Abstract
Background:
Laparoscopic gastrectomy (LG) has been widely applied in patients with gastric cancer (GC). However, the safety and application value of LG in elderly patients with GC was still unclear. In this study, we aimed to evaluate the feasibility and safety of LG for elderly patients with GC using the meta-analysis.
Methods:
Studies comparing elderly patients and nonelderly patients who underwent LG for GC were reviewed and collected from the PubMed, EBSCO, Cochrane Library, and EMBASE. Outcomes such as operative results, postoperative recovery, and morbidity were compared and analyzed. The Review Manager 5.3 was used to portray the weighted mean difference (WMD) and odds ratio (OR) with a 95% confidence interval (CI).
Results:
Eleven observational studies with a total of 3275 patients were included. Compared with nonelderly patients, elderly patients had shorter operation time (WMD −10.46; 95% CI −17.06 to −3.86; P = .002), less retrieved lymph nodes (WMD −2.34; 95% CI −3.77 to −0.92; P = .001), delayed time to first flatus (WMD 0.31; 95% CI 0.10–0.51; P = .003), longer postoperative hospital stays (WMD 1.06; 95% CI 0.07–2.05; P = .04), higher risk for overall postoperative complication (OR 1.34; 95% CI 1.08–1.67; P = .009), nonsurgical postoperative complication (OR 1.98; 95% CI 1.24–3.15; P = .004), and postoperative pulmonary complication (OR: 3.09; 95% CI 1.68–5.68; P < .001). There was no significance between nonelderly patients and elderly patients regarding the estimated blood loss, incidences of surgical postoperative complication, surgical site infection, and ileus (P > .05).
Conclusion:
Outcomes of LG for elderly patients were comparable to those in nonelderly patients. Age alone should not preclude LG in elderly patients.
Keywords: elderly patients, gastric cancer, laparoscopic gastrectomy, meta-analysis
1. Introduction
Gastric cancer (GC) represents one of the leading causes of cancer-related death worldwide, especially in Japan, Korea, and China.[1–3] Gastrectomy with adequate lymph node dissection remains the mainstay of radical treatment for GC. Laparoscopic gastrectomy (LG) has been gradually accepted since first reported in 1994 by Kitano et al.[4] Several randomized trials and meta-analysis have proved the feasibility and surgical safety of LG, along with its advantages including milder surgical trauma, faster recovery, better cosmesis, etc.[5–8]
Amount of elderly patients diagnosed with GC continues increasing. For the elderly patients with GC, proper treatments are necessary to prong the survival time and improve the quality of life. Despite this, there was limited attention focused on the elderly patients with GC. Old age was considered as a risk factor for surgical safety. Opposed to the nonelderly patients, elderly patients usually suffer comorbidities and have poor functional capacities that may not allow them to endure the severe surgical trauma. Several studies have addressed gastrectomy could be carried out in elderly patients for GC safely. This should not be considered as a contraindication.[9–11] Although, the impact of old age on patients who underwent LG is still unclear. There are also a few of studies attempting to examine the feasibility and safety of the application of LG in the elderly patients, the majority of them were noncomparative or had sample sizes which were too small to transfer their evidence to an actual group.
In our study, we aimed to evaluate the feasibility and safety of LG for elderly patients with GC by comparing the nonelderly patients with respect to operative data, postoperative recovery, and postoperative morbidity.
2. Methods
2.1. Literature search
The meta-analysis was performed in accordance with the PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses.[12] A comprehensive search was conducted in the PubMed, EBSCO, Cochrane Library, and EMBASE to identify articles comparing the elderly patients with nonelderly patients who underwent LG for GC. The latest search was conducted in September 2016. The search strategy was as following ((((gastric adenocarcinoma) OR gastric cancer)) AND ((laparoscopic) OR laparoscopy)) AND (((age) OR elderly) OR old). A manual search was also performed using “related artciles” and the reference lists of the retrieved articles to identify other potential studies. The language was limited to English.
2.2. Selection criteria
Eligibility criteria for the study included the following: all patients were confirmed to have GC, studies compared the elderly patients and nonelderly patients who underwent LG for GC, and availability of data on information of at least 3 outcome measures. Exclusion criteria included the following: open gastrectomy, hand-assisted gastrectomy, or robotic gastrectomy; including GC with distant metastasis or recurrent GC; abstracts presented at meetings, review articles, case report, or letters; and duplicated studies.
2.3. Data extraction and quality assessment
Data were independently extracted by (KC and WHY) using a standard form. Disagreements were discussed and a consensus was reached. The following data were extracted: study name, study period, sample size, mean age, mean body mass index, preoperative comorbidity, extent of lymph node dissection, tumor size, operation time, intraoperative blood loss, number of harvested lymph nodes, length of postoperative hospital stay, and postoperative complications. The qualities of studies were evaluated using the Newcastle-Ottawa Quality Assessment Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Studies with a score higher than 5 stars were included. Postoperative complications were classified into 2 categories, surgical and nonsurgical complication as defined by Jung et al.[13]
2.4. Statistical analysis
The means and standard deviations (SDs) were estimated using the method described by Hozo et al[14] in the studies with medians and ranges instead of means and SDs. Dichotomous variables were analyzed using the odds ratio (OR) with 95% confidence intervals (CIs). Continuous variables were assessed using weighted mean differences (WMDs) with a 95% CI. Statistical heterogeneity was evaluated using methods described by Higgins et al.[15]I2 values between 0% and 25% suggest low heterogeneity, values above 25% suggest moderate heterogeneity, and values above 75% suggest high heterogeneity. Pooled effects with low heterogeneity were calculated by using the Mantel–Haenszel test for fixed-effects models,[16] while those with moderate or high heterogeneity used the DerSimonian and Laird test for random-effects models.[17] The potential publication bias based on the overall postoperative complications was assessed by conducting the funnel plots. Subgroup analysis was performed based on the cutoff of ages. Data analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). P < .05 was considered statistically significant.
