite.
Interventions:
The patient stopped eating the pickled vegetables and was given rehydration, added calories and other supportive therapy without any glucocorticoids.
Outcomes:
According to his clinical manifestations, laboratory findings and imaging results, the patient was diagnosed with acute kidney injury with secondary hyperparathyroidism. He was given symptomatic supportive care therapy. After one week, the serum creatinine, parathyroid hormone (PTH), hypercalcemia, hyperphosphatemia, proteinuria, and urine red blood cell values decreased to normal range.
Lessons:
Nitrite-induced acute kidney injury with secondary hyperparathyroidism was relatively rare. After therapy, the function of the kidney and parathyroid returned to normal. This case suggests that detailed collection of medical history, physical examination and correct symptomatic treatment is very important.
Keywords: acute kidney injury, nitrite-poison, secondary hyperparathyroidism
1. Introduction
Nitrite poisoning is often caused by consuming nitrite and sometimes by consuming vegetables with enriched nitrate. The nitrate in the body can be reduced to nitrite, causing nitrite poisoning.[1] The main clinical manifestation of nitrite poisoning is functional abnormalities of the gastrointestinal and respiratory systems, as well as skin cyanosis.[2] However, reports have described renal and parathyroid damage due to nitrite poisoning.[3] In the present case report, we reported a 40-year-old man with acute kidney injury and a 1-day history of nitrite poisoning. He ate recently pickled vegetables and 1 hour later experienced nausea, vomiting, and diarrhea. Additionally, his hands and both forearms had a typical blue ash appearance. After 1 week of therapy, his serum creatinine (Scr) and parathyroid hormone (PTH) levels decreased to normal.
2. Material and methods
Acute kidney injury was diagnosed in a 40-year-old man with hyperparathyroidism and cyanosis of his hands and both forearms 1 day prior to hospital admission. The patient denied a family or personal history of methemoglobin reductase/G6PD deficiency. He had no history of chronic kidney disease, proteinuria, hematuria, diabetes, hypertension, immune disease, liver disease, heart disease, allergic diseases, thyroid disease, parathyroid disease, or malignant neoplasms. One day before admission to the hospital, the patient ate some recently pickled vegetables. One hour later, he experienced nausea, vomiting, and diarrhea. Additionally, his hands and both forearms had a typical blue ash appearance, but he did not exhibit oliguria or anuria, the volume of urine was normal compared with his history data. The results of a physical examination revealed cyanosis of the hands and both forearms. He did not have edema of the eyelids, lower limbs, or ankles; an anemic appearance; bleeding; purpura or spider nevus on body or limbs.
The pertinent laboratory findings included the following: Scr 304 μmol/L (normal reference value 53–97 μmol/L), blood urea nitrogen (BUN) 15.76 mmol/L (normal reference value 2.3–7.8 mmol/L), blood uric acid 653 μmol/L (normal reference value 210–420 μmol/L), serum calcium (Ca2+) 2.76 mmol/L (normal reference value 2.0–2.6 mmol/L), serum phosphorus (phosphate) 2.26 mmol/L (normal reference value 0.6–1.6 mmol/L), serum potassium (K+) 7.0 mmol/L (normal reference value 3.5–5.3 mmol/L), acid–base status of this patient was normal (26 mmol/L, normal reference value 22–29 mmol/L), and PTH 221.1 pg/mL (normal reference value 15–65 pg/mL). The blood methemoglobin level was determined to be 16% (normal reference value <1%) after admission to our hospital. The urinary nitrate was 1753.3 μmol/L. Laboratory tests were normal for hepatitis index, thyroid hormone, antinuclear antibodies (ANA), anti-double strain antibodies (dsDNA), anti-nucleosome antibodies, anti-histone antibodies, anti-SSA antibody, anti-neutrophil cytoplasmic antibodies, aspartate aminotransferase, and alanine aminotransferase. However, the patient exhibited slightly higher creatine kinase (5.55 pg/mL, normal 0–5 pg/mL) and lactate dehydrogenase (274 IU/mL, normal is 91–245 IU/mL) levels. His urinary albumin–creatinine ratio (ACR), electrocardiogram, red blood cells, white blood cells, hemoglobin, coagulation series, and tumor markers were all normal. Scintigraphy of the parathyroid using the Tc99m-MIBI imaging agent appeared normal (Fig. 1) to prove that there is no thyroid disease itself, for example, adenoma or hyperplasia. The dual energy x-ray absorptiometry, serum immunostaining electrophoresis, blood light chain, and urine light chain results were all normal. The results of abdominal computed tomography (CT) showed that the size, shape, and density of the kidney and adrenal gland had no obvious abnormalities, no expansion of the renal pelvis or ureter was present, and no obvious retroperitoneal lymph nodes were observed (Fig. 2).
