Abstract
Vitamin D may prevent dental caries. To date, no attempts have been made to examine the correlation between the incidence of caries and the concentrations of vitamin D in children with pituitary growth hormone deficiency.
The study observed patients of the Department of Endocrinology and Diabetology of the University Paediatric Hospital of the Medical University of Lublin treated with human recombinant growth hormone for pituitary growth hormone deficiency (GHD). The study was conducted between October 2014 and June 2015. The study group consisted of 121 children and adolescents (6–17 years old), including 56 children from rural areas and 65 children from urban areas. The study group was stratified by area of residence.
In our study, the increase in vitamin D3 [25(OH)D] levels reduced the D component by 0.66 per each 10 ng/mL of vitamin D3 concentration. The percentage of children with active caries in rural areas is 91.07% (n = 51), which is significantly higher than the percentage of children with active caries in urban areas (81.54%, n = 53).
To date, information regarding the potential possibility of reducing the incidence of dental caries by means of increasing the levels of vitamin D was sidelined by paediatricians and dentists alike. Therefore, this aspect of caries prevention should be highlighted.
Keywords: dental caries, dentistry, endocrinology, growth hormone deficiency, vitamin D
1. Introduction
Vitamin D comprises a group of chemicals with steroidal structure, acting as precursors of numerous active substances with hormonal properties. The first reports on the effects of vitamin D deficiency were based on the vitamin supply alone, not considering the blood levels of its active metabolites, which exert various genomic and extragenomic effects to regulate maturation and function of numerous tissues and organs and further affect the calcium-phosphorus metabolism and mineralization of bone tissue, including teeth. The first reports on the relationship between vitamin D deficiency and caries lesions in teeth date back to 1928.[1]