3. Result
3.1. Study characteristics
The search strategy initially retrieved 2358 hits. After exclusion of irrelevant studies by screening abstracts, full texts of 17 potentially relevant articles were obtained for assessment. Six studies were excluded due to overlapping data, statistical data unavailable, or noncomparative studies. Eleven studies were included eventually.[18–28] The PRISMA flowchart of literature review is shown in Fig. 1.
Figure 1.
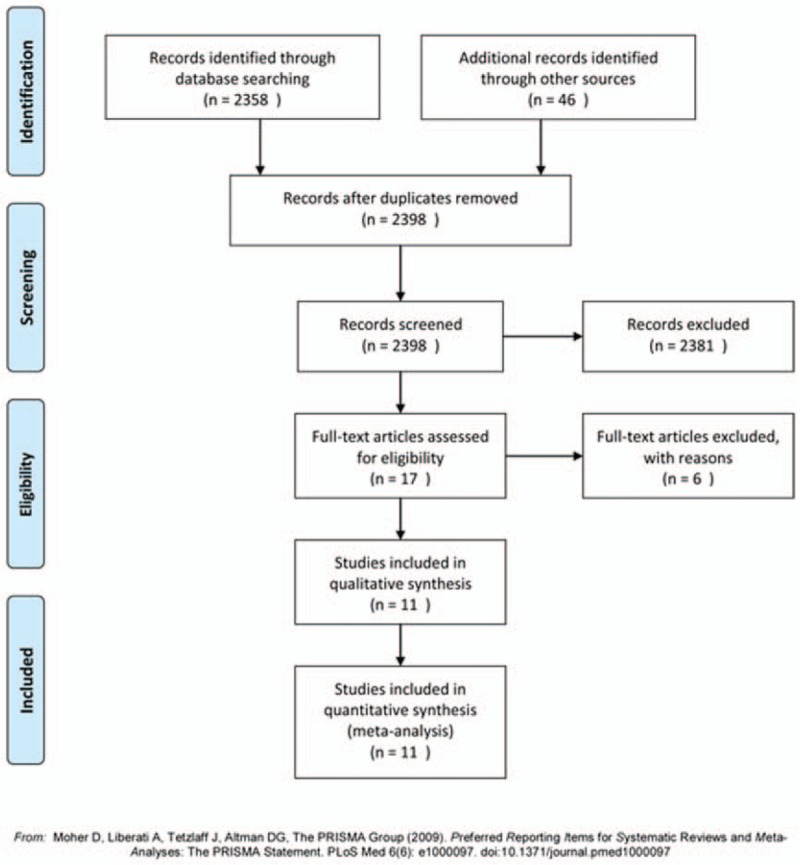
Flow chart of the studies included in the meta-analysis.
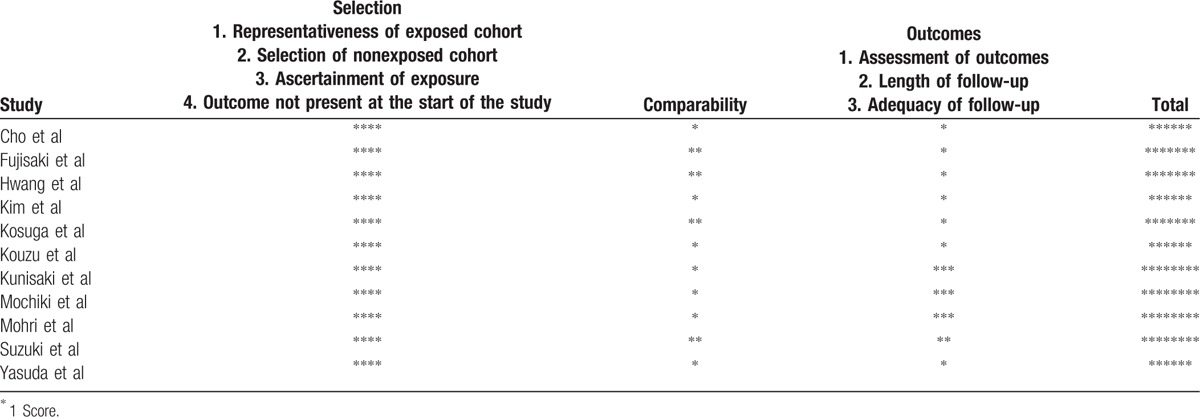
The characteristics of the included studies were summarized in Table 1. A total of 3275 patients from Japan and Korea were pooled in this meta-analysis: 796 patients were in the elderly group and 2479 in the nonelderly group. Patients who were more than 70 years old were categorized as elderly patients in 5 studies,[19,21,23,26,28] more than 65 years old in one studies,[25] and more than 75 years old in 5 studies.[18,20,22,24,27] According to the Newcastle-Ottawa Quality Assessment Scale, all 11 studies were achieved no less than 6 stars, Table 2.
