Abstract
Background:
Listening to natural sounds is applied in health contexts in order to induce relaxation. However, it remains unclear whether this effect is equally efficacious in all individuals or whether it depends on interindividual differences. Given that individuals differ in how they are impaired by somatic complaints, we investigated whether somatic complaints moderate the stress-reducing effect of listening to water sounds.
Methods:
Sixty healthy women (Mage = 25 years) were randomly allocated to 3 different conditions (listening to water sounds, a relaxing piece of music, or no auditory stimulus: n = 20 per condition) for 10 minutes before they were exposed to a standardized psychosocial stress task. Salivary cortisol was assessed before, during, and after the stress task. For binary logistic regression analyses, participants were divided into 2 groups: 1 group with a high salivary cortisol release and 1 group with low cortisol release. The Freiburg Complaints Inventory was used to assess occurrence of somatic complaints.
Results:
A significant moderating effect of somatic complaints on cortisol secretion was found in the group listening to water sounds (χ2(1) = 5.87, P < .015) but not in the other 2 groups, explaining 35.7% of the variance and correctly classifying 78.9% of the cases.
Conclusion:
The stress-reducing effect of listening to water sounds appears to depend on the occurrence of somatic complaints. This effect was not found in the music or silence condition. Individuals with somatic complaints may benefit from other, potentially more powerful forms of stress-reducing interventions, that is, combinations of visual and auditory stimuli.
Trial Registration:
not applicable (pilot study)
Keywords: acoustic stimulation, cortisol, somatic complaints, stress, water sounds
1. Introduction
Exposure to nature, such as a walk in a forest or having a scenic window view, has been found to exert beneficial effects on human beings. This includes increases in mood and self-esteem, faster recovery from surgery, and physiological and psychological stress relief.[1,2] While the positive impact of being in nature or the visual perception of it is well established, much less is known about the effect of simply listening to natural sounds.
The few existing studies that applied natural sounds in different settings, that is, during an operation,[3] before[4] or after a stressful task in the laboratory[5,6] report meaningful stress-reducing effects. In the study by Arai et al,[3] the patients underwent an operation under epidural anesthesia. They were assigned to either listening to natural sounds or they had no acoustic stimulation intraoperatively. It was reported that the activity of salivary alpha-amylase, a surrogate marker for the sympathetic nervous system (SNS) (e.g. [7]) was significantly lower in the condition with natural sounds as compared to the nonacoustic condition. Alvarsson et al[5] examined whether listening to natural sounds, as compared to noises, after a stress-provoking task was effective in reducing stress, as measured via skin conductance level (SCL) and high frequency heart rate variability (HF HRV). The authors found a faster recovery in the natural sounds condition in SCL, but not in HF HRV. It was concluded that listening to natural sounds after stress exposure is facilitating sympathetic, but not parasympathetic, recovery.[5] Annerstedt et al[6] examined stress recovery processes of natural sounds in combination with a virtual green environment (VGE). Increased parasympathetic activity was found in individuals who were listening to natural sounds in the condition with VGE after stress induction, but not in those individuals who were in the condition with VGE without sound or in control individuals who were neither exposed to VGE nor natural sounds. Finally, in an experimental study conducted by our own research group, we found that listening to water sounds before a major stress situation significantly reduced the subsequent cortisol response, an indicator for the hypothalamus-pituitary-adrenal axis activation.[4] Together, these studies suggest a stress-reducing effect of natural sounds, as reflected by changes in the endocrine and the autonomous nervous system. This implies that listening to natural sounds may be a simple and easily accessible intervention that is capable of positively affecting the major human stress systems.