The importance of vitamin D in the growth, maturation, and physiology of tissues and organs requires that its deficiency be considered in the diagnostics of skeletal system diseases. The ability to diagnose individual correlations may have a significant impact on the course of treatment and contribute to preventing delayed consequences of vitamin D deficiency. Also, there is increasing interest in the role of vitamin D for oral health.
Vitamin D impacts bone and teeth mineralization by means of vitamin D receptors (VDR). Together with parathormone (PTH) and fibroblast growth factor 23 (FGF 23), vitamin D controls the concentration of calcium and phosphorus in circulating blood and subsequent mineralization of bones.[2] Significant differences exist in recommendations regarding optimum levels of vitamin D and vitamin D supplementation, giving rise to heated disputes on the subject.[3–5] Current US guidelines include the recommendations published in 2011 in the US Institute of Medicine (IOM) report,[6] as well as the Clinical Practice Guideline recommendations from the US Endocrine Society[7] for all age groups in central European populations. The guidelines for vitamin D supplementation were formulated by a panel of experts during the conference titled “Vitamin D—minimum, maximum, optimum” in 2012.[8] Doses recommended by the IOM report/US Endocrine Society Practice Guideline and by this panel of experts are presented in Tables 1 and 2.
Table 1.
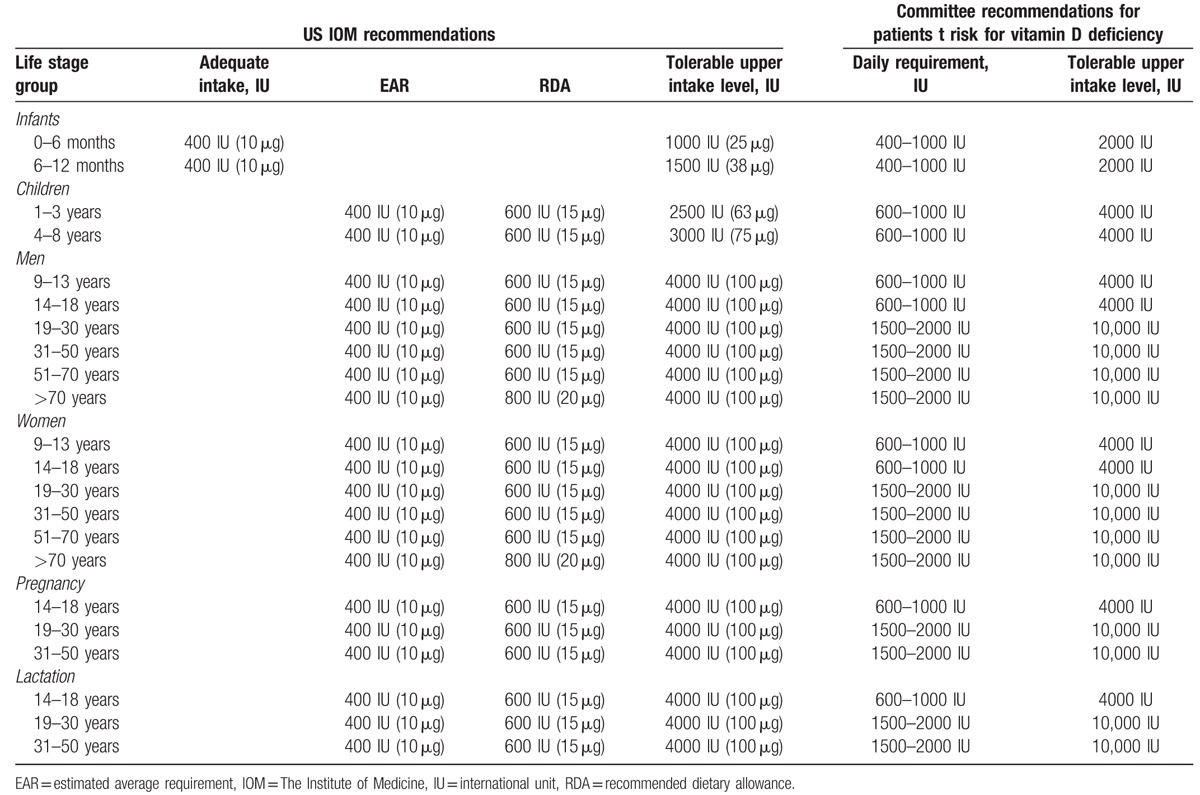
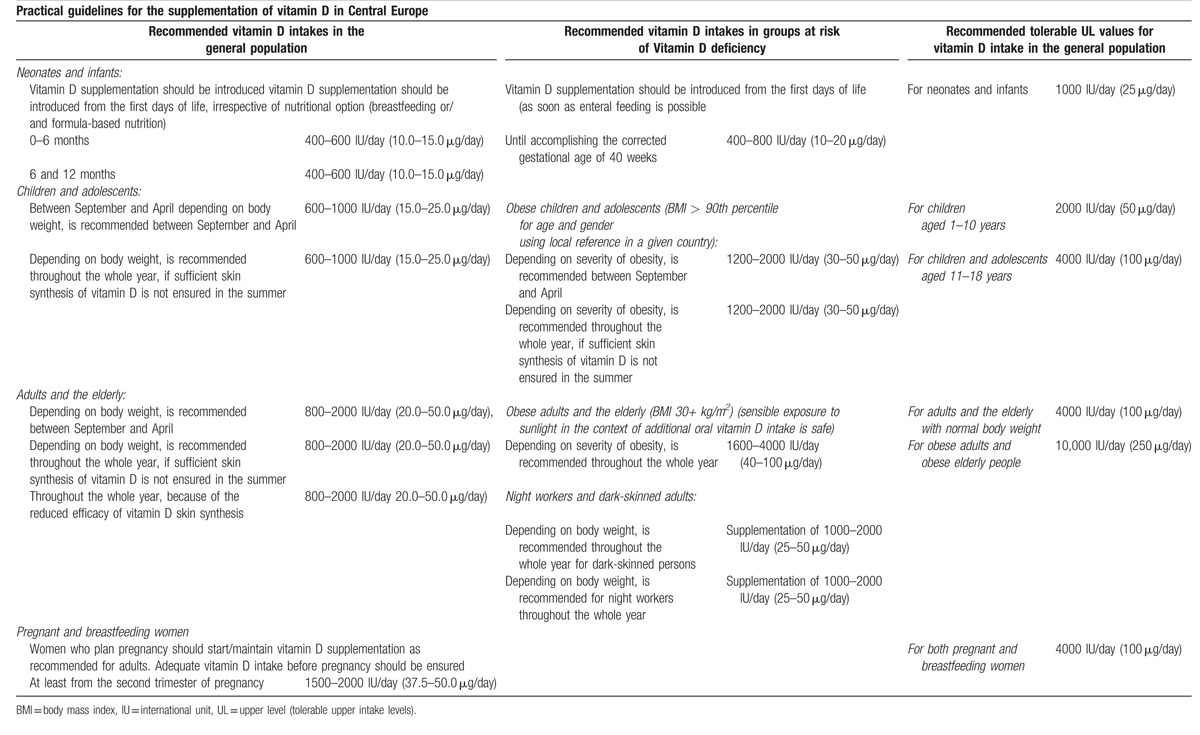
Table 2.
Practical guidelines for the supplementation of vitamin D in Central Europe[8].