Figure 1.
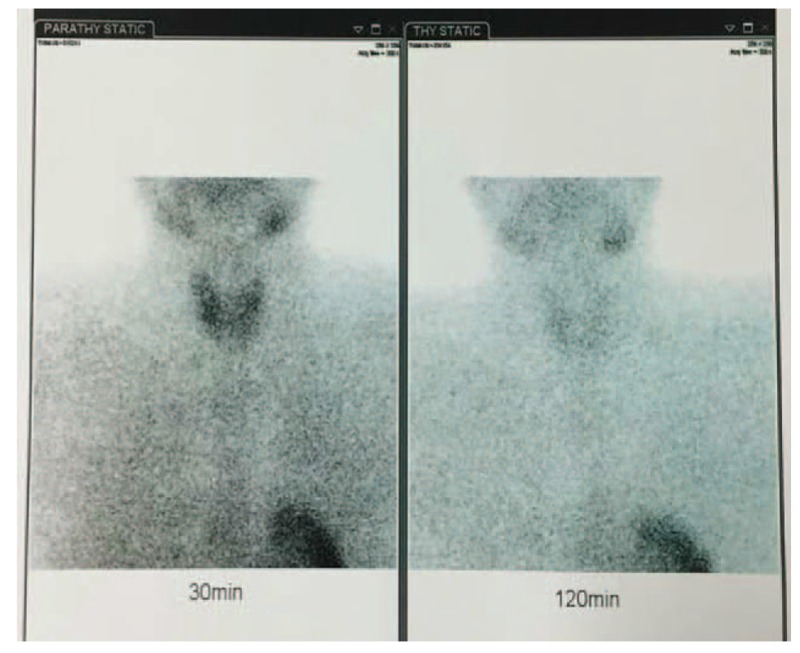
The scintigraphy of parathyroid.
Figure 2.
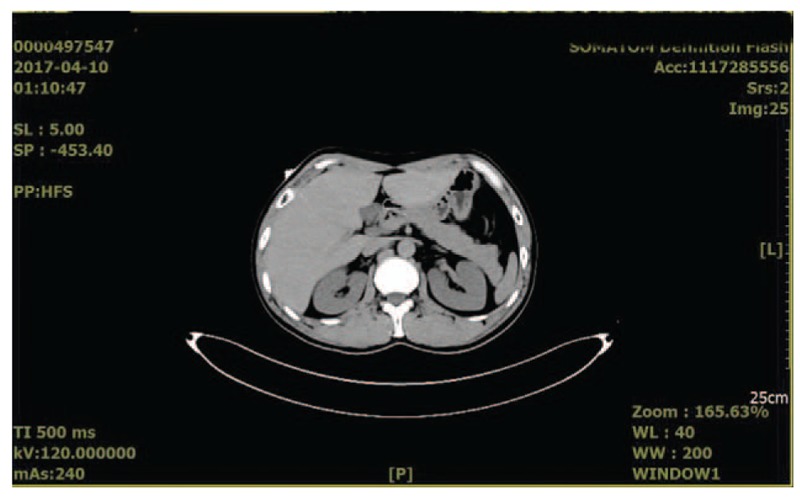
Result of abdominal computed tomography.