Given the fact that only little is known about the effect of natural sounds, it remains unclear whether the stress-reducing effect of natural sounds is universal (i.e., biophilia hypothesis) or whether it depends on interindividual differences, as is the case for music (e.g., [8]). The biophilia hypothesis posits that human beings subconsciously feel attracted to and seek nature,[9] which would imply that natural stimuli have a comparable, most likely inherent effect on all human beings. However, it is also assumed that mental or somatic conditions that affect perception, cognitive or emotional processing, or functioning of the stress systems, such as chronic stress (e.g., [10]), depressive symptomatology (e.g., [11]), or rumination (e.g., [12]) may moderate the stress-reducing effect of natural sounds. Of particular interest in this regard are somatic complaints, as they increase attention to one's own body,[13,14] in the form of an attentional or cognitive bias,[15] which might impede attention or concentration on the potentially relaxing stimulus. For instance, in a study by Eccleston et al[16], it was found that pain patients with high somatic awareness showed more attention deficits compared to those with low somatic awareness. Given that natural sounds are applied in health-related contexts, the knowledge of whether individuals with more somatic complaints benefit less from natural sounds would be of practical relevance.
To test whether somatic complaints moderate the stress-reducing effect of natural sounds, we conducted a secondary analysis of an existing data set[4] comparing healthy female participants who benefited more from the water sounds (as indicated by a lower cortisol sensitivity to the stress task) with those women who benefitted less. We hypothesized that those participants who benefited less from the stress-reducing effect of listening to water sounds are also those reporting more somatic complaints. To test whether this effect is specific to the stress-reducing effect of water sounds, exploratory analyses were computed for a music and a silence condition.
2. Methods
2.1. Characteristics of participants
Participants were considered eligible for the study if they met the following inclusion criteria: female gender, healthy body mass index (BMI), age (20–30 years), language (native Swiss German), and having a regular menstruation cycle. Female gender has been chosen as men and women differ in the stress-induced cortisol response[17,18] and the emotional and physiological response to music.[19,20]
Exclusion criteria were physical illnesses or mental disorders, use of medication (including hormonal contraceptives), and drugs. If eligibility was met, an appointment was scheduled. Participants were examined in the follicular phase of their menstrual cycle.
2.2. Design
The design of the complete study has been described extensively in the original article.[4] In the following section, we provide a short summary of the essential details of the study.
2.3. Study procedure
Sixty female participants were randomly assigned to 1 of 3 different conditions, resulting in 20 participants per group. Depending on the assigned experimental condition, they either listened to water sounds, music, or rested in silence prior to the stress induction. For the induction of a strong psychobiological stress response, all participants were subjected to the Trier Social Stress Test (TSST).[21] After arrival, participants were informed orally and in writing about the purpose and procedure of the study. After providing informed consent, a first saliva sample was taken (baseline measurement of cortisol, 30 minutes prior the onset of the TSST, t1, −30 minutes). Further samples were taken after the participants were placed in separate rooms (-20 minutes) where they listened to water sounds, music, or rested in silence for ten minutes (t2, −5 minutes); samples were taken again after the actual stress task (t3, +10 minutes), as well as 15 minutes (t4, + 25 minutes), 30 minutes (t5, + 40 minutes), 45 minutes (t6, + 55 minutes), and 60 minutes (t7, + 70 minutes) after the TSST.
All participants provided written informed consent. Ethics committees of the University of Zurich and of the Canton of Zurich approved the study protocol.
2.4. Experimental stimuli
Water sounds stimulus
In the original study, listening to water sounds was included as a control stimulus. Water sounds (unlike music) lacks melody and rhythm, but can still be listened to without boredom or stress.[19]
Music stimulus
A single standardized relaxing music stimulus (“Miserere” by Allegri) was chosen.[20] Participants were unfamiliar with this music piece.
Resting in silence
Participants sat in silence.
Participants received no further instructions prior to being exposed to the stimuli. Participants rated both acoustic stimuli as likable and relaxing using rating scales (as previously reported in[4]).