It should also be mentioned that the most recent studies suggest that vitamin D levels affect the function of the GH/IGF-I axis (GH—growth hormone). Vitamin D may cause an increase in the production and secretion of IGF-1 (insulin-like growth factor 1) and IGFBP-3 (insulin-like growth factor-binding protein 3) within the liver.[9,10] Both the growth hormone and IGF-1 lead to an increase in renal production of 1,25-(OH)2D (calcitriol) by enhancing the activity of renal 1α-hydroxylase; the blood level of vitamin D is also affected.[11]
At the time of conducting our research, the GH level was established by supplementation of recombinant human GH, which ensured proper growth and calcium phosphate metabolism. Somatotropic hypopituitarism effects were monitored every 3 months as part of a treatment program for GH insufficiency. Additional factors that influenced caries development in this group of children may include poor oral hygiene and disturbance in vitamin D levels.
Based on the available national and foreign literature, it was established that the effectiveness of the conventional methods of caries prevention is not sufficient.[12–15] Caries is a chronic disease that may cause pain, eating difficulty, and sleep disorders, as well as discomfort and reduced quality of life in affected individuals.[2,13] It is one of the most common diseases observed in paediatric patients worldwide.[14] Caries disease in Poland is relatively frequent and is differentiated in terms of sociodemographic features.[15,16] It is indispensable to define the groups most vulnerable to caries because of the uneven occurrence of this disease in the population. For the last few years, there has been a growing interest in vitamin D effects on oral health. Studies have shown that vitamin D can prevent caries.[1,17]
The aim of this cohort study is to establish any differences between children with GHD (growth hormone deficiency) in rural areas and urban areas concerning oral hygiene and caries occurrence and the relationship between caries occurrence and vitamin D concentration in children with GHD. We also investigated whether vitamin D is a potential method of caries prevention.
2. Material and methods
2.1. Study design and data collection
We studied patients of the Department of Endocrinology and Diabetology of the University Paediatric Hospital of the Medical University of Lublin treated with human recombinant growth hormone for pituitary growth hormone deficiency. The study was conducted between October 2014 and June 2015. The study group consisted of 121 children and adolescents aged 6 to 18 years, including 56 children from rural areas and 65 children from urban areas. The study group was stratified by area of residence (Table 3). The number of cases in the area during the study period determined the sample size. The children with GHD were divided by residence into 2 comparative groups—children from urban areas and from rural areas.
Table 3.
The study group according to the area of residence.
In Poland, every child who is treated with recombinant human growth hormone (rhGH) needs to qualify for the treatment program and may have conditions including growth hormone deficiency, Turner syndrome, and chronic renal insufficiency.[18] For our study, inclusion criteria were short stature (height < 3rd percentile), height velocity < 2SD (standard deviation) for age and sex, height standard deviation score (HSDS) ≤ −3, and maximal growth hormone secretion below 10 ng/mL in stimulating tests (clonidine stimulating test and insulin-stimulating test). The exclusion criteria were Turner syndrome, bone defects, celiac disease, hypothyroidism, renal insufficiency, and neoplasm. In 121 patients with pituitary growth hormone deficiency (GHD), vitamin D3 supplementation was used at the dose of 500 to 2000 IU/day. Duration of supplementation of vitamin D3 was also included. Increased growth rate, reference values of IGF-1 concentration, and upward progression on the growth chart proved the effectiveness of the treatment.
The preliminary stage consisted of obtaining written consent from the guardians of children and adolescents taking part in the study. The first part of the study was based on a survey containing questions about the hygienic and nutritional habits. The second stage consisted of a clinical trial including epidemiological dental examinations and laboratory investigations to determine serum levels of vitamin D3. In addition, computed tomography (CT) or magnetic resonance imaging (MRI) scans were acquired to rule out proliferative disorder in-line with the recommendations from the Polish program of short stature treatment with human growth hormone in adolescents and children. The guardians were informed about the objectives of the study.
Dental assessment was carried out using the WHO (World Health Organization) criteria for epidemiological trials.[19] We compared the intensity of caries (mean DMFT index) for patients from different residential areas (urban and rural areas). The study was carried out in accordance with all sanitary requirements using sterile diagnostic kits (i.e., disposable mirrors and probes in artificial lighting). The examiner was assisted by the recording clerk. The results of the dental assessment were recorded in standard denture diagrams prepared for each patient.
Using the “vitamin D Total” (ELECSYS Roche Diagnostics), blood serum 25(OH)D (vitamin D3) levels were measured by a chemiluminescence assay (CLIA).