3. Results
According to his clinical manifestations, laboratory findings and imaging results, the patient was diagnosed with acute kidney injury with secondary hyperparathyroidism. He stopped eating the pickled vegetables and was given rehydration, added calories, and other supportive therapy without any glucocorticoids. Throughout the course of treatment, no oliguria or anuria occurred. One day later, the typical blue ash appearance and cyanosis of the hands and both forearms disappeared. Two days later, the Scr level decreased level to 243 μmol/L. After 1 week, Scr level had decreased to 86 μmol/L, and the following results were obtained: BUN 4.87 mmol/L, blood uric acid 247 μmol/L, serum Ca2+ 2.30 mmol/L, serum phosphate 0.94 mmol/L, serum K+ 3.96 mmol/L, PTH 33.87 pg/mL, and serum methemoglobin 1%, and the urinary nitrate decreased significantly, under 20 μmol/L.
The patient and his family agreed the authors of this manuscript to freely apply his personal and medical information, including published articles and further scientific research, and the patient already signed the informed consent.
4. Discussions
Acute kidney injury is a group of clinical syndromes that manifest as a sudden (from 1 to 7 days) and sustained (>24 hours) decreased in renal function, defined as an elevation of the Scr level by at least 0.5 mg/d (<400 mL/24 h or 17 mL/h) or anuria (<100 mL/24 h). (Quote the international guidelines KDIGO,[4,5]). According to the lesion and cause, acute kidney injury can be divided into 3 categories: prerenal, renal, and postrenal, and each has a different etiology and pathophysiology.[6]
The causes of prerenal acute kidney injury are effective blood volume loss. The mechanism of renal acute kidney injury includes glomerular disease, renal interstitial disease, renal vascular disease, acute tubular necrosis, infection, and infiltration of other diseases. Postrenal acute kidney injury is often caused by urinary tract obstruction.[7,8] In many of cases, the decreased effective blood volume and presence of substances harmful to the kidney are the main causes of acute kidney injury in clinical practice.
Sodium nitrite is a powder or granule with a white to pale yellow color. It tastes slightly salty and is water soluble. Nitrate and nitrite are widely found in the human environment.[9,10] The appearance and taste of sodium nitrite are similar to that of salt, and it is widely used in industry and construction. Sodium nitrite is also used in limited amounts as a coloring agent in meat products. A high concentration of nitrate or nitrite is present in many pickled meat products, pickles, and deteriorating vegetables.[11] The highest nitrite content appears approximately 1 week after pickling. The probability of food poisoning caused by nitrite is high, and ingesting 0.3 to 0.5 g of nitrite can cause poisoning; 3 g can lead to death.
The latent time of nitrite poisoning varies depending on the amount and concentration of nitrite and ranges from 10 minutes to 2 days. If a patient has recently consumed vegetables or recently pickled vegetables and has the above symptoms, including skin and mucous membranes with a typical blue ash, blue or blue-black appearance, they should be highly suspected of having nitrite poisoning,[12–14] and the urinary nitrate increased. We reported a 40-year-old man with acute kidney injury and a 1-day history of nitrite poisoning. The patient consumed a greater than usual amount of recently pickled vegetables that contained a high concentration of nitrates/nitrites. The patient denied having a family history of methemoglobin reductase or G6PD deficiency (methemoglobin reduction test, reduction rate 83%; detection was performed after the return of normal renal function and PTH levels). The Scr level increased due to secondary hyperparathyroidism, and cyanosis of the hands and both forearms were noticed 1 day prior to hospital admission[15] and the urinary nitrate was higher than normal (1753.3 μmol/L). After 1 week of therapy, the Scr, urinary nitrate, and PTH levels decreased to normal.