Table 1.
The basic characteristics of included studies.
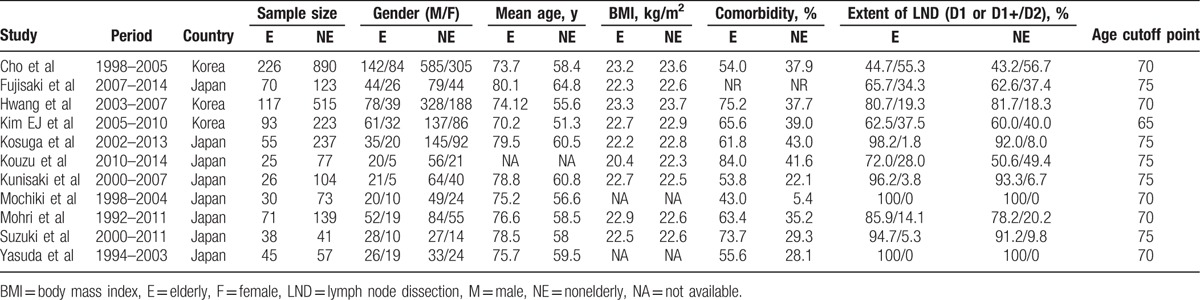
Table 2.
The qualities of included studies evaluated using the Newcastle-Ottawa Quality Assessment Scale.
3.2. Intraoperative outcomes
All 11 pooled studies reported the operation time. Compared with nonelderly patients, elderly patients had shorter operation time (WMD −10.46; 95% CI −17.06 to −3.86; P = .002; Fig. 2A). According to 10 studies reporting estimated blood loss, our meta-analysis found there was no difference between elderly and nonelderly patients (WMD: −6.05; 95% CI: −42.18–30.07; P = .74; Fig. 2B). Moreover, elderly patients achieved less lymph nodes compared with nonelderly patients (WMD −2.34; 95% CI −3.77 to −0.92; P = .001; Fig. 2C).
Figure 2.
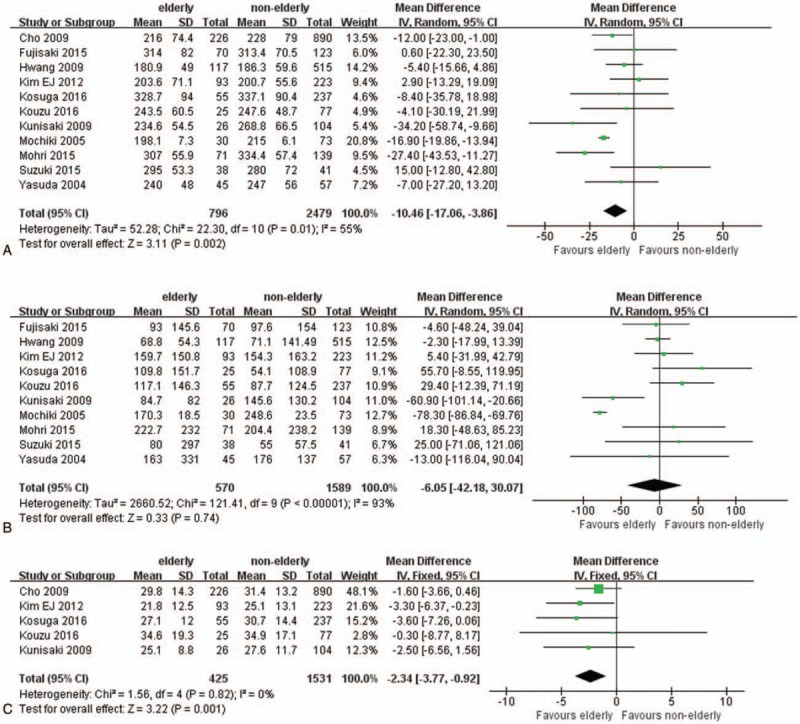
Forest plots of operative outcomes: (A) operation time, (B) estimated blood loss, and (C) number of retrieved lymph nodes.
3.3. Postoperative outcomes
The first flatus postoperatively in elderly patients was delayed (WMD.31; 95% CI 0.10–0.51; P = .003; Fig. 3A). Longer postoperative hospital stays were observed in elderly patients (WMD 1.06; 95% CI 0.07–2.05; P = .04; Fig. 3B).
Figure 3.
