Abstract
To determine the diagnostic accuracy of pelvic magnetic resonance imaging (MRI), transvaginal sonography (TVS), and transrectal sonography (TRS) in diagnosis of deep infiltrating endometriosis (DIE).
This diagnostic accuracy study was conducted during a 2-year period including a total number of 317 patients with signs and symptoms of endometriosis. All the patients were evaluated by pelvic MRI, TVS, and TRS in the same center. The criterion standard was considered to be the laparoscopy and histopathologic examination.
Of 317 patients being included in the present study, 252 tested positive for DIE. The sensitivity, specificity, positive predictive value, and negative predictive value of TVS was found to be 83.3%, 46.1%, 85.7%, and 41.6%, respectively. These variables were 80.5%, 18.6%, 79.3%, and 19.7% for TRS and 90.4%, 66.1%, 91.2%, and 64.1% for MRI, respectively. MRI had the highest accuracy (85.4%) when compared to TVS (75.7%) and TRS (67.8%). The sensitivity of TRS, TVS, and MRI in uterosacral ligament DIE was 82.8%, 70.9%, and 63.6%, respectively. On the contrary, specificity had a reverse trend, favoring MRI (93.9%, 92.8%, and 89.8% for TVS and TRS, respectively).
The results of the present study demonstrated that TVS and TRS have appropriate diagnostic accuracy in diagnosis of DIE comparable to MRI.
Keywords: deep infiltrating endometriosis, laparoscopy, magnetic resonance imaging, transrectal sonography, transvaginal sonography
1. Introduction
Endometriosis is a major gynecological health problem being associated with infertility, chronic pelvic pain, and dysmenorrhea with an estimated prevalence of 6.1% of women in reproductive age.[1] The presence of endometrial tissue, fibrosis, and hyperplasia below the peritoneum is defined as the deep infiltrating endometriosis (DIE) which accounts for approximately 15% to 30% of all diagnosed endometriosis cases.[2,3] The most common sites of the DIE have been reported to be uterosacral ligaments, the rectosigmoid colon, the vagina, and the bladder.[4] Clinical examination is of less value in evaluating patients with DIE and thus there is a need for additional diagnostic studies.[5–8] Surgical (laparoscopic) resection of the deep endometrial lesion remain the mainstay of the treatment of the patients with DIE.[9,10] However, in patients with DIE, especial procedures are required to excise the deep lesion in specific locations such as vaginal, rectal, or bladder wall[11] being associated with increased risk of complications.[12] Thus, precise preoperative assessment of the DIE is necessary for appropriate surgical planning.[13]
The criterion standard for diagnosis of DIE is laparoscopic observation and biopsy of intraperitoneal cavity lesions which is invasive and does not provide the ability for preoperative planning.[14,15] Various noninvasive imaging modalities such as magnetic resonance imaging (MRI), transvaginal sonography (TVS), transrectal sonography (TRS), and 3D ultrasound are currently available for the diagnosis of DIE.[14–21] The diagnostic accuracy of these modalities have been investigated in various settings with different results. TRS precludes the limitation of the virginity, whereas TVS is limited to married patients with DIE. The MRI is a noninvasive but costly modality of diagnosis of the DIE with the ability to evaluate the whole peritoneal cavity with acceptable accuracy. The performance and interpretation of all these modalities depend on the experience and expertise of the interpreter.[22] Despite these investigations, selecting the most preferred imaging modality for the diagnosis and pre-operative assessment of patients with DIE has remained a challenge. The aim of the present study was to determine the diagnostic accuracy of MRI, TRS, and TVS in patients with DIE.
2. Materials and methods
2.1. Study population
This prospective longitudinal diagnostic accuracy study was conducted during a 2-year period from March 2013 to February 2015 in private clinics and Mother and Child hospital, a tertiary healthcare center affiliated with Shiraz University of Medical Sciences. We included those patients referring to our centers with primary impression of endometriosis. The condition was suspected based on the clinical symptoms (chronic pelvic pain, dyspareunia, and dysmenorrhea) and physical examination finding (localized tenderness in the posterior cul-de-sac or uterosacral ligament; palpable tender nodules in retrocervical position; tender enlarged adnexal mass). We excluded those with claustrophobia, renal failure or any other contraindication for gadolinium contrast medium injection, malignancy, history of any metallic implants, or prostheses preventing MRI study, structural anomalies of the reproductive system, pregnancy, refusal, or lack of compliance with TVS or TRS. All the patients underwent MRI, TRS, and TVS before surgery. We also excluded the virgin subjects as these could not undergo TVS. The study protocol was approved by the institutional review board and the medical ethics committee of Shiraz University of Medical Sciences and all the participants provided their informed written consents before inclusion in the study.