After proliferative disorders were ruled out by computed tomography (CT) or magnetic resonance imaging (MRI), qualifying GHD patients received treatment with recombinant human growth hormone. All children received rhGH at the dose of 0.5 to 1.0 IU per kilogram of body weight per day. The patients presented with normal IGF1 levels and growth rates according to the percentile mesh appropriate for their sex and age, indicating correct levels of the growth hormone in blood. This study conformed to the STROBE guidelines for reporting observational studies (www.strobe-statement.org).[20]
2.2. Statistical analysis
The intensity of dental caries was assessed using the mean DMFT index, where D component is the number of teeth with active decay, M component is the number of missed teeth, and F component is the number of teeth with fillings. The DMFT index was calculated for each patient and each result was considered a dependent variable. Independent variables included serum levels of vitamin D3 and the hygienic and nutritional habits of the participants. Also examined was the relationship between the independent variables and sex, age, and area of residence, as well as the relationship between the dependent variables and classification criteria used in the study. Data were analyzed with the Statistica 10 software package using Student's t-test, Mann–Whitney's U-test, Spearman's rank correlation coefficient, Pearson's Chi2 test, Kruskal–Wallis test, and Pearson's linear correlation coefficient. A P value of ≤0.05 was considered statistically significant.
2.3. Ethical approval
The study was approved by the Medical Ethics Committee of the Medical University of Lublin, Poland.
3. Results
Due to the lack of correlation between the independent variables (answers to survey questions) and the sex and age of patients, these analyses were not considered in the results and discussion.
The mean concentrations of vitamin D3 [25(OH)D] were not significantly different in children from rural areas (29.03 ± 11.23 ng/mL) and children from urban areas (26.17 ± 9.43 ng/mL). Vitamin D3 concentration in particular categories (0–10 ng/mL, 11–20 ng/mL, 21–30 ng/mL, above 30 ng/mL) varies statistically (Pearson's Chi2 test = 8.90; df = 3; P = .031; Fig. 1). A higher percentage of patients with vitamin D3 concentration between 21 and 30 ng/mL was significantly more often observed in urban areas, while the concentration above 30 ng/mL was reported more frequently in rural areas. No significant impact of vitamin D3 concentration on mean DMFT and its components was observed in urban areas. In contrast, the statistically significant impact of vitamin D3 concentration on the average value of DMFT number and its component DT (decayed teeth) was noted in rural areas. When vitamin D3 concentration was higher, values of mentioned caries indicators were lower (P < .05; Table 4). Our statistical analysis showed a significant impact of vitamin D3 concentration on the value of DT component (r = −0.31; P = .023). There is a weak negative correlation (Fig. 2). The analysis revealed a correlation between increases in vitamin D3 concentration by 10 units and decreases in the value of DT component by 0.66 in children from rural areas (r = -0.31; P = .023; corrected Fisher's Z = 0.67 for H0: Ro = 0). There is a correlation between increases in increases in vitamin D3 concentration by 10 units and decreases in the value of DMFT by 0.82 in children from rural areas (r = −0.33; α=0.05; Fisher's Z = 0.73 for H0: Ro = 0).
Figure 1.
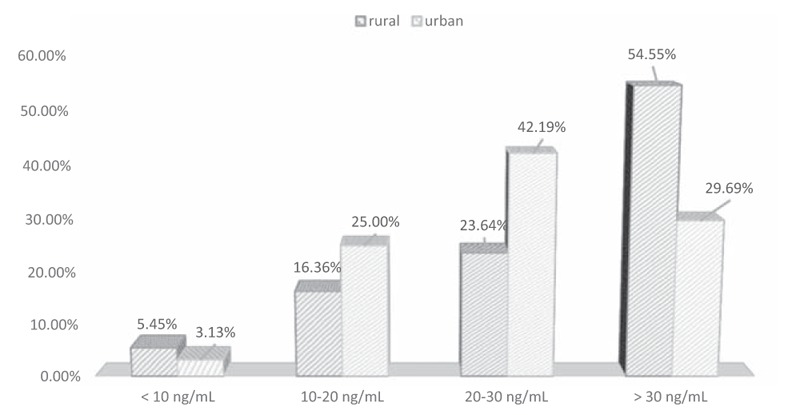
Vitamin D3 concentration in four categories in patients’ blood serum according to their site of residence.
Table 4.
The influence of vitamin D concentration on mean DMFT index and its components in population of children with GHD from rural areas.
