Supplemental Digital Content is available in the text
Keywords: Alagille syndrome, bronchoscopic surgical procedures, bronchoscopy, Dieulafoy, hemoptysis
Abstract
Rationale:
Dieulafoy lesions are aberrantly large submucosal arteries most frequently associated with gastrointestinal hemorrhage. They are rarely identified in the bronchial submucosa and can cause massive hemoptysis.
Patient concerns:
We present three episodes of massive hemoptysis in two patients, the first with comorbid Alagille syndrome including multiple cardiac and pulmonary vascular abnormalities and the second with thyroid cancer metastatic to the mediastinum.
Diagnoses:
All episodes were due to Dieulafoy lesions of the bronchus based on bronchoscopic appearance.
Interventions:
Bronchoscopic ablation using Nd:YAP laser was attempted both patients.
Outcomes:
Nd:YAP laser successfully ablated the Dieulafoy lesion in the first case with long-term relief from recurrent hemoptysis. The first episode in the second patient responded to bronchial artery embolization; laser ablation of a different Dieulafoy lesion responsible for the second episode was unsuccessful but additional bronchial artery embolization has provided relief from further episodes.
Lessons:
Bronchoscopic ablation of Dieulafoy lesions of the bronchus can provide durable relief from recurrent symptoms. Clinical and anatomical features should be considered carefully before intervention, which should only be attempted by experienced operators with appropriate ancillary support available.
1. Introduction
French physician Paul-Georges Dieulafoy pathologically characterized the lesion that now bears his name in a series of 3 patients with massive hematemesis published in 1898.[1] Each was found to have an aberrantly large artery in the gastric submucosa with overlying mucosal erosion. This lesion most frequently occurs near the gastroesophageal junction and is uncommonly identified elsewhere in the gastrointestinal tract. Even more rare are analogous lesions reported in the bronchial submucosa. There have been approximately 40 such reports associated with hemoptysis that is characteristically episodic and massive, and associated with significant morbidity and mortality.[2] Both the bronchial and pulmonary circulations have been implicated, the former most frequently.[2–5] Most reported cases occurred in the setting of chronic inflammatory lung disease secondary to chronic infection or smoking, although cases in otherwise healthy individuals as young as 13 years old have occurred.[3,6] Accordingly, there are several hypotheses regarding the origin of these lesions, including hypertrophy of submucosal vasculature in response to chronic inflammation or the possibility of congenital malformation.[3,6] Management has included surgical lung resection, bronchial artery embolization, and bronchoscopic ablation.[3,7–9]
We report 2 cases consistent with Dieulafoy lesions of the bronchus thought to be attributable to medical comorbidities not previously reported in association with these lesions. In both cases, bronchoscopic ablation using Nd:YAP laser was attempted, which was successful in one case. This report was approved by the Vanderbilt University Medical Center Institutional Review Board (#171315).
2. Case #1
A 51-year-old woman with Alagille syndrome presented to the Emergency Department after an episode of hemoptysis of an estimated 300 to 400 mL of frank blood over 20 minutes. Manifestations of her rare genetic disorder included branch pulmonary artery stenoses, patent ductus arteriosus and large ventricular septal defect with predominantly left-to-right shunt, severe pulmonary hypertension with preserved right ventricular function, and stage IV chronic kidney disease related to renal dysplasia and renal artery stenosis. She was admitted to the intensive care unit with acute hypoxemic respiratory failure requiring 80% FiO2 at 60 L per minute via high flow nasal cannula. CT scan with contrast was not performed due to renal dysfunction; flexible bronchoscopy was instead pursued to further characterize her bleeding source. This was performed in a dedicated bronchoscopy operating room under general anesthesia via an endotracheal tube. Brisk bleeding was encountered upon clearance of left-sided clot. A 5 mm nodular lesion ejecting bright red blood was identified in the left lower lobe (Fig. 1, panel A). After hemostasis, a pulsatile superficial vessel running along a segmental carina with protruding tortuous loop or aneurysmal dilation was clearly visible, with white cap at the bleeding site (Fig. 1, panels B–D; Video 1). After transiently reducing inspired FiO2 to 40%, the lesion was ablated with Nd:YAP laser at 25 W (Fig. 1, panels E and F; Video 2), starting with the afferent limb of the vessel near the 3 o’clock position. The patient discharged the next day and has not had further hemoptysis at 2 years follow-up.
