Abstract
Patients with peritoneal metastases (PM) are generally considered incurable; therefore, the presence of PM is a critical factor in deciding between palliative surgery and curative resection as a therapeutic strategy. Previous studies have not determined the predictive value of ascites detected on computed tomography (CT) for the presence of PM. We aimed to analyze the factors that are associated with PM in patients with CT-detected ascites.
A total of 2207 consecutive patients who were diagnosed with gastric cancer between 2004 and 2013 were identified. Eleven patients with liver cirrhosis or chronic renal insufficiency with ascites and 57 patients who received previous treatment were excluded. Ninety-eight patients who had definite evidence of distant metastasis or PM on CT and 64 patients who did not undergo surgery were excluded. A total of 91 patients were enrolled in the study to analyze the association between CT-detected ascites and surgically confirmed PM.
Seventy-six patients underwent curative resection and 15 patients underwent palliative surgery. Twelve patients exhibited peritoneal seeding and 37 patients showed regional lymph node metastasis. Regional lymph node metastasis, advanced gastric cancer, undifferentiated pathology, and the amount of ascites were significantly associated with PM. Multivariable logistic regression analysis identified the amount of ascites to be an independent risk factor for the presence of PM.
Regional lymph node metastasis, advanced gastric cancer, undifferentiated pathology, and the amount of ascites were associated with PM. The amount of ascites was found to be an independent risk factor for PM.
Keywords: ascites, computed tomography, gastric cancer, peritoneal metastases
1. Introduction
Gastric cancer is one of the leading causes of cancer-related deaths in the world,[1,2] and Helicobacter pylori infection is a major cause of gastric cancer.[3] Although the incidence and mortality rates have steadily declined over the past 4 decades, gastric cancer is still the second most common cancer in Korea.[4] As surgical resection is the only curative therapeutic option in the treatment of gastric cancer, accurate preoperative staging of gastric cancer is essential to determine the optimal therapeutic strategy. Computed tomography (CT) is an accurate and effective imaging technique for the preoperative staging of gastric cancer and has been used reliably to detect tumors and to assess metastases to regional lymph nodes, the liver, and distant metastases resulting from gastric cancer.[5–7] However, the sensitivity of CT for the detection of peritoneal metastases (PM) is lower (13%–30%) than its sensitivity for the detection of other distant metastases.[8,9] There are limitations in detecting PM by using CT, and therefore PM are often only detected intraoperatively.[8,10,11] Previous reports have suggested that nodules on the peritoneal surface, soft-tissue stranding in intraabdominal fat, omental cake, and peritoneal thickening with abnormal contrast enhancement are suggestive of PM.[12–14] Because patients with PM are generally deemed incurable, the presence of PM is a critical factor to be considered when choosing between palliative surgery and curative resection as a therapeutic strategy.[15] Some reports have suggested that the presence of ascites on a CT scan suggests the presence of PM and indicates a poor prognosis.[16–18] However, the predictive value of CT-detected ascites for the presence of PM has not been determined.[19] Some studies, which investigated the association between the volume of ascites detected by CT and PM, concluded that there was no significant association between the ascites and PM or survival outcomes.[20,21] However, those studies were limited by small sample sizes. In this study, we aimed to analyze the clinical significance of CT-detected ascites and determine if it is a predictive factor for PM in gastric cancer patients.
2. Materials and methods
2.1. Patients
This study was approved by the Institutional Review Board of Seoul National University Boramae Medical Center. A total of 2207 consecutive patients who were diagnosed with gastric cancer based on histological examinations of endoscopically biopsied gastric mucosal tissues between 2004 and 2013 were identified. Their electronic medical records and CT scans were retrospectively reviewed. Of the 2207 patients, ascites was detected on the CT scans of 321 patients. Eleven patients with liver cirrhosis or chronic renal failure with ascites and 57 patients who underwent previous treatment were excluded from the study. Ninety-eight patients whose CT images showed definite evidence of distant metastases or PM (contrast-enhanced density in peritoneal adipose tissues and nodules on the peritoneum, mesentery, and omentum) and 64 patients who did not undergo surgery were excluded. As a result, 91 patients were included in the study (Fig. 1).
Figure 1.