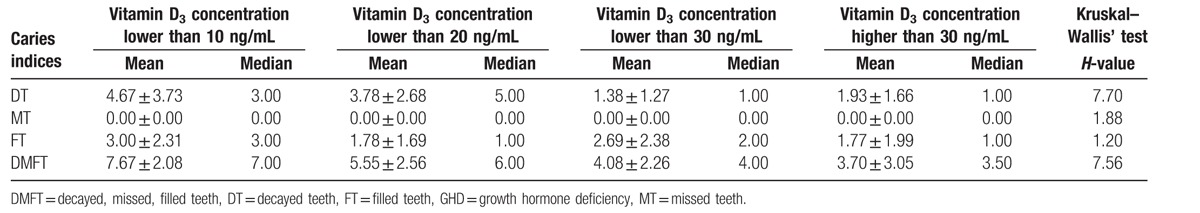
Figure 2.
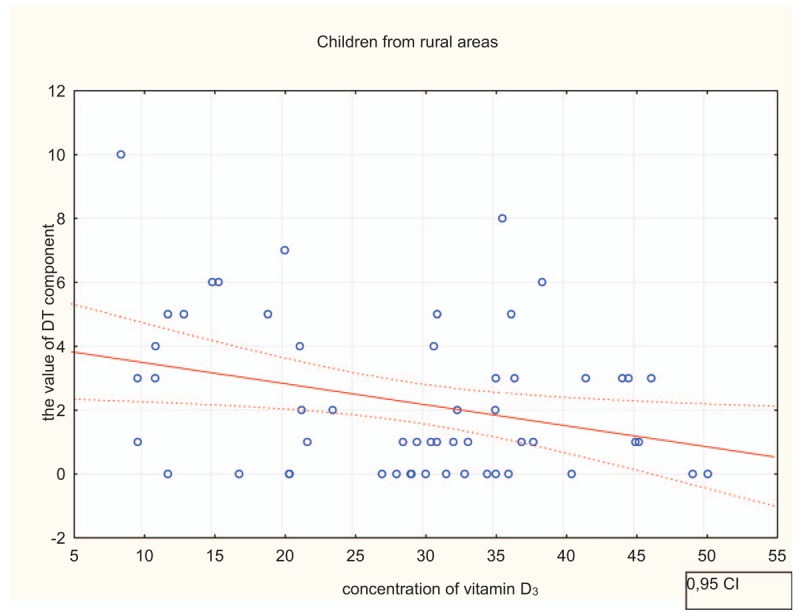
Influence of vitamin D3 concentration on the value of D component in children from rural areas. CI = confidence interval.
No statistically significant correlations were observed between the overall DMFT and DT component and the duration of vitamin D3 supplementation. However, a reduction in the DMFT index was observed along with increased vitamin D3 levels in children from rural areas; the difference was statistically significant. These results show that the increase in vitamin D3 levels reduces the number of decayed teeth in children from rural areas, while no such correlation can be observed in children from urban areas (Table 5). Table 6 shows the DT component and DMFT index in children according to their site of residence.
Table 5.
The influence of vitamin D concentration on mean DMFT index and its components in population of children with GHD from urban areas.
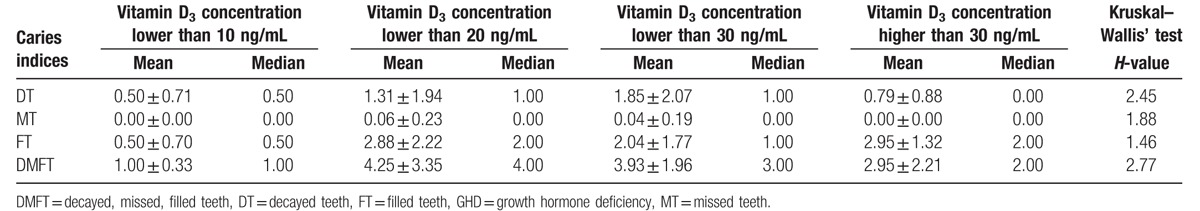
Table 6.
D component and DMFT index in children according to their site of residence.

The survey questionnaire consisted of questions regarding the hygienic and nutritional habits of patients. The frequency of toothbrush change had no statistically significant impact on the DMFT index or the number of decayed teeth (DT component) in children from urban and rural areas. No statistically significant differences were observed between the frequency of teeth brushing and number of decayed teeth (DT component) and mean DMFT index in children from urban and rural areas. Brushing teeth after meals, as well as forgetting to brush teeth, had no effect on the number of decayed teeth (DT component) or the mean DMFT index in children from urban as well as rural areas. Using dental floss had no significant impact on the number of decayed teeth (DT component) as well as the mean DMFT index in children from rural areas. Lastly, the frequency of dental visits and consumption of sweetened beverages had no significant impacts on number of decayed teeth (DT component) or total DMFT index.