2.2. Transvaginal sonography
All the TVS examinations were performed by the same operator (SA) who was blinded to the clinical findings of the subjects (he is a board certificate gynecologic ultrasonologist with 30 years of experience in the field). A 7.5 MHz probe (UltrasonixOP machine; British Columbia, Canada) was used and the evaluation was done on nonmenstrual days of the cycle. Patients were asked to have semifilled bladder and bowel prep to ensure better visualization of the pelvic organs upon TVS and TRS evaluations. Interpretations were done in real-time and sonograms were documented in each patient's file. The examination protocol comprised visualization compartments, of the peritoneum and structures in the anterior and posterior as well as the uterus and ovaries. Nodular, hypo-echoic solid lesions with and without cystic components, in different structures of the pelvic cavity were considered highly suggestive for DIE. Likewise, hyper-echogenic abnormal thickening of the peritoneum was considered as a sign of DIE (Fig. 1).
Figure 1.
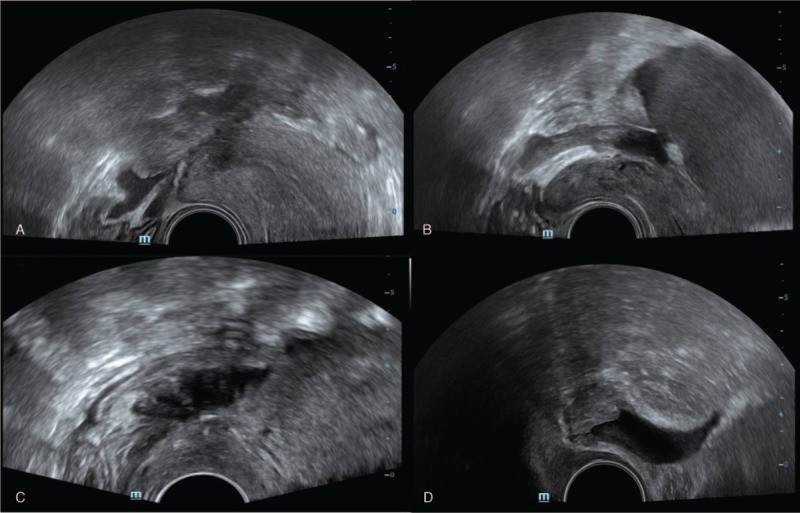
Transvaginal sonography for diagnosis of deep infiltrative endometriosis. A nodular, hypoechoic solid lesion in uterosacral ligament in favor of deep infiltrating endometriosis (DIE) (A); A nodular, cystic solid hypoechoic lesion in retrocervical region in favor of DIE (B); A lesion with similar pattern in rectal wall (C); and a patient with bladder DIE (D).
2.3. Transrectal sonography
All the TRS examinations were performed by the same operator (SA) who was blinded toward the clinical findings of the patients. TRS was performed 2 weeks after TVS evaluation. The evaluation was done by the same gynecologist using a 7.5 MHz linear probe (UltrasonixOP machine; British Columbia, Canada) following bowel prep. To ensure a proper bowel prep, each individual was instructed to have a soft diet on the day before sonography, having 2 spoonful milk of magnesium syrup orally after lunch and using 2 suppositories of 10 mg bisacodyl (Temad Co, Tehran, Iran) at 6 pm and 12 midnight on the day before the procedure. Patients were asked to skip breakfast and take other 2 bisacodyl suppositories at 6 am on the day of procedure. The procedure was performed with empty bladder, using lubricant gel and without administration of sedatives. Interpretation was done in real-time and sonograms were documented in each patient file for future reference. The examination protocol was similar to that of TVS and the same diagnostic criteria were applied (Fig. 2).
Figure 2.
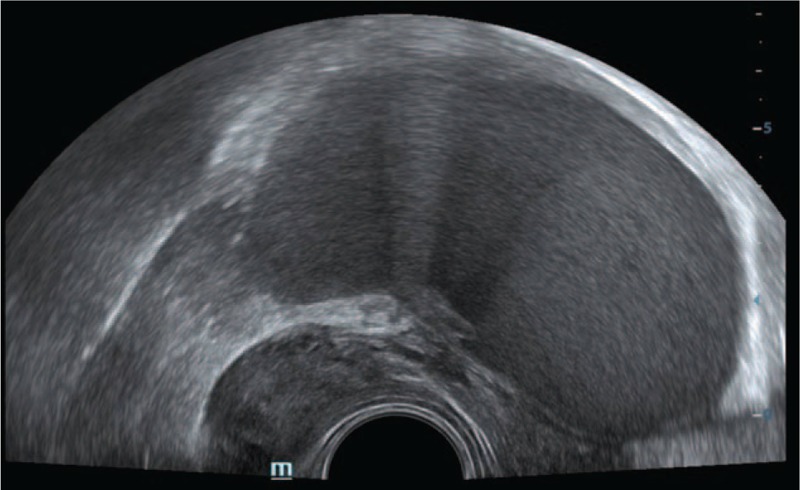
A nodular solid lesion in the ovarian fossa visualized in transrectal sonography in a patient with dysmenorrhea and chronic pelvic pain in favor of ovarian fossa deep infiltrating endometriosis (DIE).