Figure 1.
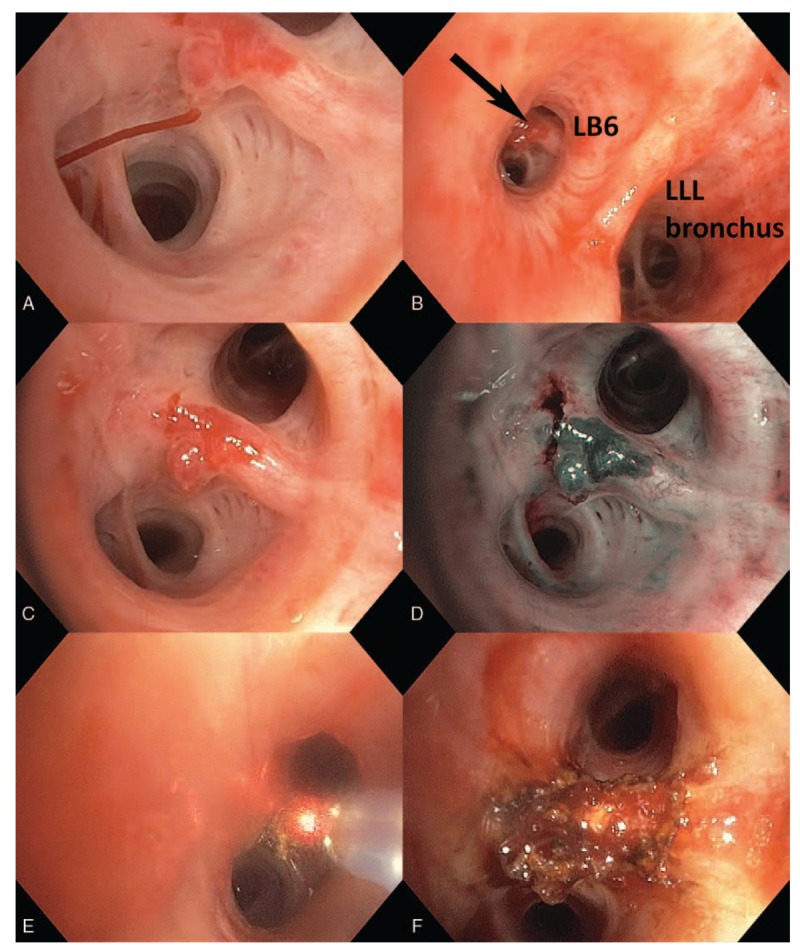
Active bleeding into saline-filled bronchus (panel A). Lesion location on proximate secondary carina of the superior segment of the left lower lobe (panel B). White light (panel C) and narrow-band imaging (panel D) of the lesion after hemostasis was achieved. Destruction of the lesion with Nd:YAP laser, starting with the afferent region at 3 o’clock (panel E). Final result (panel F).
3. Case #2
A 76-year-old man presented to the Emergency Department with episodic hemoptysis. His medical history was notable for follicular thyroid carcinoma for which he had undergone laryngectomy 5 years prior with subcarinal mediastinal recurrence, atrial fibrillation on apixaban, coronary artery disease on clopidogrel, and remote smoking history. The night before presentation, he abruptly developed hemoptysis with spontaneous resolution 30 minutes later after producing approximately 100 to 200 mL of frank red blood. Computed tomography scan of the chest with intravenous contrast revealed a hypertrophied bronchial artery supplying his large subcarinal metastasis (Fig. 2). Flexible bronchoscopy was performed in the operating room via laryngectomy stoma under moderate sedation, revealing clotted blood in both mainstem bronchi. Suctioning of clot prompted pulsatile bright red blood to erupt from a small 1 to 2 mm nodular lesion along the posterior membrane of the proximal right mainstem bronchus (Fig. 3, Video 3). Bleeding stopped spontaneously after several minutes. Ablative bronchoscopic intervention was felt to be prohibitively risky given its posterior membrane location just anterior to the esophagus. He underwent successful embolization of the branches of the bronchial artery supplying this region, with resolution of bleeding. His apixaban and clopidogrel were discontinued.