Patients from urban areas significantly more often declared brushing their teeth from the first year of life, while a significant percentage of patients from rural areas started to brush their teeth in the second year of life (Pearson's chi2 test = 15.46, df = 2, P = .0004; Fig. 3). There was a significant impact on the DMFT index for the age at which brushing teeth was initiated in children from rural areas (Fig. 4). The percentage of urban children brushing their teeth from the age of 1 year was significantly higher than the corresponding percentage of rural children (63.32% vs. 27.59%). Children from rural areas usually started brushing their teeth at the age of 2 years. In both rural and urban areas, the most common frequency of toothbrush change is 3 months. Frequency of toothbrush replacement did not significantly influence caries frequency in children from rural areas.
Figure 3.
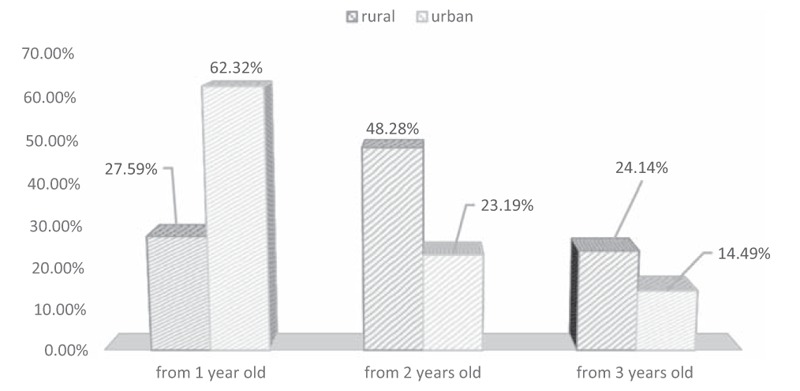
Children with GHD with regard to initiation of tooth brushing and inhabitation area. GHD = growth hormone deficiency.
Figure 4.
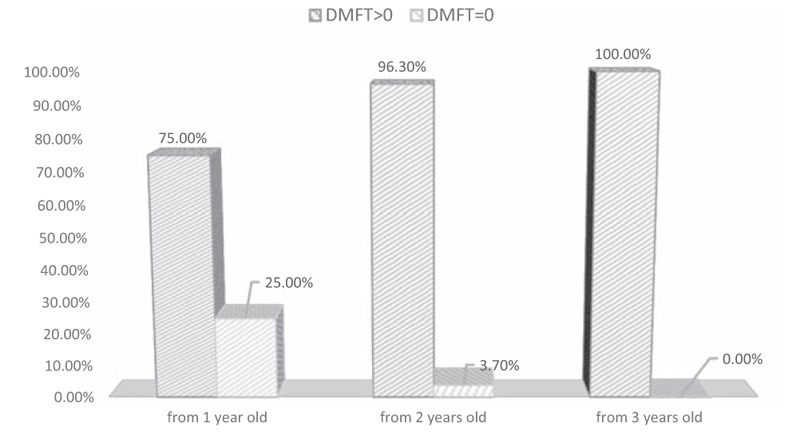
Caries frequency in a group of children from rural areas dependent on the age of starting tooth brushing. DMFT = decayed, missed, filled teeth.
However, the study showed a significant correlation between the age at which tooth brushing began and caries frequency in children from rural areas. Children and adolescents who started to brush their teeth earlier had lower caries frequency. Similarly, there was a significantly higher number of fillings in children from rural areas who declared oral hygiene self-care. Moreover, higher values of DMFT were observed in children from rural areas who brushed their teeth irregularly or before eating breakfast.
4. Discussion
To date, only several have been conducted in a larger group of paediatric patients to evaluate the correlation between vitamin D levels and dental caries. Thus, few publications are available regarding this relationship.
Schroth et al evaluated the relationship between vitamin D levels and dental caries in Canadian school students participating in the Canadian Health Measures Survey (CHMS). Total of 1017 children aged 6 to 11 years were included in the study.[24] The authors observed dental caries in 56.4% of children, with a mean DMF of 2.47. The presence of caries was associated with 25(OH)D levels (vitamin D3) below 30 ng/mL (optimum level as defined by the authors) and below 20 ng/mL (optimum level as defined by IOM), failure to brush teeth twice per day and attend annual dental visits.[17] In our study, the D value was 1.39 and 2.23 in urban and rural children, respectively. The percentage of children with active caries in rural areas (91.07%, n = 51) was higher than the percentage of children with active caries in urban areas (81.54%, n = 53; Pearson's chi2 = 2.26, df = 1, P = .13). The results obtained by Schroth et al suggest that the failure to achieve the optimum vitamin D levels (below 30 ng/mL) is associated with the likelihood of dental caries being increased by 39%, while failure to achieve the optimum vitamin D levels as defined by IOM[6] (below 20 ng/mL) may increase the likelihood of dental caries by 47%.[17] As shown by our study, the increase in vitamin D3 levels reduces the D value by 0.66 per each 10 ng/mL of vitamin D3 concentration.