3. Material
Depressive symptomatology was assessed with the Beck Depression Inventory (BDI).[22] Chronic stress over the last 3 months was assessed with the Trier Inventory for the Assessment of Chronic Stress (TICS).[23] The Freiburg Complaint List (Freiburger Beschwerdeliste, FBL)[24] was used to assess somatic complaints clustered in ten syndromes (general condition, emotional reactivity, cardiovascular, gastrointestinal, head–throat syndrome, tenseness, sensory, pain, motor function, skin). The frequency of 55 somatic complaints (e.g., “do you have pain in the legs?”) was rated on a frequency scale comprised of 5 categories (“almost every day,” “about 3 times/week,” “about 2 times/month,” “about 2 times/year,” “hardly ever”). In addition, 25 somatic complaints (e.g., “are you sensitive to cold?”) were rated on an intensity scale. For the statistical analyses, sum scores for the ten syndromes and a total score of somatic complaints (= sum of all ten scores) were calculated.
3.1. Biological assessment
Small cotton rolls (Salivettes, Sarstedt, Sevelen, Switzerland) were used for the collection of stimulated saliva for the analysis of salivary free cortisol. Until biochemical analysis, samples were stored at −20°C. A commercial chemiluminescence immunoassay (LIA) (IBL, Hamburg, Germany) was used to determine cortisol. Inter- and intra-assay coefficients of variation were below 10%. All samples from each participant were analyzed in the same run.
3.2. Statistical analyses
All statistical analyses were performed using SPSS Statistics 23 for Mac OSX (IBM, Chicago, IL). A measure for area under the curve with respect to increase (AUCI) was calculated for cortisol to receive an index for cortisol stress sensitivity, which will be referred to as AUCI sensitivity from now on. The AUCI was calculated according to the formula provided by Pruessner et al.[25] The AUCI typically includes several time points and is calculated with respect to the first value (= difference between the baseline and subsequent measures) and is thus a measure that is indicative of a system's sensitivity.[25] We included the four measurement time points from T1, −30 minutes (= baseline), to T4, +25 (= peak) to receive an index that is particularly sensitive to the reactivity of the system, in analogy to cortisol reactivity, which is a delta measure (peak minus baseline values) (e.g., [26]). We observed a pattern of high versus low-reactive individuals. We interpreted a negative cortisol response, that is, a decrease in cortisol relative to the baseline (= AUCI with a negative value, index of decrease) (see [25]), as the low cortisol responding group (LOW CORT); and a positive cortisol response, that is, an increase in cortisol relative to the baseline (= AUCI with a positive value), as the high cortisol responding group (HIGH CORT).
To test whether LOW and HIGH CORT groups significantly differed with regard to cortisol stress sensitivity, univariate analyses were conducted. Independent t tests were computed for the assessment of differences in the FBL scores between groups. To test whether somatic complaints were predictive for cortisol AUCI sensitivity, a binary logistic regression was computed. For the exploratory analysis of whether this effect is specific to water sounds, we computed univariate analysis of variance (ANOVA).
4. Results
4.1. Sample characteristics
All sixty participants (Mage = 25.3 years, SD = 3.21) were included in the secondary analyses for this report. Groups did not differ with regard to basic characteristics, such as age, BMI, depression (as measured with the BDI), or chronic stress (as measured with the TICS) (see [4]). The HIGH and LOW CORT participants in the water sounds condition (n = 20) did not differ with regard to participant characteristics (all n.s., data not shown).
The following presented analyses are based on data of the water sound condition (n = 20) to test whether somatic complaints moderate the stress-reducing effect of water sounds.