2.4. Magnetic resonance imaging
MRI was performed for all patients after 4 hours of fasting with semifilled bladder, before and after the injection of gadolinium contrast medium at the dose of 0.01 mmol/kg, using 1.5 Tesla (Avento Seimens Machine, Erlangen, Germany) machine through the body pelvic but not endovaginal coil. For better delineation of rectal and vaginal walls, 60 cm3 lubricant gel was inserted into the vaginal cuff and 1 ampule of hyoscine was injected intramuscularly. To capture details on anatomy and pathology, the protocols comprised axial, coronal, and sagittal T1- and T2-weighted images. T1 axial and sagittal fat saturation technique with and without contrast were also performed. The bladder wall and rectovaginal septum were evaluated in T2 sagittal and axial images. Uterosacral ligaments and rectal wall were mostly evaluated in the coronal and axial T2-weighted images. Endometriomas were characterized by high signal in T1- and low signal in T2-weighted images. On the contrary, DIE was low signal or signal void in T2-weighted images. Thickening of the walls were in favor of involvements (Fig. 3). All MRI evaluations were reported by a board-certified radiologist with MRI fellowship, blinded to patients’ history and physical examination.
Figure 3.
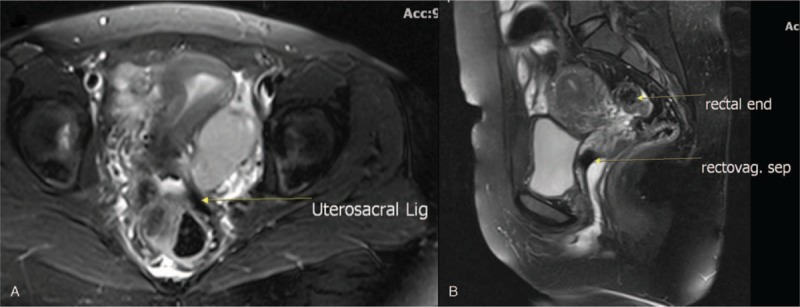
Magnetic resonance imaging (MRI) characteristics of deep infiltrative endometriosis (DIE); axial T2-weighted images of the pelvic cavity demonstrating low signal intensity on the uterosacral ligament in favor of DIE (A); a lesion in rectovaginal septum with hypointese signal change in sagittal T2-weighted MRI in favor of DIE of the region (B).
2.5. Laparoscopy
All operative laparoscopy interventions were performed by the same gynecologist (SA) after whole bowel prep under general anesthesia using the endoscopic instruments. He was aware of the TVS and TRS results but unaware of the MRI results and thus the surgical planning was based on the sonography results. Uterine manipulation was not used and hystrometer was the only applied measure. The pelvis was systematically assessed in all laparoscopies performed following the routine protocol at our center.[9,10] The pelvic cavity was explored and endometriosis was classified according to the revised American Society for Reproductive Medicine classification.[23] The lesion location was matched intraoperatively with TVS and TRS results. Depending on the pathology location, pararectal and paravesical and rectovaginal spaces were dissected when necessary. All adhesions were released with sharp dissection and all DIE-suspected lesions were resected to possibly restore normal anatomic relations. The excised tissues were then sent for pathologic investigation. Meticulous hemostasis was achieved with bipolar coagulation and when necessary by suturing. For rectal lesions, pararectal and rectovaginal spaces were dissected and inspected for suspicious areas. The suspicious lesions were excised, disk resection or segmental resection was done, and reanastomosis of bowel was performed where necessary. Ureterolysis and excision/reanastomosis were done in patients with extrinsic and intrinsic ureteral lesions, respectively. In patients who presented with bladder lesions, based on the depth of lesions, either shaving or partial cystectomy was performed. Colorectal surgeon and urologist were to be involved in the event of notable colorectal and urinary system lesions.[3] Laparoscopy with histopathologic examination was considered the criterion standard for diagnosis of DIE.
2.6. Histopathologic evaluation
All the biopsies were studied in laboratory after hematoxylin and eosin staining by the same pathologist who was unaware of the patients clinical and imaging findings. Diagnosis of endometriosis was confirmed for all resected tissue samples after evaluating both glands and stroma.
2.7. Statistical analysis
The sample size was calculated according to the formula n = Z2pq/d2, based on the binomial distribution. In this formula, n stands for the minimum required population and p represents the attributable accuracy of MRI; q = 1 – p. The P value was considered to be .73 according to a previous study.[15] The precision of the estimates, d, was set at 5%, and Z (the normal deviate) was given a value of 1.96. By solving this formula for n, it was shown that 302 individuals were needed for the study. In order to compensate for nonevaluable patients, we included 317 patients. All statistical analyses were performed with the Statistical Package for Social Sciences version 18.0 (SPSS Inc, Chicago, IL). The results are expressed as mean ± standard deviation or proportions. The definitive diagnosis of DIE as well as the size and location of the pathology were defined based on the laparoscopic findings and histopathologic examination. Meanwhile, findings from the preoperative imaging techniques were compared with surgical observations as the criterion standard of diagnosis. In addition, each modality was assessed for its sensitivity, specificity, negative (NPV), and positive predictive values (PPV), as well as the accuracy. We also calculated the positive and negative likelihood ratios (LR+ and LR−). The sensitivity and specificity was compared between 2 study groups using Mann–Whitney test. A 2-sided P value of <0.05 was considered statistically significant.