In 2013, Hujoel published a systematic review and meta-analysis of 24 clinical trials on vitamin D3 or D2 supplementation and the received doses of ultraviolet radiation (UV radiation). The study showed that vitamin D supplementation resulted in a 47% reduction in dental caries.[21] In 2011, Grant published an article highlighting the role of UVB radiation in the reduction of dental caries, probably by means of vitamin D production, leading to the induction of cathelicidins and defensins characterised by antibacterial properties. Grant suggested that vitamin D levels higher than 30 to 40 ng/mL should significantly reduce the incidence of dental caries.[22] Schroth et al ascribe the incidence of early childhood caries (ECC) to low vitamin D levels.[13] In addition, they demonstrated that levels of vitamin D in pregnant women may impact dental development in the fetus and bear responsibility for future incidence of ECC in the progeny.[23]
Contemporary medical knowledge shows that vitamin D deficiency is not a only nutrition problem. In view of its pleiotropic effect, it also became an endocrinological and dental problem. The proper supplementation of vitamin D has a preventive effect on various health problems, including caries. The individualization of vitamin D dose is considered by several authors.[4,24] Our study shows that in spite of age-appropriate vitamin D supplementation, the majority of GHD patients suffer from vitamin D deficiency. This is an argument for the individualization of vitamin D dosages. The study shows that 70.31% of GHD children from urban areas and 45.45% of GHD children from rural areas suffer from vitamin D deficiency. The duration of vitamin D supplementation has not significantly impacted vitamin D concentration in patients. This may have a positive effect on this population's health status by improvement of bone metabolism.
4.1. Strengths and bias
As far as we are aware, our study is the first study to show a correlation between the incidence of dental caries and the concentrations of vitamin D3 in children with pituitary growth hormone deficiency. Strengths of this study include the fact that dental examinations were performed by trained dentist and examination of the patient's blood serum 25(OH)D levels, which is considered to be the best metabolite to demonstrate vitamin D status. It shows the sum of 25(OH)D from diet and endogenous synthesis.[25]
However, we acknowledge that there are some limitations in this study. We did not consider some factors which also have an impact on the concentration of vitamin D3, such as body mass index (BMI) of children, reduced exposure to sunlight, winter season, sunscreen use, being indoors, and clothing coverage.[25]
Further studies with a control group and larger sample size to improve power analysis are necessary to better understand the correlation between vitamin D levels in blood serum and incidence of dental caries in children with GHD.
5. Conclusions
-
1.
It was determined that there were significant differences in oral hygiene between GHD children who inhabit rural areas and urban areas. The assessment showed that oral hygiene in children suffering from GHD who live in rural areas is unsatisfactory. There are significant differences when it comes to DMFT and the DT component between children suffering from GHD who inhabit urban and rural areas. Caries in dentition was observed more frequently in children who lived in rural areas than in those who lived in urban areas
-
2.
It was observed that higher vitamin D3 concentration reduces the intensity of active caries in children who suffer from GHD, which could suggest that caries may constitute a symptom of vitamin D deficiency.
-
3.
The data concerning possibilities of reducing caries occurrence by increasing vitamin D concentration have been sidelined by paediatricians and dentists. Therefore, we recommend emphasis on vitamin D intake, which may possibly support caries prevention.