PTH is an alkaline single-chain peptide hormone mainly produced by parathyroid chief cells. It is composed of 84 amino acids, and its main function is to regulate Ca2+ in the vertebrate body. In some patients with chronic kidney disease, PTH increased due to compensation, and in some patients with AKI, increased levels of PTH was also seen.[16] In the present case report, the PTH and Scr levels were significantly increased at admission, but scintigraphy of the parathyroid appeared normal and there was no adenoma or hyperplasia. The PTH level decreased to normal after therapy, and the event was considered secondary hyperparathyroidism. The proposed mechanism of the secondary hyperparathyroidism was that nitrate/nitrite poisoning caused parathyroid injury; therefore, the PTH that was stored in parathyroid was rapidly released into the blood. Because the short-term damage did not cause parathyroid chief cell hyperplasia, when the cause of injury was removed and timely treatment was received, the PTH level in the blood quickly returned to normal levels. At the same time, nitrite poisoning caused renal dysfunction with an elevated creatinine level and internal environment disorders (abnormal levels of Ca2+, phosphate, and K+), which can stimulate the release of PTH. The 2 factors caused PTH levels to increase over a short time and decrease rapidly to normal after treatment.
Primary hyperparathyroidism is due to parathyroid adenoma, hypertrophy, or adenocarcinoma, and the main clinical manifestations is the over secretion of parathyroid hormone and hypercalcemia. A variety of non-parathyroidic factors stimulate parathyroid hormone secretion, known as secondary hyperparathyroidism. In the present case report, the parathyroid itself does not have a related lesion, and was stimulated to release over normal parathyroid hormone, when the stimulating factor disappears, PTH is normal.
In some reports,[17,18] after ingestion of nitrate, urinary nitrate concentrations increased. With a single high dose of dietary nitrate, concentrations of urinary nitrate returned to baseline levels in several days. The length of time for these concentrations to return to baseline levels after a nitrate intake is undetermined. In the present study, the urinary nitrate was higher than normal in the baseline after admission, and after 1 week treatment, the concentration of urinary nitrate decreased significantly, under 20 μmol/L.
Treatment of acute kidney injury includes removal of the cause, maintaining the stability of the internal environment, nutritional supporting, treatment of complications, and blood purification. In the present study, we separated the patient from pickled vegetables and avoided this continued damage of poison on the kidney. After admission, we gave the patient enough liquid to replenish the blood volume for reducing kidney damage caused by prerenal causes because of nausea, vomiting, and diarrhea. At the same time, the case was given enough calories (including glucose and fat emulsion) to increase the body's resistance to the disease, and the patient was given alprostadil injection to ameliorate kidney blood circulation. In some cases with acute kidney disease, because of high serum creatitine, the patients perhaps needed to accept the hemodialysis and glucocorticoids, but in the present study, the enrolled patient has no experience of hemodialysis and glucocorticoids because of rapid recovery to normal, and after 1 week, the indicator became normal.
In the present case report, the patient ate recently pickled vegetables, and later he was diagnosed acute kidney disease according to the clinical manifestations, laboratory findings, and imaging results. These findings indicated a diagnosis of nitrite-induced acute kidney injury with secondary hyperparathyroidism. In the future study, we will collect more cases, similar to the reported in the present study, and investigate the mechanism of acute kidney injury with secondary hyperparathyroidism.
Footnotes
Abbreviations: ACR = urinary albumin-creatinine ratio, ANA = antinuclear antibodies, BUN = blood urea nitrogen, Ca2+ = serum calcium, CT = computed tomography, dsDNA = anti-double strain antibodies, K+ = serum potassium, PTH = parathyroid hormone, Scr = serum creatinine.
This study was supported by Shandong Province Outstanding Young Scientist Research Award Fund Project, No. BS2013YY042. Natural Science Foundation of Shandong Province, No. ZR2013HM106.
No conflicts of interest are declared.