4.2. Cortisol response
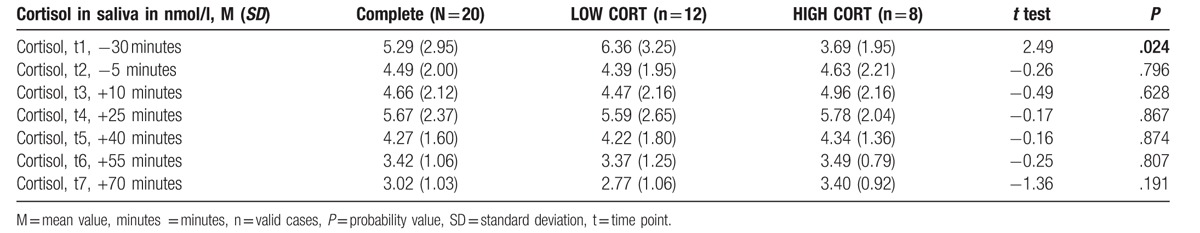
The stress task induced a significant cortisol stress response in our participants (see [4]). The HIGH CORT participants (n = 8, M = 74.56 nmol/L, SD = 79.65, 95% CI [25.58 to 123.54]) in the water sounds condition had a significantly higher AUCI sensitivity: F(1/19) = 16.23, P = .001) as compared to the LOW CORT participants (n = 12, M = -46.70 nmol/L, SD = 55.48, 95% CI [−86.69 to −6.71]). The 2 groups did not differ with regard to their mean cortisol levels per measurement time points with the exception of t1, baseline (Table 1).
Table 1.
Values for cortisol separately for each measurement time point for the complete water sound group (n = 20), and for the low and high cortisol responders (means, standard deviations).
4.3. Comparison of somatic complaints between high and low cortisol responders
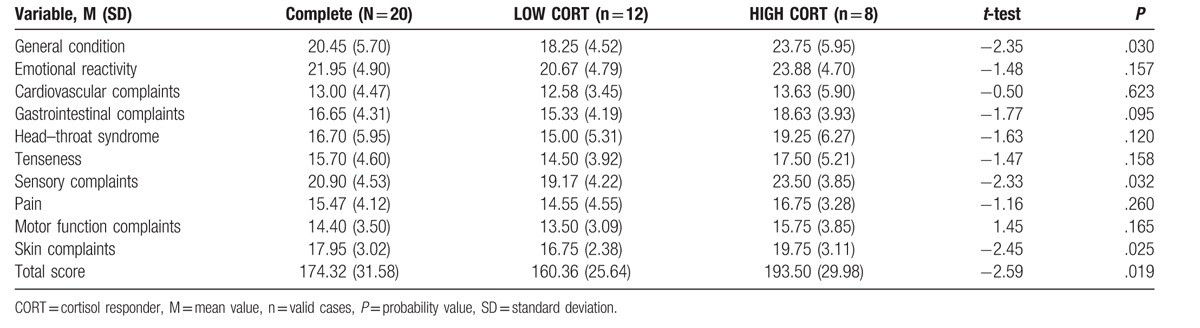
The HIGH CORT participants showed significantly higher scores in the FBL total score (t = −2.59, P = .019), as well as in the subscales “general condition” (t = −2.35, P = .030), “sensory complaints” (t = −2.33, P = .032), and “skin complaints” (t = −2.45, P = .025), compared to the LOW CORT participants (Table 2). No significant differences in the FBL total score or in the FBL subscales were found between HIGH and LOW CORT participants in the music or silence condition (not shown here).
Table 2.
Values for the Freiburg Complaints List subscales presented separately for the complete water sounds group, and for the low and high cortisol responders (means, standard deviations); and group differences (t test, P-value) for each subscale, and the total score.
4.4. Prediction of cortisol AUCI sensitivity in the water sounds condition
The model which included the total score of somatic complaints (FBL) as a predictor explained 35.7% of the variance in cortisol AUCI sensitivity (Nagelkerke R2) and correctly classified 78.9% of the cases (χ2(1) = 5.87, P = .015). The Hosmer–Lemeshow goodness of fit test indicates that the model is correctly specified (χ2(8) = 5.90, P = .659). Participants with more somatic complaints were slightly more likely to exhibit an increased cortisol AUCI sensitivity (B = 0.043, SE = 0.021, Wald = 4.18, P = .041, 95% CI 1.00 to 1.09).
We then computed separate binary logistic regression analyses for the FBL subscales; first testing the predictive effect of those subscales in which the groups differed significantly (skin, sensory, and general condition). The model which included the FBL subscale skin explained 31.8% of the variance and correctly classified 80% of the cases (χ2(1) = 5.37, P = .021). Participants with more skin complaints were 50% more likely to exhibit an increased cortisol AUCI sensitivity (B = 0.41, SE = 0.202, Wald = 4.10, P = .043, 95% CI 1.01 to 2.23).