3. Results
We assessed >500 patients for eligibility of whom 317 (only nonvirgins) were enrolled in this study through consecutive sampling method. The mean age of the subjects was 31 ± 5.4 (ranging from 19 to 49). The diagnosis of DIE was confirmed in 252 (79.5%) patients. These 252 patients had total number of 350 DIE lesions in different locations. The baseline characteristics of the patients as well as the laparoscopic and histopathologic examination results are summarized in Table 1.
Table 1.
The baseline characteristics of 317 patients suspected for deep infiltrative endometriosis enrolled in current study.
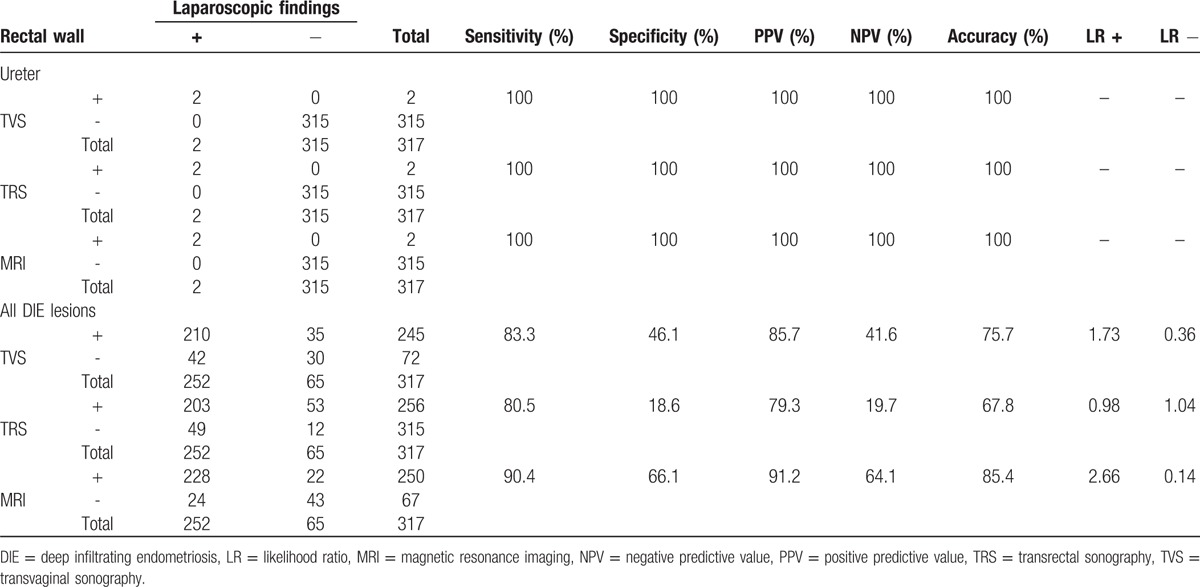
Of the 317 patient, 245 (77.3%) had DIE lesions on TVS examination, and 210 of them had positive pathologic findings (PPV = 85.7%). A total of 72 women showed normal findings on the TVS examination, and 30 of them had normal pathology (NPV = 41.6%). However, 42 of the 72 women with normal TVS examinations had pathologic abnormalities. The sensitivity and specificity of TVS for diagnosis of DIE were 83.3% and 46.1%, respectively. The diagnostic accuracy of TVS, TRS, and MRI for DIE in different locations is summarized in Table 2 . As demonstrated, MRI had higher sensitivity and accuracy compared to TVS and TRS in diagnosis of DIE.
Table 2.
The diagnostic accuracy of the transvaginal sonography, transrectal sonography, and magnetic resonance imaging for deep infiltrative endometriosis of different locations in 317 patients with suspected symptoms.