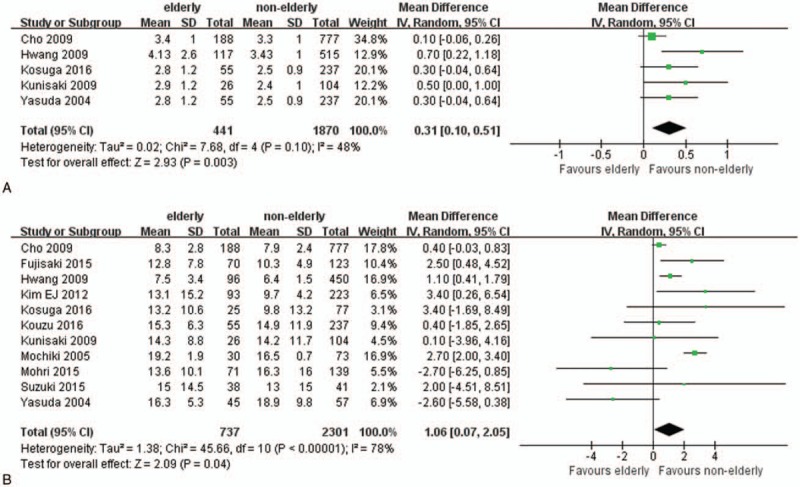
Forest plots of postoperative recovery: (A) time to first flatus, (B) length of postoperative hospitalization.
Postoperative complications were recorded in all studies, Table 3. Elderly patients had higher risk for overall postoperative complication (OR 1.34; 95% CI 1.08–1.67; P = .009; Fig. 4A). As for surgical complications, there were no significant differences between 2 groups (OR: 1.20; 95% CI 0.94–1.53; P = .14; Fig. 4B). Nonsurgical postoperative complication significantly increased in elderly patients (OR 1.98; 95% CI 1.24–3.15; P = .004; Fig. 4C). Further analysis revealed an association between higher postoperative pulmonary complication (PPC) rate and the elderly patients (OR: 3.09; 95% CI 1.68–5.68; P < .001; Fig. 4D). Incidences of surgical site infection (OR: 1.47; 95% CI 0.98–2.21; P = .06; Fig. 4E) and ileus (OR: 1.24; 95% CI 0.44–3.51; P = .68; Fig. 4F) were comparable in elderly patients and nonelderly patients.
Table 3.
Surgical and long-term outcomes of included studies.
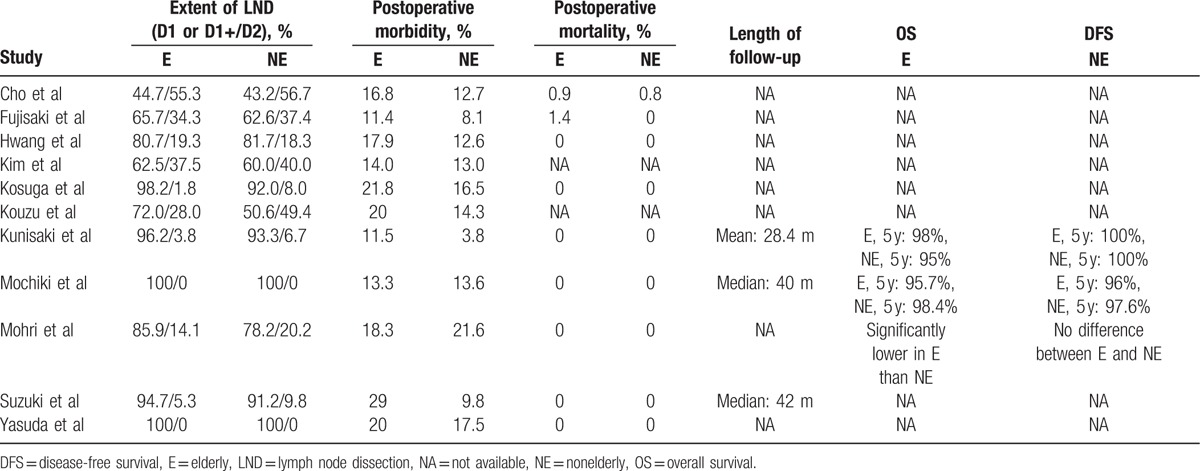
Figure 4.
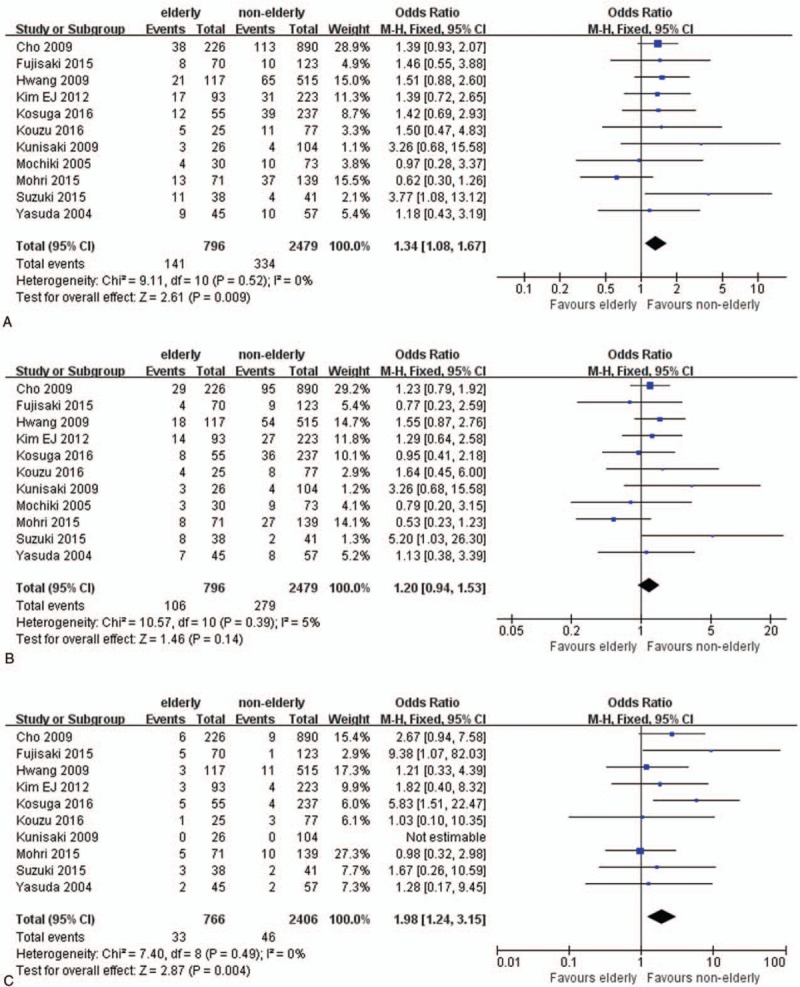
Forest plots of postoperative complications: (A) overall complication, (B) surgical complication, and (C) nonsurgical complication.