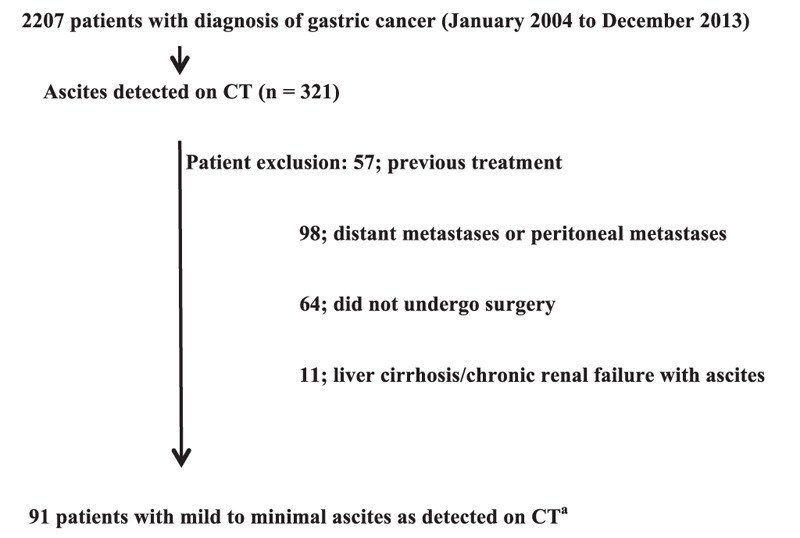
Patients enrolment.
2.2. Radiology
Contrast-enhanced CT examinations were performed on the 91 enrolled patients by using a Somatom Plus-4 scanner (Siemens Medical System, Erlangen, Germany). The helical technique was applied during the scanning of the abdomen and pelvis, and images were reconstructed with 10-mm-thick sections. A total of 80 to 120 mL of iopromide contrast medium (Ultravist 370, Schering, Berlin, Germany) was administered by using a mechanical power injector.
The CT findings were interpreted by an experienced radiologist (YHC). Tumor status, lymph node metastases, evidence of ascites, adjacent organ invasion, distant metastases, and PM were diagnosed. Ascites was diagnosed when a low radiologic density of ≤10 Hounsfield number was detected within the abdominopelvic cavity external to the abdominal or pelvic organs. The volume of ascites was calculated by applying ruler grids to the CT images, as described in previous reports.[19,21] Minimal ascites was defined as an ascites volume of <50 mL, and mild ascites was defined as a volume of 50 to 200 mL.
Based on a previous study, lymph nodes were considered significantly enlarged when the long axis diameter was ≥10 mm.[21] Other criteria for malignant involvement of the lymph nodes included a nearly round shape, loss of the normal fatty hilum, and marked heterogeneous enhancement. The lymph nodes located along the lesser or greater curvatures of the stomach or at the common hepatic, gastric, celiac, and splenic arteries were classified as regional, whereas intraabdominal nodes beyond these regions were defined as distant lymph nodes.[22]
2.3. Statistical analyses
Statistical analyses were performed by using IBM SPSS version 20.0 statistical software (IBM Corp., Armonk, NY). A 2-tailed P < .05 was considered statistically significant. The categorical variables of the CT findings were summarized as frequencies and proportions, and were evaluated by using the χ2 test or Fisher exact test. Continuous variables were expressed as the mean ± standard deviation (SD) and were compared by performing a t test. To assess which variables were related to PM, we conducted a logistic regression analysis with Firth's penalized likelihood, which is used in the presence of small number of events.[23] First, a univariable logistic regression model was developed. Then, variables with P < .20 from the univariable analyses were included in the multivariable logistic regression analysis. The final model was developed using a backward elimination procedure.