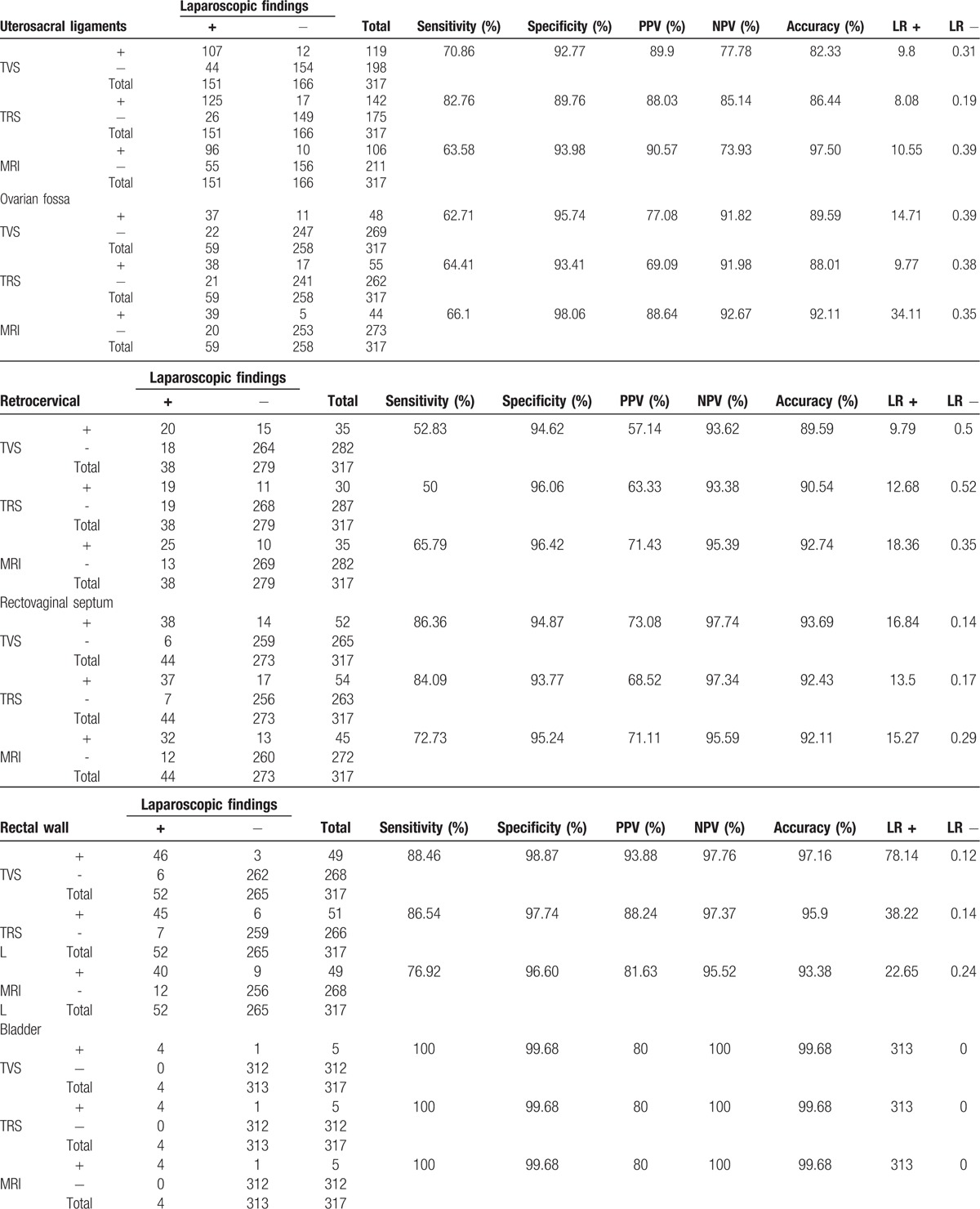
We also compared the diagnostic accuracy of TVS, TRS, and MRI in different locations to determine the best diagnostic modality (Table 3). The sensitivity of TRS, TVS, and MRI in uterosacral ligament DIE was 82.8%, 70.9%, and 63.6%, respectively. On the contrary, specificity had a reverse trend and was favoring MRI (93.9%, 92.8%, and 89.8% for TVS and TRS, respectively). For rectovaginal septum DIE, the sensitivity and accuracy were comparable in TVS, TRS, and MRI (86.4%, 93.7% vs 84%, 92.4% and 72.7%, 92.1%, respectively). Similarly, the specificity of MRI was comparable to TVS and TRS (95.2% vs 94.9% and 93.8%, respectively). MRI was found to be superior to TRS and TVS in terms of sensitivity, specificity, and accuracy for retrocervical DIE lesions (Table 3). For bladder and uretral DIE, the 3 modalities were comparable.
Table 2 (Continued).
The diagnostic accuracy of the transvaginal sonography, transrectal sonography, and magnetic resonance imaging for deep infiltrative endometriosis of different locations in 317 patients with suspected symptoms.
4. Discussion
In the present study, we investigated the diagnostic accuracy of 3 noninvasive modalities for DIE. Although laparoscopy and histopathologic examination is the criterion standard for DIE, but it is invasive and requires preoperative planning. MRI, TVS, and TRS are noninvasive methods, which are available and feasible. In addition, early diagnosis of DIE is an important predictor of outcome and quality of life as well as fertility.[24] In this large series of patients with symptoms of infiltrative endometriosis, we found that the diagnostic accuracy of MRI was higher than TVS and TRS in diagnosis of DIE especially in rectovaginal and ureter locations. But the TVS and TRS both had high diagnostic accuracy for DIE indicating them as appropriate modalities of choice for DIE. Taking into account the fact that TRS could be performed in virgin individuals where TVS is not applicable, TRS remains an important noninvasive modality for diagnosis of DIE.