The extents of lymph node dissection were comparable between elderly and nonelderly patients in each included studies, as showed in Table 3. Moreover, 3 included studies reported the long-term outcomes, which revealed similar overall survival rates and disease-free survival rate between elderly and nonelderly patients (Table 3).
3.4. Sensitivity analysis, subgroup analysis, and publication bias
Sensitivity analyses were conducted by excluding the highest weighted study in each pooled analysis. In addition, further analyses were conducted by exclusion of the studies by due to the unbalance of surgical extent between the elderly patients and nonelderly patients.[21,27] These exclusions did not alter the results obtained in cumulative analyses. Subgroup analysis based on the cutoff of age showed similar trends as the overall effects. Details of subgroup analysis were showed in Table 4. A funnel plot based on the overall postoperative complication was performed to assess publication bias. No significant publication bias was detected by visual inspection of the funnel plot in which the pooled studies were almost symmetry and none of them were outside the 95% CI (Fig. 5).
Table 4.
Subgroup analysis of outcomes based on the cutoff ages.
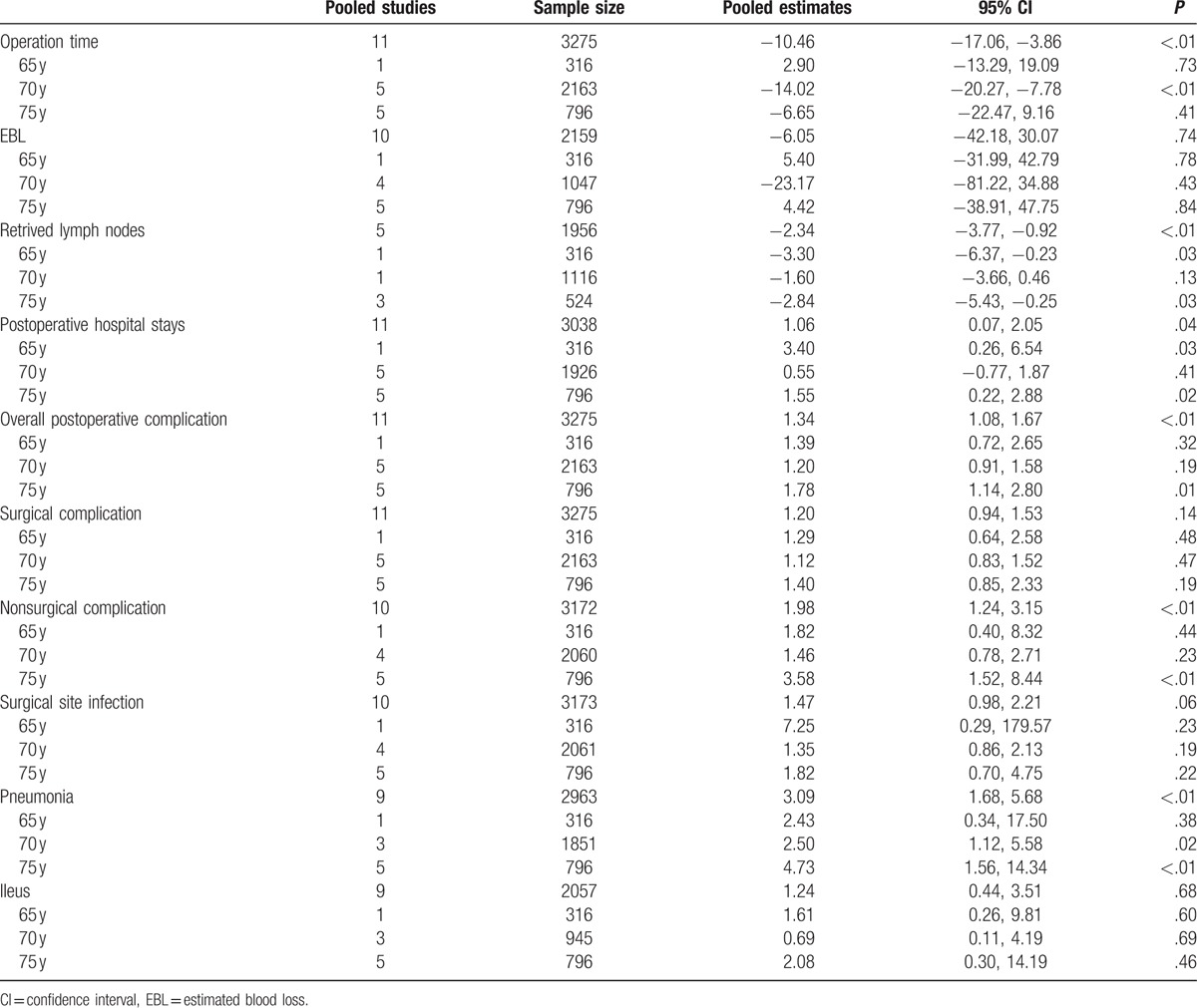
Figure 5.