3. Results
3.1. Baseline characteristics and clinical and surgical outcomes
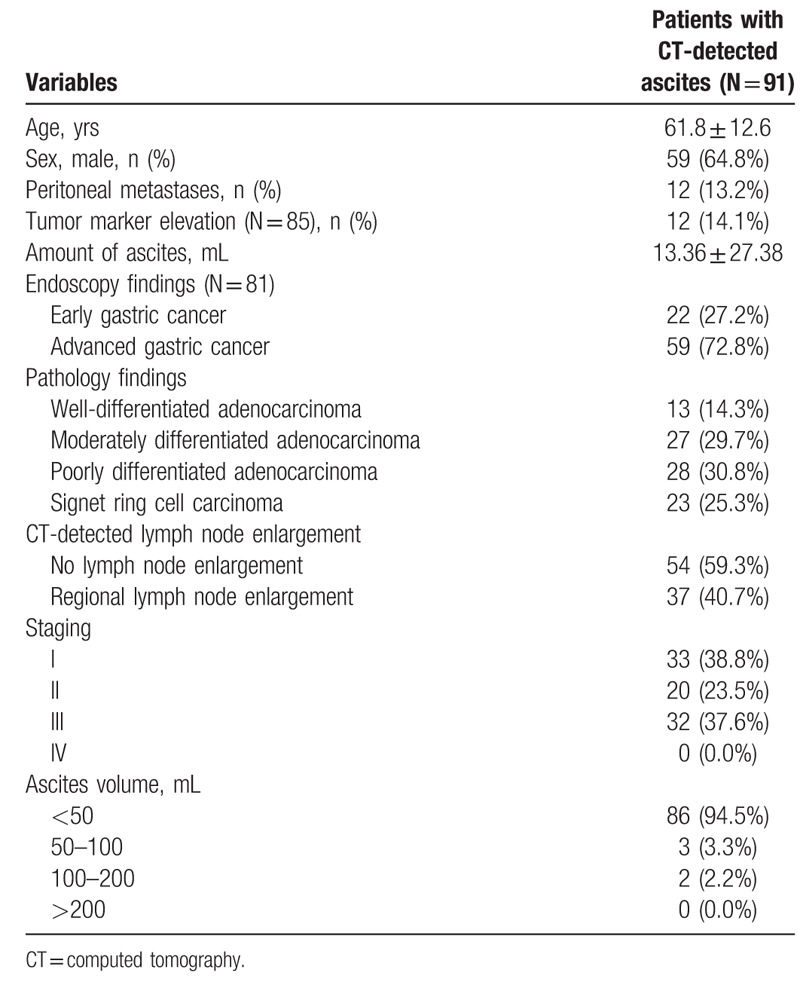
A total of 91 patients (men, 59; women, 32; mean age, 61.8 yrs) were enrolled in the study to analyze the association between CT-detected ascites and surgically detected PM. Endoscopy results were available for 81 patients, of which 22 patients had early gastric cancer (EGC) and 59 patients had advanced gastric cancer (AGC). Of the 91 patients who underwent surgery, 76 patients underwent curative resection and 15 patients underwent palliative gastrectomy. Pathology findings revealed peritoneal seeding in 12 patients and regional lymph node metastasis in 37. No patient had distant lymph node metastasis (Table 1).
Table 1.
Baseline characteristics of the patients with CT-detected ascites.
3.2. Factors associated with PM
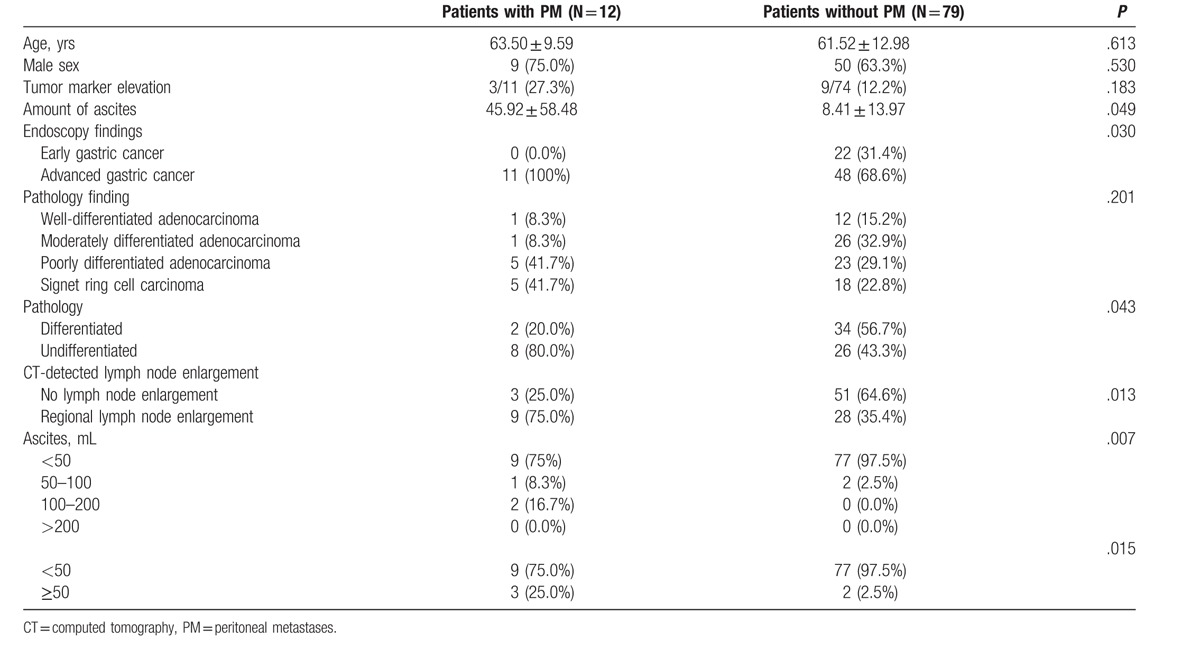
The relationship between the detection of ascites on CT and PM at surgery is shown in Table 2. PM was detected during surgery in only 12 patients (13.2%). We found that endoscopic diagnosis of AGC (P = .030) and the presence of regional LN metastases (P = .013) were associated with PM. Further, the amount of ascites was higher in patients with PM than in those without PM (P = .049). The χ2 test comparison indicated that the incidence of PM was higher in patients with mild ascites (P = .015) as compared with those with minimal ascites. Elevation of tumor marker levels was not significantly associated with PM. When a comparison was performed between patients with differentiated and undifferentiated pathology, PM was found to be significantly associated with undifferentiated pathology (P = .043) (Table 2).