In a second model, we included the FBL scale sensory as a predictive factor. This model explained 30.3% of the variance and correctly classified 70% of the cases (χ2(1) = 5.08, P = .024). Participants with more sensory complaints were 31% more likely to exhibit an increased cortisol AUCI sensitivity (B = 0.27, SE = 0.14, Wald = 3.87, P = .049, 95% CI 1.00 to 1.71).
The model which included the FBL scale general condition explained 30.1% of the variance and correctly classified 70% of the cases (χ2(1) = 5.04, P = .025). Participants with more general somatic complaints were, with a statistical trend, 24% more likely to exhibit an increased cortisol AUCI sensitivity (B = 0.21, SE = 0.11, Wald = 3.67, P = .055, 95% CI 1.00 to 1.53).
We also computed regression analyses for the FBL subscales with no significant group differences. Computed models and predictors were all non-significant (data not shown).
4.5. Exploratory analyses: test for specificity
We calculated all of the above described analyses for the music and silence condition. All analyses were non-significant (not described here). To test for the specificity of the effect, we ran a univariate ANOVA with the FBL total score, the experimental conditions, as well as an interaction term (experimental conditions∗FBL total score) as variables. Neither of the entered variables were significantly related to AUCI (all n.s.). Correspondingly, we ran univariate ANOVAs for all FBL subscales (all n.s.). We additionally ran linear regressions with the predictors: somatic complaints (total score), experimental conditions and an interaction term. Again, we found no meaningful effect of the experimental condition (all n.s.). Correspondingly, we ran linear regressions for all FBL subscales (all n.s.).
5. Discussion
Although staying in a natural surrounding may be an optimal environment for stress relief, it is often of little practical relevance for individuals living in an urban environment. Only recently, some studies have shown that the mere act of listening to natural sounds is capable of inducing a state of relaxation via stress relieving effects within the endocrine and autonomous nervous system.[3–6] It is yet unknown whether all individuals do respond to natural sounds equally, as can be assumed based on the biophilia hypothesis, or rather individually different, such as in the case of music. On the basis of current results from a secondary analysis, we were able to preliminary demonstrate that the stress-reducing effect of water sounds, as indicated by a reduced cortisol output after stress induction, depends on the occurrence of somatic complaints. Healthy individuals with more somatic complaints benefited less from listening to water sounds when compared to individuals with fewer somatic complaints. Exploratory analyses revealed that while the moderating effect of somatic complaints was not found in either the music or the silence condition, analyses of specificity do not suggest a meaningful effect of the experimental condition.
This study provides the first, preliminary evidence for a differential effect of listening to water sounds in healthy female individuals depending on reported somatic complaints. Given the limited number of studies examining the stress-reducing effect of natural sounds, we are left speculating about the reason for this finding. It may be that the increased attention towards somatic complaints[13,14] may impede attention or concentration on the potentially stress-reducing effect by the natural sounds. However, given the fact that the current study is a re-analysis of an existing data set, we did not control, manipulate or measure attention or concentration on the acoustic stimuli. Future studies have to use a study design that help to answer the differing effect of natural sounds.
Our findings suggest the importance of acknowledging even minor somatic complaints when giving medical advice or treating individuals seeking care for somatic complaints, which is a common phenomenon in primary care practices.[27] Around 30%[28] to 64%[29] of the presented somatic complaints are idiopathic, that is, medically unexplained. The impact of medically unexplained complaints is comparable to complaints with a medical explanation.[30] Given the increasing risk for mental disorders, such as depression and anxiety, with a higher number of somatic complaints,[29] it is of utmost importance to find adequate treatment for those individuals. While we still know little about the phenomenon of somatic complaints,[31] stress relief interventions may be effective in the treatment of somatic complaints. Our results suggest that individuals with more somatic complaints may not benefit from the use of natural sounds as relaxation stimulation. It might be the case that these individuals need alternative stimuli in order to induce stress reduction. These alternative stimuli should be designed in a way that would allow subjects not to focus too intensively on their bodily symptoms. This might be achieved by combining an auditory stimulus with additional visual stimulation in form of a corresponding virtual stimulation of the natural environment, as a bimodal stimulation may enhance effects (e.g., [32]).