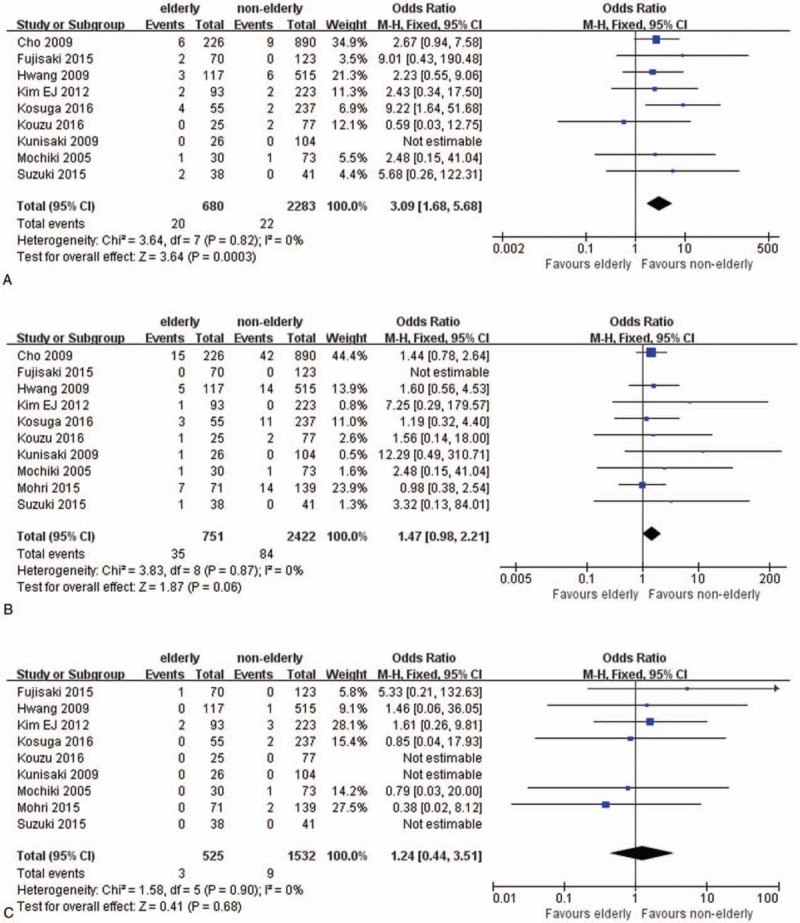
Details of postoperative complications: (A) postoperative pulmonary complications, (B) surgical site infections, and (C) ileus.
4. Discussion
Nowadays LG has been widely adopted owning to its minimally invasiveness as compared to open gastrectomy. Increasing elderly patients with GC accepts operation to achieve better prognosis with the improvement of surgical techniques and perioperative care. Till now, results of randomized studies and reviews focus on the elderly patients underwent LG have not yet been reported. To evaluate the feasibility and safety of LG in elderly patients, we conducted this study by reviewing and analyzing the previous studies using the meta-analysis method.
Figure 6.
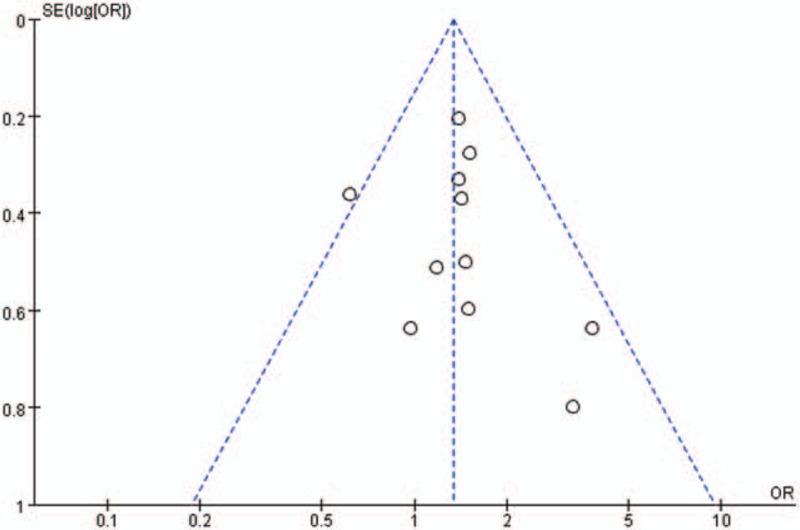
Funnel plots of overall postoperative complication.