Figure 2.
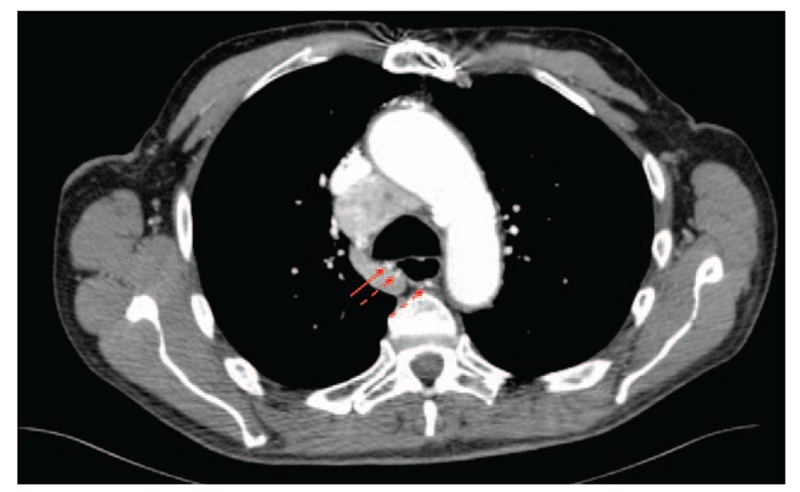
Axial cut of an intravenous contrast-enhanced computed tomography scan of the chest, demonstrating a tortuous hypertrophied bronchial artery (3 arrows) supplying a large subcarinal metastasis not visualized in this image. The vessel approximates the posterior membrane of the proximal right mainstem bronchus (solid arrow) at the location of the bleeding lesion identified at bronchoscopy.
Figure 3.
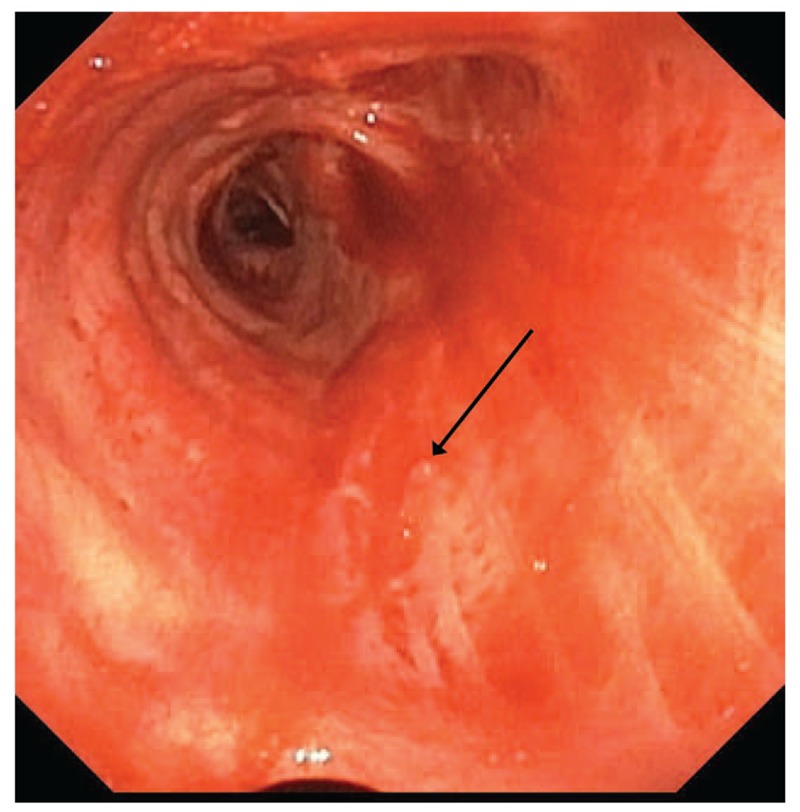
Small nodular lesion along the posterior membrane of the proximal right mainstem bronchus (arrow), visualized after spontaneous hemostasis.