Footnotes
Abbreviations: 1,25-(OH)2D = calcitriol, 25(OH)D = vitamin D3, BMI = body mass index, CHMS = Canadian Health Measures Survey, CLIA = chemiluminescence assay, CT = computed tomography, DMFT = index where D component is the number of teeth with active decay, M component is the number of missed teeth, and F component is the number of teeth with fillings, ECC = early childhood caries, FGF 23 = fibroblast growth factor 23, GH = growth hormone, GHD = growth hormone deficiency, HSDS = height standard deviation score, IGF-1 = insulin-like growth factor, IGFBP-3 = insulin-like growth factor-binding protein 3, IOM = Institute of Medicine, MRI = magnetic resonance imaging, PTH = parathormone, rhGH = recombinant human growth hormone, SD = standard deviation, UV radiation = ultraviolet radiation, VDR = vitamin D receptors, WHO = World Health Organization.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Mellanby M, Pattison CL. The action of vitamin D in preventing the spread and promoting the arrest of caries in children. Br Med J 1928;2:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol 2015;25:620–5. [DOI] [PubMed] [Google Scholar]
- [3].Theodoratou E, Tzoulaki I, Zgaga L, et al. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035–12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pramyothin P, Holick MF. Vitamin D supplementation: guidelines and evidence for subclinical deficiency. Curr Opin Gastroenterol 2012;28:139–50. [DOI] [PubMed] [Google Scholar]
- [5].Veugelers P, Pham T-M, Ekwaru J. Optimal vitamin D supplementation doses that minimize the risk for both low and high serum 25-hydroxyvitamin D concentrations in the general population. Nutrients 2015;7:10189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ross AC. Institute of Medicine (U. S.), eds. Dietary Reference Intakes: Calcium, Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- [7].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [8].Płudowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol 2013;64:319–27. [DOI] [PubMed] [Google Scholar]
- [9].Ameri P, Giusti A, Boschetti M, et al. Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol 2013;169:767–72. [DOI] [PubMed] [Google Scholar]
- [10].Bogazzi F, Rossi G, Lombardi M, et al. Vitamin D status may contribute to serum insulin-like growth factor I concentrations in healthy subjects. J Endocrinol Invest 2011;34:e200–3. [DOI] [PubMed] [Google Scholar]
- [11].Ciresi A, Cicciò F, Giordano C. High prevalence of hypovitaminosis D in Sicilian children affected by growth hormone deficiency and its improvement after 12 months of replacement treatment. J Endocrinol Invest 2014;37:631–8. [DOI] [PubMed] [Google Scholar]
- [12].Mejàre IA, Klingberg G, Mowafi FK, et al. A systematic map of systematic reviews in pediatric dentistry—what do we really know? PLoS One 2015;10:e0117537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schroth RJ, Levi JA, Sellers EA, et al. Vitamin D status of children with severe early childhood caries: a case–control study. BMC Pediatr 2013;13:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bagramian RA, Garcia-Godoy F, Volpe AR. others. The global increase in dental caries. A pending public health crisis. Am J Dent 2009;22:3–8. [PubMed] [Google Scholar]
- [15].Rodakowska E, Wilczynska-Borawska M, Baginska J, et al. Epidemiological analysis of dental caries in 12-year-old children residing in urban and rural settings in the Podlaskie region of north-eastern Poland. Ann Agric Environ Med 2013;20:325–8. [PubMed] [Google Scholar]
- [16].Gaszynska E, Wierzbicka M, Marczak M, et al. Thirty years of evolution of oral health behaviours and dental caries in urban and rural areas in Poland. Ann Agric Environ Med 2014;21:557–61. [DOI] [PubMed] [Google Scholar]
- [17].Schroth RJ, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res 2015;95:173–9. [DOI] [PubMed] [Google Scholar]
- [18].Tomasz E. Romer, Mieczysław Walczak, Andrzej Lewiński, Maria Roszkowska-Blaim. Ogólnopolski program leczenia niedoboru wzrostu u dzieci i młodzieży w następstwie somatotropinowej niedoczynności przysadki, zespołu Turnera i przewlekłej niewydolności nerek, przez zastosowanie hormonu wzrostu, 2002. Available at: http://www2.mz.gov.pl/wwwfiles/ma_struktura/docs/ogolnopolski_program_niedoborwzrostu.doc. Accessed December 12, 2017. [Google Scholar]
- [19].World Health Organization. Oral Health Surveys: Basic Methods. World Health Organization; 2013. Available at: https://scholar.google.pl/scholar?q=World+Health+Organization.+Oral+Health+Surveys:+Basic+Methods.+World+Health+Organization%3B+2013&hl=pl&as_sdt=0&as_vis=1&oi=scholart&sa=X&ved=0ahUKEwip8ZPFyYXZAhXEWCwKHTXkBbQQgQMIKDAA. [Google Scholar]
- [20].Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hujoel PP. Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev 2013;71:88–97. [DOI] [PubMed] [Google Scholar]
- [22].Grant WB. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol 2011;3:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics 2014;133:e1277–84. [DOI] [PubMed] [Google Scholar]
- [24].Hoel DG, Berwick M, de Gruijl FR, et al. The risks and benefits of sun exposure 2016. Dermatoendocrinol 2016;8:e1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Langlois K, Greene-Finestone L, Little J, et al. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2010;21:47. [PubMed] [Google Scholar]