Table 2.
Comparison of clinical characteristics between patients with and without peritoneal metastases.
3.3. Risk factors for PM
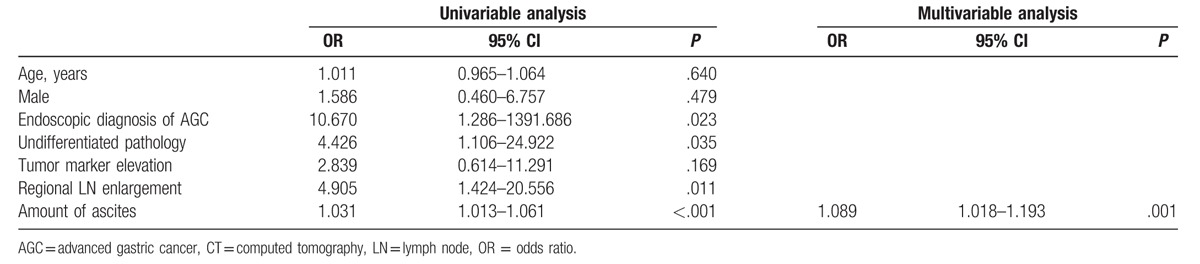
Univariable logistic regression analyses revealed that regional lymph node metastasis (OR, 4.905; 95% CI 1.424–20.556; P = .011), undifferentiated pathology on endoscopic biopsy (OR, 4.426; 95% CI 1.106–24.922; P = .035), and the amount of ascites (OR, 1.031; 95% CI 1.013–1.061; P < .001) were statistically significant factors associated with PM. In addition, endoscopic diagnosis of AGC was significantly associated with PM (OR, 10.670; 95% CI 1.286–1391.686; P = .023). In the multivariable logistic regression analysis with backward elimination, the amount of ascites was only identified as an independent risk factor for PM (OR, 1.089; 95% CI 1.018–1.193; P = .001; Table 3).
Table 3.
Univariable and multivariable analyses to determine risk factors of peritoneal metastases in patients with CT-detected ascites.
4. Discussion
Patients with PM are usually deemed incurable despite some recent studies showing survival benefits of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy.[24,25] Therefore, the detection of PM is crucial in guiding therapeutic strategy. Previous studies have reported conflicting results on the association between CT-detected ascites and PM.[16,17,20,21] In our study, we found an association between the amount of ascites and the presence of PM in gastric cancer patients who did not exhibit definite distant metastasis or PM on CT. As expected, univariable analysis revealed advanced gastric cancer and lymph node metastasis to be predictors. Similarly, undifferentiated pathology, which is a well-known factor associated with poor clinical outcome, was also found to be a factor.[26,27] In our study, which excluded patients with liver cirrhosis or chronic renal insufficiency, 10.5% of the patients with an ascites volume of < 50 mL had PM, whereas 60% of those with >50 mL of ascites had PM. Therefore, an ascites volume of >50 mL in gastric cancer patients without liver cirrhosis or chronic renal insufficiency may suggest PM.
CT-detected ascites was reported to be a specific indicator of PM in previous studies; however, the amount of ascites was not specified.[16–18] A recent study investigated patients with CT-detected ascites volume of <50 mL and concluded that there is no relation between minimal ascites and survival outcomes in patients with gastric cancer; however, patients with ascites volumes of >50 mL were not addressed in this study.[20] Chang et al[21] analyzed CT-detected ascites in gastric cancer patients, but the sample size was small. We investigated the factors associated with PM in gastric cancer patients and found the amount of ascites to be an independent risk factor. To our knowledge, this is the largest study to investigate the predictors of surgically proven PM in gastric cancer patients with ascites and without CT-detected distant metastasis or PM.