Three weeks later he was readmitted with similar episodic hemoptysis. Computed tomography scan of the chest did not demonstrate obvious targets for bronchial artery embolization; no flow was noted in the previously treated vessels. Flexible bronchoscopy was again performed, with similar initial findings. No nodules were present in the right mainstem bronchus and no bleeding could be elicited from this region. However, suctioning clot from the left mainstem bronchus prompted pulsatile bright red bleeding from a 3 mm nodular lesion of the proximal aspect of the bronchus adjacent to the subcarinal space (Fig. 4, Video 4). Bleeding was significant and required adjunctive suctioning with a 3 mm rigid bronchoscopy suction catheter advanced through the laryngectomy stoma. Ablation was attempted using the Nd:YAP laser at 15 W with concentric burn around the bleeding nodule followed by ablation of the nodule itself, without diminishing the rate of hemorrhage (Video 5). Bleeding ultimately stopped spontaneously after 10 minutes with total blood loss of about 250 mL. Emergent angiography of the bronchial circulation was performed, with several additional branches of the same hypertrophied artery seeming to supply the region in question, which were successfully embolized without further bleeding episodes at 6 months follow-up.
Figure 4.
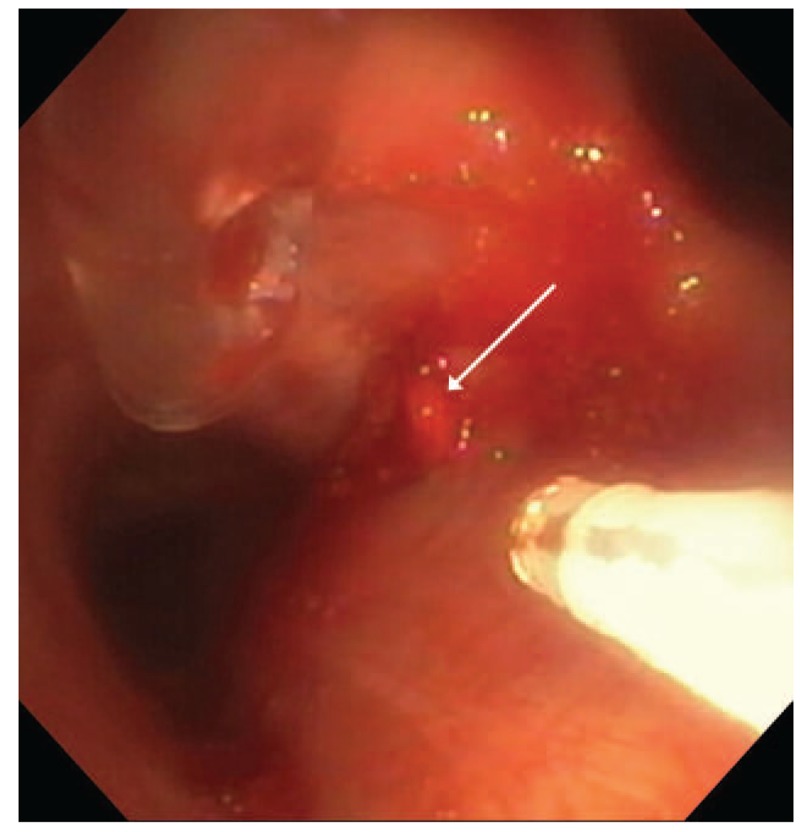
Nodular lesion with erupting pulsatile blood, medial proximal left mainstem bronchus (arrow). YAP laser fiber (white) and 3 mm rigid suction catheter (clear) are also in view.