Limitations of our study include the relatively small size of our subgroups that prevented controlling for multiple-testing and the influence of co-varying factors in the statistical analyses and the very homogeneous sample (only young women in the follicular phase of the menstrual cycle not taking oral contraceptives) which limits generalization and findings cannot be transferred to men. In addition, we chose a between-subjects as compared to a within-subjects study design. Although comparable to other stress studies, cortisol levels at baseline, that is, prior to the actual stress induction, were higher than normally expected in a (nonlaboratory) resting situation. The waiting for an anticipated stressor, in combination with being in a new and unfamiliar environment may be a stressor by itself, which may have accounted for the slightly increased cortisol levels at baseline. However, this applied to all participants in all three conditions. Finally, a limitation of our study is the categorization of participants into 2 groups (HIGH vs LOW), which resulted in smaller subsamples and consequently reduced power. Clearly, our observations should be corroborated in a bigger sample, ideally using dimensional measures of cortisol responses. We hope that our current preliminary findings will inform future research endeavors in this area.
6. Conclusion
In conclusion, with this work we were able to show for the first time, preliminary evidence that the stress-reducing effect of listening to water sounds may dependent on the presence of somatic complaints. Future studies are needed to test our exploratory analyses with regard to the specificity of the moderating effect of somatic complaints on the stress-reducing effect of water sounds using a within-subjects study design in a larger sample.
Footnotes
Abbreviations: AUCI = area under the curve with respect to increase, BDI = Beck Depression Inventory, BMI = body mass index, FBL = Freiburg Complaint List, HF HRV = high-frequency heart rate variability, HIGH CORT = high cortisol responding group, LOW CORT = low cortisol responding group, SCL = skin conductance level, SNS = sympathetic nervous system, TICS = Trier Inventory for the Assessment of Chronic Stress, TSST = Trier Social Stress Test, VGE = virtual green environment.
This study was supported by a grant from the Young Investigator Grant of the University of Zurich, grant no: 56233208 (MVT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Bratman GN, Hamilton JP, Daily GC. The impacts of nature experience on human cognitive function and mental health. Ann N Y Acad Sci 2012;1249:118–36. [DOI] [PubMed] [Google Scholar]
- [2].Velarde MD, Fry G, Tveit M. Health effects of viewing landscapes—landscape types in environmental psychology. Urban Forestry & Urban Greening 2007;6:199–212. [Google Scholar]
- [3].Arai YC, Sakakibara S, Ito A, et al. Intra operative natural sound decreases salivary amylase activity of patients undergoing inguinal hernia repair under epidural anesthesia. Acta Anaesthesiol Scand 2008;52:987–90. [DOI] [PubMed] [Google Scholar]
- [4].Thoma MV, La Marca R, Bronnimann R, et al. The effect of music on the human stress response. PLoS One 2013;8:e70156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alvarsson JJ, Wiens S, Nilsson ME. Stress recovery during exposure to nature sound and environmental noise. Int J Environ Res Public Health 2010;7:1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Annerstedt M, Jönsson P, Wallergård M, et al. Inducing physiological stress recovery with sounds of nature in a virtual reality forest—results from a pilot study. Physiol Behav 2013;118:240–50. [DOI] [PubMed] [Google Scholar]
- [7].Thoma MV, Kirschbaum C, Wolf JM, et al. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol 2012;91:342–8. [DOI] [PubMed] [Google Scholar]