Pronged operation time and elevated blood loss were reported to increase surgical stress and risks of postoperative complications. Huang et al[29] reviewed 2170 patients who underwent LG and identified intraoperative blood loss more than 75 mL as an independent risk factor for major complications. Park et al[30] also reported pronged operation time was an important risk factor for the 30-day mortality rate. Characteristics of LG including amplified operating view and meticulous manipulation helped to reduce the blood loss, which also led to longer operation time. Many surgeons had gradually overcome this drawback as they passed the learning curves and utilized more advanced instruments. In our analyses, the operation time and estimated blood loss of elderly patients was comparable with that of nonelderly patients. In fact, the operation time was slightly less in elderly patients (nearly 10 minutes). Operation time and estimated blood loss might not be specific disadvantages accompanied with the elderly patients.
Postoperative complications of LG are the major concern among the elderly. Elderly patients usually have increased severity of associated comorbidities and decreased functional reservation. Malignancies like GC could damage the balanced immune and nutrient status in elderly patients. In the study, we found elderly patients had more postoperative complications than the nonelderly patients (17.7% vs 13.5%). Hager et al[11] reported the overall complication in elderly patients who underwent open gastrectomy was 27.6% with an in-hospital mortality of 12%. In a study including 2014 patients (ranged from 12 to 91 years) underwent LG, Lin et al[31] revealed that 13.6% of the patients suffered postoperative complications. Thus, the overall complication rate in elderly patients was acceptable as compared with historical reports. We also demonstrated there was no difference between elderly patients and nonelderly patients in surgical complications, while elderly patients had more nonsurgical complications. In detail, elderly patients had comparable surgical site infections rate and ileus rate, but a higher pulmonary complications rate. Further analysis also showed in subgroup elder cutoff age (75 years), elderly patients suffered higher risk of pneumonia, nonsurgical complications, and subsequently overall complications. These results also corroborated the point that elderly patients were associated with higher pulmonary complications rate.
Pneumoperitoneum during LG was considered as an adverse factor, which may exacerbate the preexistent pulmonary comorbidities and bring new pulmonary complications including pneumonia, atelectasis, pleural effusion, etc. Cho et al[28] reported higher incidence of postoperative respiratory complications were observed among elderly patients with preoperative pulmonary diseases who underwent LG. Conversely, Suzuki et al[20] argued the transitory cardiopulmonary adverse effects by pneumoperitoneum could normalize during the intraoperative period even among decrepit elderly patients with cardiopulmonary disease. In a study by Hamakawa et al,[32] chronic obstructive pulmonary disease was significantly associated with the occurrence of postoperative complications. Thus, we speculated the severity of the preoperative comorbidities and the patients’ physiological statuses were the main risk factors for PPCs. Proper interventions are recommended for preventing PPCs including medicine, nutrient-supporting treatment, and inspiratory muscle training.[33,34]
Our study also found elderly patients had delayed first flatus, which indicated the bowel function recovery of elderly patients was slower than younger patients. In consistent with this, elderly patients had a longer length of hospitalization, which was likely a result of slower recovery and higher postoperative morbidity.
An unexpected result of our study was that the retrieved lymph nodes in elderly patients were less than younger patients. In some pooled studies, elderly patients were less likely to undergo D2 or even D1+ lymphadenectomy. Performing extended lymph node resection meant a higher risk of postoperative complications. Previous studies reported that there were no significant survival benefits of D2 over D1 in elderly patients.[35] Furthermore, Takeshita et al[36] reported that limited lymph node dissection on elderly patients may reduce life expectancy, especially in stage I and II patients. For this population gastrectomy with limited lymph node dissection is recommended. In the present study, the oncological outcomes as reported by several pooled studies showed no differences between the elderly and nonelderly patients. The residual life expectancy of elderly patients is short. The true value of extended lymphadenectomy in this population needs more well-designed studies to evaluate.
Our studies also had some limitations needed to be noted. First, all the pooled studies were retrospective, which had bias in patients selection, surgeons techniques, surgical extents and regional differences, etc. Second, heterogeneity in the studies with different cutoff ages of elderly patients may also bring the bias. Third, the inclusion of some studies not reporting the means and SDs and estimate the data using the method described by Hozo et al may also result in bias.
5. Conclusion
In conclusion, LG for elderly patients is a feasible and safe approach for GC. Despite of delayed recovery and higher risks of postoperative complications, old age should not be considered as the absolute contraindication for LG.
Acknowledgement
The authors thank Medical and health technology project of Zhejiang Province (grant No.2014KYB138) for the support.
Footnotes
Abbreviations: CI = confidence interval, GC = gastric cancer, LG = laparoscopic gastrectomy, OR = odds ratio, PPC = postoperative pulmonary complication, SD = standard deviation, WMD = weighted mean difference.
YP and KC equally contributed to this work.
Ethics statement: There is no need to seek informed consent from patients, since this is a meta-analysis based on the published data, without any potential harm to the patients.
Funding/support: This work was supported by Medical and health technology project of Zhejiang Province (grant No.2014KYB138).