References
- [1].Wu CK, Tseng PT, Chen YW, et al. Significantly higher peripheral fibroblast growth factor-2 levels in patients with major depressive disorder: a preliminary meta-analysis under MOOSE guidelines. Medicine (Baltimore) 2016;95:e4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Katabami K, Hayakawa M, Gando S. Severe methemoglobinemia due to sodium nitrite poisoning. Case Rep Emerg Med 2016;2016:9013816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bahadoran Z, Mirmiran P, Ghasemi A, et al. Association between dietary intakes of nitrate and nitrite and the risk of hypertension and chronic kidney disease: Tehran Lipid and Glucose Study. Nutrients 2016;8: pii: E811. doi: 10.3390/nu8120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Filipczak PT, Senft AP, Seagrave J, et al. NOS-2 inhibition in phosgene-induced acute lung injury. Toxicol Sci 2015;146:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ciccia E, Devarajan P. Pediatric acute kidney injury: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 2017;10:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keenswijk W, Vanmassenhove J, Raes A, et al. Epidemiology and outcome of acute kidney injury in children, a single center study. Acta Clin Belg 2017;72:405–12. [DOI] [PubMed] [Google Scholar]
- [7].Potočnjak I, Domitrović R. Carvacrol attenuates acute kidney injury induced by cisplatin through suppression of ERK and PI3K/Akt activation. Food Chem Toxicol 2016;98(Pt B):251–61. [DOI] [PubMed] [Google Scholar]
- [8].Wan H, Hu Z, Wang J, et al. Clindamycin-induced kidney diseases: a retrospective analysis of 50 patients. Intern Med 2016;55:1433–7. [DOI] [PubMed] [Google Scholar]
- [9].Yoshioka H, Nonogaki T, Fukuishi N, et al. Chronotoxicity of bromobenzene-induced hepatic injury in mice. J Toxicol Sci 2017;42:251–8. [DOI] [PubMed] [Google Scholar]
- [10].Lavon O. Early administration of isosorbide dinitrate improves survival of cyanide-poisoned rabbits. Clin Toxicol (Phila) 2015;53:22–7. [DOI] [PubMed] [Google Scholar]
- [11].Seel DJ, Kawabata T, Nakamura M, et al. N-nitroso compounds in two nitrosated food products in southwest Korea. Food Chem Toxicol 1994;32:1117–23. [DOI] [PubMed] [Google Scholar]
- [12].Cui N, Su L, Wang H, et al. A case report of Churg-Strauss syndrome presenting with cardiogenic shock treated with extracorporeal membrane oxygenation. Medicine (Baltimore) 2015;94:e1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raei N, Behrouz B, Zahri S, et al. Helicobacter pylori infection and dietary factors act synergistically to promote gastric cancer. Asian Pac J Cancer Prev 2016;17:917–21. [DOI] [PubMed] [Google Scholar]
- [14].Fang F, Feng T, Du G, et al. Evaluation of the impact on food safety of a Lactobacillus coryniformis strain from pickled vegetables with degradation activity against nitrite and other undesirable compounds. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2016;33:623–30. [DOI] [PubMed] [Google Scholar]
- [15].Arcêncio L, Vento DA, Bassetto S, et al. Exhaled nitrite/nitrate levels as a marker of respiratory complications after heart valve surgery. J Crit Care 2013;28:533.e1–7. [DOI] [PubMed] [Google Scholar]
- [16].Peng T, Hu Z, Gao Z, et al. Acquired ichthyosis and secondary hyperparathyroidism with systemic lupus erythematosus. Lupus 2015;24:218–21. [DOI] [PubMed] [Google Scholar]
- [17].Pannala AS, Mani AR, Spencer JP, et al. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med 2003;34:576–84. [DOI] [PubMed] [Google Scholar]
- [18].Bondonno CP, Liu AH, Croft KD, et al. Short-term effects of a high nitrate diet on nitrate metabolism in healthy individuals. Nutrients 2015;7:1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