Several previous studies have investigated the diagnostic accuracy of these modalities with various results.[7,8,14–16,18,19,21,25–32] We have summarized the results of these studies in Table 4. As demonstrated there is a wide variability regarding the diagnostic accuracy of these modalities between different studies mainly because of variability of techniques and experience. The diagnostic accuracy also varies between different anatomic locations. Abrao et al[7] demonstrated that TVS has better sensitivity, specificity, NPV, PPV, and accuracy than MRI for diagnosis of rectovaginal sonography. In contrast, our study found MRI to have a better diagnostic performance than TVS (sensitivity of 65.8% and 52.6%, specificity of 96.4% and 94.6%, PPV of 71.4% and 57.1%, NPV of 95.4% and 93.6%, and accuracy of 92.7% and 89.6%, respectively) for rectovaginal septum DIE. In addition, Bazot et al[8] demonstrated that the diagnostic accuracy of the TVS was higher than MRI and TRS for rectovaginal septum DIE.
Table 3.
Comparing the diagnostic accuracy of transvaginal sonography, transrectal sonography, and magnetic resonance imaging in diagnosis of deep infiltrating endometriosis of various locations in 317 patients.
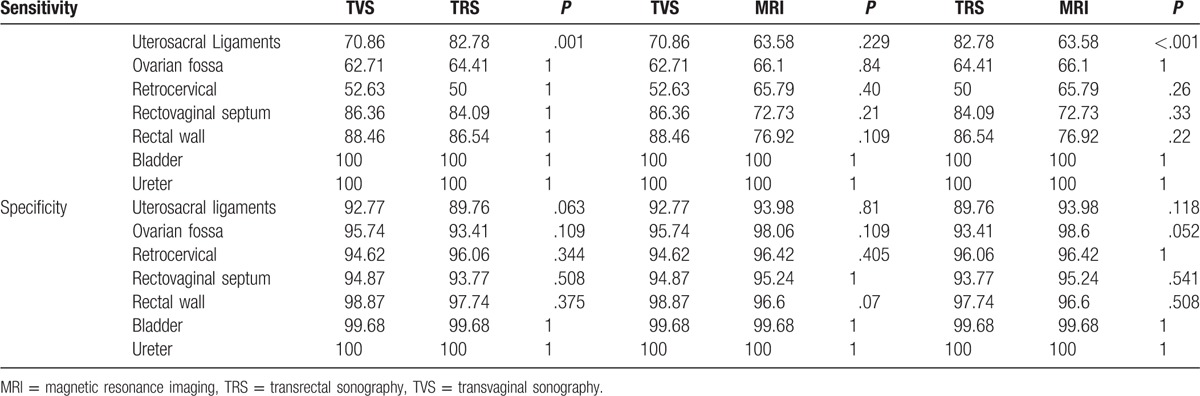
Table 4.
Comparing the diagnostic accuracy of magnetic resonance imaging, transvaginal sonography, and transrectal sonography in deep infiltrating endometriosis being reported in different studies.
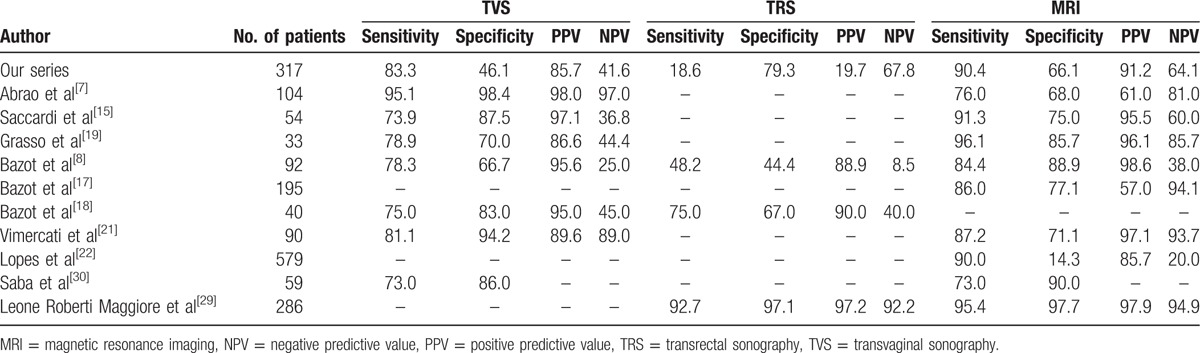
Previously Bazot et al[8] reported that TRS was inaccurate for diagnosis of uterosacral ligament DIE, whereas we demonstrated that TRS has appropriate diagnostic accuracy in different locations comparable to TVS. The main advantage of TRS is that it could be performed in virgin individuals where TVS in not applicable. In line with our findings, Bazot et al[8] demonstrated that TVS was a little less sensitive (78.3%) than MRI (84.4%) for the diagnosis of uterosacral ligament DIE. Dysmenorrhea or deep dyspareunia were always present, but physical examination was negative in more than one third of patients.[8] These data support the use of MRI for all symptomatic patients, even when physical examination and TVS are not contributive, although cost-effectiveness studies are needed. Accordingly, Guerriero et al[33] suggested a new approach entitled “tenderness-guided” to be used systematically to increase the value of TVS for the diagnosis of DIE.