The authors have no conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Graham DY. Roadmap for elimination of gastric cancer in Korea. Korean J Intern Med 2015;30:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maruyama K, Katai H. Surgical treatment of gastric cancer in Japan, trend from standardization to individualization. Chirurgia (Bucur) 2014;109:722–30. [PubMed] [Google Scholar]
- [4].Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146–8. [PubMed] [Google Scholar]
- [5].Vinuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446–56. [DOI] [PubMed] [Google Scholar]
- [6].Haverkamp L, Brenkman HJ, Seesing MF, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer 2015;15:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report – a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417–20. [DOI] [PubMed] [Google Scholar]
- [8].Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350–7. [DOI] [PubMed] [Google Scholar]
- [9].Otsuji E, Fujiyama J, Takagi T, et al. Results of total gastrectomy with extended lymphadenectomy for gastric cancer in elderly patients. J Surg Oncol 2005;91:232–6. [DOI] [PubMed] [Google Scholar]
- [10].Zhou CJ, Chen FF, Zhuang CL, et al. Feasibility of radical gastrectomy for elderly patients with gastric cancer. Eur J Surg Oncol 2016;42:303–11. [DOI] [PubMed] [Google Scholar]
- [11].Hager ES, Abdollahi H, Crawford AG, et al. Is gastrectomy safe in the elderly? A single institution review. Am Surg 2011;77:488–92. [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [13].Jung MR, Park YK, Seon JW, et al. Definition and classification of complications of gastrectomy for gastric cancer based on the accordion severity grading system. World J Surg 2012;36:2400–11. [DOI] [PubMed] [Google Scholar]
- [14].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Kosuga T, Ichikawa D, Okamoto K, et al. Impact of age on early surgical outcomes of laparoscopy-assisted gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer. Anticancer Res 2015;35:2191–8. [PubMed] [Google Scholar]
- [19].Yasuda K, Sonoda K, Shiroshita H, et al. Laparoscopically assisted distal gastrectomy for early gastric cancer in the elderly. Br J Surg 2004;91:1061–5. [DOI] [PubMed] [Google Scholar]
- [20].Suzuki S, Nakamura T, Imanishi T, et al. Carbon dioxide pneumoperitoneum led to no severe morbidities for the elderly during laparoscopic-assisted distal gastrectomy. Ann Surg Oncol 2015;22:1548–54. [DOI] [PubMed] [Google Scholar]
- [21].Mohri Y, Yasuda H, Ohi M, et al. Short- and long-term outcomes of laparoscopic gastrectomy in elderly patients with gastric cancer. Surg Endosc 2015;29:1627–35. [DOI] [PubMed] [Google Scholar]
- [22].Kunisaki C, Makino H, Takagawa R, et al. Efficacy of laparoscopy-assisted distal gastrectomy for gastric cancer in the elderly. Surg Endosc 2009;23:377–83. [DOI] [PubMed] [Google Scholar]
- [23].Mochiki E, Ohno T, Kamiyama Y, et al. Laparoscopy-assisted gastrectomy for early gastric cancer in young and elderly patients. World J Surg 2005;29:1585–91. [DOI] [PubMed] [Google Scholar]
- [24].Kouzu K, Tsujimoto H, Hiraki S, et al. Efficacy of totally laparoscopic distal gastrectomy for gastric cancer in elderly patients. Mol Clin Oncol 2016;4:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim EJ, Seo KW, Yoon KY. Laparoscopy-assisted distal gastrectomy for early gastric cancer in the elderly. J Gastric Cancer 2012;12:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hwang SH, Park DJ, Jee YS, et al. Risk factors for operative complications in elderly patients during laparoscopy-assisted gastrectomy. J Am Coll Surg 2009;208:186–92. [DOI] [PubMed] [Google Scholar]
- [27].Fujisaki M, Shinohara T, Hanyu N, et al. Laparoscopic gastrectomy for gastric cancer in the elderly patients. Surg Endosc 2016;30:1380–7. [DOI] [PubMed] [Google Scholar]
- [28].Cho GS, Kim W, Kim HH, et al. Multicentre study of the safety of laparoscopic subtotal gastrectomy for gastric cancer in the elderly. Br J Surg 2009;96:1437–42. [DOI] [PubMed] [Google Scholar]
- [29].Huang CM, Tu RH, Lin JX, et al. A scoring system to predict the risk of postoperative complications after laparoscopic gastrectomy for gastric cancer based on a large-scale retrospective study. Medicine 2015;94:e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park JM, Jin SH, Lee SR, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc 2008;22:2133–9. [DOI] [PubMed] [Google Scholar]
- [31].Lin JX, Huang CM, Zheng CH, et al. Surgical outcomes of 2041 consecutive laparoscopic gastrectomy procedures for gastric cancer: a large-scale case control study. PloS One 2015;10:e0114948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hamakawa T, Kurokawa Y, Mikami J, et al. Risk factors for postoperative complications after gastrectomy in gastric cancer patients with comorbidities. Surg Today 2016;46:224–8. [DOI] [PubMed] [Google Scholar]
- [33].Katsura M, Kuriyama A, Takeshima T, et al. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev 2015;CD010356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li W, Zhao Y, Sun Z, et al. Lung protective effects of budesonide nebulization during perioperative period of thoracolumbar fusion. J Thorac Dis 2014;6:1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liang YX, Deng JY, Guo HH, et al. Characteristics and prognosis of gastric cancer in patients aged >/=70 years. World J Gastroenterol 2013;19:6568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takeshita H, Ichikawa D, Komatsu S, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg 2013;37:2891–8. [DOI] [PubMed] [Google Scholar]