Preoperative identification of PM is critical in deciding therapeutic strategy. However, despite the recent developments in CT technology, the sensitivity of CT for PM detection is limited.[12] It is easy to diagnose PM when definite indicators such as soft tissue nodules, small bowel wall thickening, intraabdominal fat stranding, and peritoneal thickening/enhancement are identified on CT,[22] and poor survival outcomes of patients with definite PM have been reported.[28,29] However, in cases where CT shows minimal ascites without additional indicators such as distant metastasis or PM, determining the therapeutic strategy is difficult. In cases with massive ascites, a cytological examination via paracentesis may aid in diagnosing PM. However, a cytological diagnosis cannot be performed in patients with minimal or mild ascites, making it more difficult for clinicians to establish a treatment plan in cases when CT does not show PM. Our study indicated that the risk of PM differs depending on the amount of ascites in patients with minimal or mild ascites. Our study may aid surgeons in deciding between curative resection and palliative surgery.
Previous studies have described that endoscopic ultrasonography (EUS) is a superior technique for the local staging of gastric cancer and the detection of ascites.[30–32] However, EUS is limited because of its low sensitivity in diagnosing liver or distant metastases.[19] In addition, the detection of fluid in the lower abdomen and the Douglas pouch by EUS is limited.[19,33] CT is very effective in the preoperative staging of gastric cancer, such as tumor detection and assessment of regional lymph node and liver metastases. However, CT has lower sensitivity in diagnosing PM as compared with diagnosing other distant metastases.[8,9] The findings of our study can be used by clinicians in cases where PM are not detected on CT.
Our study has some limitations. First, this study was a retrospective study. Because we included patients with CT-detected ascites and surgically confirmed PM, the size of this study is limited. Further, some of the enrolled patients lacked medical records such as endoscopic biopsy and tumor marker elevation results. However, this is the largest study to investigate the relation between CT-detected ascites and PM in patients with gastric cancer. Second, clinicopathological factors such as body mass index, H. pylori infection, and tumor size were not analyzed in this study. Third, the results of the peritoneal washing cytology performed during surgery were not available for most of the patients. Fourth, our study was conducted in a single academic teaching hospital.
5. Conclusions
Regional lymph node metastasis, advanced gastric cancer, undifferentiated pathology, and the amount of ascites were associated with PM. The amount of ascites was an independent risk factor for PM. Our study warrants further investigations with a larger sample size to elucidate the factors that are associated with PM in gastric cancer patients with ascites.
Footnotes
Abbreviations: AGC = advanced gastric cancer, CT = computed tomography, EGC = early gastric cancer, EUS = endoscopic ultrasonography, PM = peritoneal metastases.
This study was reviewed and approved by the Institutional Review Board of Seoul Metropolitan Government Seoul National University Boramae Medical Center and was conducted according to the Declaration of Helsinki.
SHK and YHC contributed equally to this work. JWK contributed to study conception and design; SHK contributed to data analysis, data interpretation, and writing of article. YHC contributed to the interpretation of CT findings; SL contributed to data acquisition and data analysis; SHK, JWK, SL, BGK, KLL, and YHC contributed to editing, reviewing, and final approval of article.