Concerning bladder DIE, our study suggested similar results for the 3 modalities. Fedele et al[13] reported TVS superiority to MRI and transabdominal sonography in determining site of the lesions; however, they studied a small series of patients and their investigation dates back to the time when the use of these modalities for the diagnosis of DIE were in their prime.[13,34] Meanwhile, our findings were in agreement with other reports,[8,17,18] introducing TVS as an accurate method in the diagnosis of DIE within bladder. An earlier study by Grasso et al[19] found the sensitivity of 83.3%, specificity of 100%, PPV of 100%, and NPV of 92.5% for MRI in detection of bladder DIE, whereas our investigation reported a higher sensitivity (100%) and lower precision (PPV = 80%) for the same. According to Balleyguier et al,[16] MRI provides more accurate results than TVS in detecting the DIE lesions of bladder, especially in deeply extended posterior DIEs and in small lesions missed by TVS. Our study supported the findings from a recent investigation which reported the sensitivity of 100%, specificity of 96.8%, PPV of 72.7%, and NPV of 100% for TVS.[34]
The observed TVS, TRS, and MRI sensitivity of 100% for the detection of ureteral DIE in our study, was not aligned with the results from Chamie et al[28] and Grasso et al[19] (MRI sensitivity of 66.6% and 50%, respectively). Bazot et al[8] suggested using TVS as the first-line screening imaging technique and save MRI for symptomatic women with normal TVS findings, and ultimately use TRS for the individuals who have discrepancy in TVS and MR results. Interestingly, our results found that TRS retains a comparable sensitivity to TVS and MRI considering its total performance in the diagnosis of DIE lesions (sensitivity of 81.1% vs 80.1% and 77.9%, respectively). Being less costly, though with a comparable sensitivity to MRI, TRS can be considered as a modality of choice for the diagnosis of DIE before MRI. Moreover, given the limitation of using TVS for virgin subjects mainly in our practice, we propose TRS as a reasonable alternative for diagnosing DIE when a transvaginal approach is not available or acceptable. Moreover, TRS is the modality of choice for virgin individuals in whom using TVS is not acceptable.
In conclusion, the results of the present study demonstrated that TVS and TRS have appropriate diagnostic accuracy in diagnosis of DIE comparable to MRI. Given the comparable performance of TVS and TRS in diagnosing DIE and their availability and affordability compared to MRI, they are both considered as reasonable diagnostic modalities, whereas TRS is preferred in virgin individuals. Accordingly, MRI could be considered as the modality of choice for preoperative diagnosis and planning of patient with DIE. All these 3 modalities are experience dependent and their interpretation depends on the interpreter.
Acknowledgments
The authors would like to thank all the patients and their families who participated in the present study. We also would like to acknowledge the editorial assistant of Diba Negar Research Institute. This article is the result of a thesis project submitted to Shiraz school of medicine in partial fulfillment of the requirements for specialty in Obstetrics and Gynecology by Dr. Mahboobeh Kazemi.
Footnotes
Abbreviations DIE = deep infiltrating endometriosis, LR = likelihood ratio, MRI = magnetic resonance imaging, NPV = negative predictive value, PPV = positive predictive value, TRS = transrectal sonography, TVS = transvaginal sonography.
This study was funded by a grant from Vice Chancellor of research of Shiraz University of Medical Sciences (grant number 83-798).
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
None of the authors have any conflict of interest to declare regarding the manuscript.