- [8].Cassidy G, MacDonald RA. The effect of background music and background noise on the task performance of introverts and extraverts. Psychol Music 2007;35:517–37. [Google Scholar]
- [9].Kellert S. R., Wilson E. O. (Eds.). (1995). The Biophilia Hypothesis. Island Press. [Google Scholar]
- [10].Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010;35:2–16. [DOI] [PubMed] [Google Scholar]
- [11].Burke HM, Davis MC, Otte C, et al. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 2005;30:846–56. [DOI] [PubMed] [Google Scholar]
- [12].Lyubomirsky S, Kasri F, Zehm K. Dysphoric rumination impairs concentration on academic tasks. Cogn Ther Res 2003;27:309–30. [Google Scholar]
- [13].Eriksen H, Ursin H. Subjective health complaints, sensitization, and sustained cognitive activation (stress). J Psychosom Res 2004;56:445–8. [DOI] [PubMed] [Google Scholar]
- [14].Martin A, Rief W. Relevance of cognitive and behavioral factors in medically unexplained syndromes and somatoform disorders. Psychiatr Clin North Am 2011;34:565–78. [DOI] [PubMed] [Google Scholar]
- [15].Brosschot JF. Cognitive emotional sensitization and somatic health complaints. Scand J Psychol 2002;43:113–21. [DOI] [PubMed] [Google Scholar]
- [16].Eccleston C, Crombez G, Aldrich S, et al. Attention and somatic awareness in chronic pain. Pain 1997;72:209–15. [DOI] [PubMed] [Google Scholar]
- [17].Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med 1992;54:648–57. [DOI] [PubMed] [Google Scholar]
- [18].Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry 2002;52:318–27. [DOI] [PubMed] [Google Scholar]
- [19].McFarland RA, Kadish R. Sex differences in finger temperature response to music. Int J Psychophysiol 1991;11:295–8. [DOI] [PubMed] [Google Scholar]
- [20].Nater UM, Abbruzzese E, Krebs M, et al. Sex differences in emotional and psychophysiological responses to musical stimuli. Int J Psychophysiol 2006;62:300–8. [DOI] [PubMed] [Google Scholar]
- [21].Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76–81. [DOI] [PubMed] [Google Scholar]
- [22].Beck AT. Beck-Depressions-Inventar. Bern: Huber; 1961. [Google Scholar]
- [23].Schulz P, Schlotz W, Becker P. Das Trier Inventar zum chronischen Stress—Manual. Göttingen: Hogrefe; 2004. [Google Scholar]
- [24].Fahrenberg J. FBL–Freiburger Beschwerdeliste. Göttingen: Hogrefe; 1994. [Google Scholar]
- [25].Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–31. [DOI] [PubMed] [Google Scholar]
- [26].Thoma MV, Gianferante D, Hanlin L, et al. Stronger hypothalamus-pituitary-adrenal axis habituation predicts lesser sensitization of inflammatory response to repeated acute stress exposures in healthy young adults. Brain Behav Immun 2017;61:228–35. [DOI] [PubMed] [Google Scholar]
- [27].Jackson JL, Kroenke K. Prevalence, impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med 2008;70:430–4. [DOI] [PubMed] [Google Scholar]
- [28].Khan AA, Khan A, Harezlak J, et al. Somatic symptoms in primary care: etiology and outcome. Psychosomatics 2003;44:471–8. [DOI] [PubMed] [Google Scholar]
- [29].Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics 2011;52:263–71. [DOI] [PubMed] [Google Scholar]
- [30].Klaus K, Rief W, Brähler E, et al. The distinction between “medically unexplained” and “medically explained” in the context of somatoform disorders. Int J Behav Med 2013;20:161–71. [DOI] [PubMed] [Google Scholar]
- [31].Salmon P. Conflict, collusion or collaboration in consultations about medically unexplained symptoms: the need for a curriculum of medical explanation. Patient Educ Couns 2007;67:246–54. [DOI] [PubMed] [Google Scholar]
- [32].Nordahl R. Evaluating environmental sounds from a presence perspective for virtual reality applications. EURASIP Journal on Audio, Speech, and Music Processing 2010;1:426937. [Google Scholar]