The authors declare no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50. [DOI] [PubMed] [Google Scholar]
- [3].Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2014;9:303–9. [DOI] [PubMed] [Google Scholar]
- [4].Oh CM, Won YJ, Jung KW, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat 2016;48:436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol 2007;25:2107–16. [DOI] [PubMed] [Google Scholar]
- [6].Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 2004;230:465–71. [DOI] [PubMed] [Google Scholar]
- [7].Andaker L, Morales O, Hojer H, et al. Evaluation of preoperative computed tomography in gastric malignancy. Surgery 1991;109:132–5. [PubMed] [Google Scholar]
- [8].Kayaalp C, Arda K, Orug T, et al. Value of computed tomography in addition to ultrasound for preoperative staging of gastric cancer. Eur J Surg Oncol 2002;28:540–3. [DOI] [PubMed] [Google Scholar]
- [9].D’Elia F, Zingarelli A, Palli D, et al. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol 2000;10:1877–85. [DOI] [PubMed] [Google Scholar]
- [10].Stell DA, Carter CR, Stewart I, et al. Prospective comparison of laparoscopy, ultrasonography and computed tomography in the staging of gastric cancer. Br J Surg 1996;83:1260–2. [PubMed] [Google Scholar]
- [11].Walkey MM, Friedman AC, Sohotra P, et al. CT manifestations of peritoneal carcinomatosis. AJR Am J Roentgenol 1988;150:1035–41. [DOI] [PubMed] [Google Scholar]
- [12].Kim SJ, Kim HH, Kim YH, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 2009;253:407–15. [DOI] [PubMed] [Google Scholar]
- [13].Yoshikawa T, Kanari M, Tsuburaya A, et al. Clinical and diagnostic significance of abdominal CT for peritoneal metastases in patients with primary gastric cancer. Gan To Kagaku Ryoho 2002;29:1925–8. [PubMed] [Google Scholar]
- [14].Halvorsen RA, Jr, Panushka C, Oakley GJ, et al. Intraperitoneal contrast material improves the CT detection of peritoneal metastases. AJR Am J Roentgenol 1991;157:37–40. [DOI] [PubMed] [Google Scholar]
- [15].Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364–7. [DOI] [PubMed] [Google Scholar]
- [16].Yajima K, Kanda T, Ohashi M, et al. Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg 2006;192:185–90. [DOI] [PubMed] [Google Scholar]
- [17].Cheong JC, Choi WH, Kim DJ, et al. Prognostic significance of computed tomography defined ascites in advanced gastric cancer. J Korean Surg Soc 2012;82:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pongpornsup S, Neungton P, Chairoongruang S, et al. Diagnostic performance of multidetector computed tomography (MDCT) in evaluation for peritoneal metastasis in gastric cancer. J Med Assoc Thai 2014;97:863–9. [PubMed] [Google Scholar]
- [19].Lee YT, Ng EK, Hung LC, et al. Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients. Gut 2005;54:1541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee H, Hwang HS, Chang DK, et al. Clinical significance of minimal ascites of indeterminate nature in gastric adenocarcinoma without peritoneal carcinomatosis: long-term follow-up study. Hepatogastroenterology 2011;58:137–42. [PubMed] [Google Scholar]
- [21].Chang DK, Kim JW, Kim BK, et al. Clinical significance of CT-defined minimal ascites in patients with gastric cancer. World J Gastroenterol 2005;11:6587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee MH, Choi D, Park MJ, et al. Gastric cancer: imaging and staging with MDCT based on the 7th AJCC guidelines. Abdom Imaging 2012;37:531–40. [DOI] [PubMed] [Google Scholar]
- [23].Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38. [Google Scholar]
- [24].Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Benizri EI, Bereder JM, Rahili A, et al. Ascites and malnutrition are predictive factors for incomplete cytoreductive surgery for peritoneal carcinomatosis from gastric cancer. Am J Surg 2013;205:668–73. [DOI] [PubMed] [Google Scholar]
- [26].Patel PR, Yao JC, Hess K, et al. Effect of timing of metastasis/disease recurrence and histologic differentiation on survival of patients with advanced gastric cancer. Cancer 2007;110:2186–90. [DOI] [PubMed] [Google Scholar]
- [27].Han YH, Park JK, Kwon JS, et al. Liver metastasis of early gastric cancer with mixed histology after endoscopic submucosal dissection. Clin Endosc 2015;48:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jeon HH, Park CH, Park JC, et al. Carcinomatosis matters: clinical outcomes and prognostic factors for clinical success of stent placement in malignant gastric outlet obstruction. Surg Endosc 2014;28:988–95. [DOI] [PubMed] [Google Scholar]
- [29].Park CH, Park JC, Kim EH, et al. Impact of carcinomatosis and ascites status on long-term outcomes of palliative treatment for patients with gastric outlet obstruction caused by unresectable gastric cancer: stent placement versus palliative gastrojejunostomy. Gastrointest Endosc 2015;81:321–32. [DOI] [PubMed] [Google Scholar]
- [30].Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. J Clin Gastroenterol 2002;35:321–7. [DOI] [PubMed] [Google Scholar]
- [31].Cho JW. The Role of Endosonography in the Staging of Gastrointestinal Cancers. Clin Endosc 2015;48:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim EY. Application of endoscopic ultrasonography in the diagnosis and treatment of lower gastrointestinal disease. Intest Res 2015;13:101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nozoe T, Matsumata T, Sugimachi K. Usefulness of preoperative transvaginal ultrasonography for women with advanced gastric carcinoma. Am J Gastroenterol 1999;94:2509–12. [DOI] [PubMed] [Google Scholar]