References
- [1].Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the united states: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest 2017;82:453–61. [DOI] [PubMed] [Google Scholar]
- [2].Chapron C, Fauconnier A, Vieira M, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod 2003;18:157–61. [DOI] [PubMed] [Google Scholar]
- [3].Benacerraf BR, Groszmann Y, Hornstein MD, et al. Deep infiltrating endometriosis of the bowel wall: the comet sign. J Ultrasound Med 2015;34:537–42. [DOI] [PubMed] [Google Scholar]
- [4].Raiza L, Bianchi P, Cordioli E, et al. Prevalence of sonographic signs of deep infiltrative endometriosis among women submitted to routine transvaginal sonography. J Minim Invasive Gynecol 2016;23:S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod 2014;29:1666–76. [DOI] [PubMed] [Google Scholar]
- [6].Johnston JL, Reid H, Hunter D. Diagnosing endometriosis in primary care: clinical update. Br J Gen Pract 2015;65:101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abrao MS, Goncalves MO, Dias JA, Jr, et al. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod 2007;22:3092–7. [DOI] [PubMed] [Google Scholar]
- [8].Bazot M, Lafont C, Rouzier R, et al. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 2009;92:1825–33. [DOI] [PubMed] [Google Scholar]
- [9].Alborzi S, Keramati P, Younesi M, et al. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril 2014;101:427–34. [DOI] [PubMed] [Google Scholar]
- [10].Alborzi S, Momtahan M, Parsanezhad ME, et al. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril 2004;82:1633–7. [DOI] [PubMed] [Google Scholar]
- [11].Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod 2011;26:274–81. [DOI] [PubMed] [Google Scholar]
- [12].Kondo W, Bourdel N, Tamburro S, et al. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG 2011;118:292–8. [DOI] [PubMed] [Google Scholar]
- [13].Fedele L, Bianchi S, Raffaelli R, et al. Pre-operative assessment of bladder endometriosis. Hum Reprod 1997;12:2519–22. [DOI] [PubMed] [Google Scholar]
- [14].Ito TE, Abi Khalil ED, Taffel M, et al. Magnetic resonance imaging correlation to intraoperative findings of deeply infiltrative endometriosis. Fertil Steril 2017;107:e11–2. [DOI] [PubMed] [Google Scholar]
- [15].Saccardi C, Cosmi E, Borghero A, et al. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2012;40:464–9. [DOI] [PubMed] [Google Scholar]
- [16].Balleyguier C, Chapron C, Dubuisson JB, et al. Comparison of magnetic resonance imaging and transvaginal ultrasonography in diagnosing bladder endometriosis. J Am Assoc Gynecol Laparosc 2002;9:15–23. [DOI] [PubMed] [Google Scholar]
- [17].Bazot M, Darai E, Hourani R, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 2004;232:379–89. [DOI] [PubMed] [Google Scholar]
- [18].Bazot M, Detchev R, Cortez A, et al. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod 2003;18:1686–92. [DOI] [PubMed] [Google Scholar]
- [19].Grasso RF, Di Giacomo V, Sedati P, et al. Diagnosis of deep infiltrating endometriosis: accuracy of magnetic resonance imaging and transvaginal 3D ultrasonography. Abdom Imaging 2010;35:716–25. [DOI] [PubMed] [Google Scholar]
- [20].Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2011;37:257–63. [DOI] [PubMed] [Google Scholar]
- [21].Vimercati A, Achilarre MT, Scardapane A, et al. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2012;40:592–603. [DOI] [PubMed] [Google Scholar]
- [22].Lopes L, Hindman N, Huang K. Accuracy of magnetic resonance imaging (MRI) in the diagnosis of endometriosis—evaluation of an institutional protocol. J Minim Invasive Gynecol 2015;22:S52–4. [DOI] [PubMed] [Google Scholar]
- [23].Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–21. [DOI] [PubMed] [Google Scholar]
- [24].Dubernard G, Piketty M, Rouzier R, et al. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod 2006;21:1243–7. [DOI] [PubMed] [Google Scholar]
- [25].Bazot M, Malzy P, Cortez A, et al. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007;30:994–1001. [DOI] [PubMed] [Google Scholar]
- [26].Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med 2012;31:651–3. [DOI] [PubMed] [Google Scholar]
- [27].Chamie LP, Blasbalg R, Goncalves MO, et al. Accuracy of magnetic resonance imaging for diagnosis and preoperative assessment of deeply infiltrating endometriosis. Int J Gynaecol Obstet 2009;106:198–201. [DOI] [PubMed] [Google Scholar]
- [28].Chamie LP, Blasbalg R, Pereira RM, et al. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics 2011;31:E77–100. [DOI] [PubMed] [Google Scholar]
- [29].Goncalves MO, Dias JA, Jr, Podgaec S, et al. Transvaginal ultrasound for diagnosis of deeply infiltrating endometriosis. Int J Gynaecol Obstet 2009;104:156–60. [DOI] [PubMed] [Google Scholar]
- [30].Leone Roberti Maggiore U, Biscaldi E, Vellone GV, et al. Magnetic resonance enema versus rectal water contrast transvaginal ultrasonography in the diagnosis of rectosigmoid endometriosis. Ultrasound Obstet Gynecol 2016;49:524–32. [DOI] [PubMed] [Google Scholar]
- [31].Saba L, Guerriero S, Sulcis R, et al. MRI and “tenderness guided” transvaginal ultrasonography in the diagnosis of recto-sigmoid endometriosis. J Magn Reson Imaging 2012;35:352–60. [DOI] [PubMed] [Google Scholar]
- [32].Kasraeian M, Asadi N, Ghaffarpasand F, et al. Value of transvaginal ultrasonography in endometrial evaluation of non-bleeding postmenopausal women. Climacteric 2011;14:126–31. [DOI] [PubMed] [Google Scholar]
- [33].Guerriero S, Ajossa S, Gerada M, et al. Tenderness-guided” transvaginal ultrasonography: a new method for the detection of deep endometriosis in patients with chronic pelvic pain. Fertil Steril 2007;88:1293–7. [DOI] [PubMed] [Google Scholar]
- [34].Exacoustos C, Malzoni M, Di Giovanni A, et al. Ultrasound mapping system for the surgical management of deep infiltrating endometriosis. Fertil Steril 2014;102:143.e142–50.e142. [DOI] [PubMed] [Google Scholar]