4. Discussion
Fewer than 40 cases of Dieulafoy lesions of the bronchus have been reported. Bronchoscopic appearance and clinical history in the aforementioned cases are consistent with prior reports.[2–4,6,7,10] Chronic airway inflammation, related to smoking or chronic infection, has been most commonly associated with these lesions in existing cases.[3] Association with cardiac disease and mediastinal malignancy has not been previously reported.
Most bronchial Dieulafoy lesions appear to be bronchial arteries, with few reported cases derived from the pulmonary circulation.[2,4,5] The Dieulafoy lesions in Case 2 appeared bronchial in origin. We hypothesize that the large subcarinal tumor burden in this patient was responsible for his bronchial artery hypertrophy, perhaps driven by tumor-secreted angiogenesis factors. Interestingly, shortly after embolization of the initial lesion, a second aberrantly large submucosal arterial lesion developed in the contralateral mainstem. Whether this resulted from simple shunting of blood through the nonembolized branches of the same artery which supplied this region or angiogenesis-driven hypertrophy is unclear. Existing literature suggests recurrent hemoptysis after initial bronchial artery embolization is common, in some cases prompting lobectomy.[2,4] It is not clear in such cases whether this represents recanalization at the site of therapeutic embolization or hypertrophy of nearby submucosal vasculature, similar to our Case 2.
Delineating the circulation responsible for the Dieulafoy lesion in Case 1 is more challenging. Pulsatile bright red blood in this patient with a left-to-right shunt and severe pulmonary hypertension did not allow a reliable distinction between circulations on a visual basis, and advanced renal disease precluded contrasted imaging or angiography. We suggest a submucosal vessel hypertrophied to provide collateral circulation for pulmonary (bypassing branch pulmonic stenosis) or bronchial (compensating from impaired pulmonic flow) systems is possible, and hypothesize this lesion is related to the underlying cardiac malformations via either of these 2 biologically plausible mechanisms.
Bronchoscopic ablation has been attempted in a minority of cases. Stoopen et al[7] report a case in which contact electrocautery temporized a bleeding lesion, but rebleeding shortly thereafter necessitated emergent lobectomy with definitive control. Argon plasma coagulation has been used to successfully manage 2 cases.[8,9] Our cases are the first in which the Nd:YAP laser has been employed to manage these lesions. Our success in Case 1 supports the use of this and similar ablative technologies, but such intervention should only be performed in select cases. Conditions for attempted bronchoscopic intervention were ideal in Case 1: a length of the submucosal artery was clearly visible, it was running atop a cartilaginous segmental carina, the bronchoscope was wedged in the segmental bronchus, and the lesion was not bleeding at time of ablation. In contrast, the initial lesion in Case 2 was poorly situated for an ablation attempt, given its location on the posterior membrane with esophagus running immediately adjacent posteriorly. Ablation along the posterior membrane must be performed extremely cautiously to avoid full-thickness burn through the thin 3 to 4 mm thick tissue. Mediastinitis, esophageal injury, and bronchoesophageal fistula are all potential complications. Additionally, only the small nodule producing pulsatile blood could be visualized, unlike in Case 1, where the entire vessel could be delineated. Attempted ablation of this “tip of the iceberg” might only have accomplished enlarging the defect in the vessel wall leading to more massive hemorrhage. The recurrent lesion in Case 2, while partially located along the posterior membrane, was adjacent to the subcarinal tumor itself which acted a back-stop for ablative energy. In this case, we suspect a “tip of the iceberg” effect and active bleeding account for failed ablation; only a short segment of vessel was visible and accessible to ablative energy, some of which was absorbed by the active hemorrhage. This clearly demonstrates that ablation is not always successful. Other ablative tools may have been more successful, such as argon plasma coagulation (excellent coagulation, able to more rapidly ablate a larger area) or Holmium:YAG laser (deeper tissue penetration than Nd:YAP, for this mostly hidden vessel); neither were readily available.
Bronchoscopy in the setting of massive hemoptysis carries significant risk of precipitating life-threatening bleeding, as evidenced by both cases presented here. Therefore it should only be performed by experienced operators adept at managing major airway hemorrhage. Equipment and personnel such as rigid bronchoscopy, occlusion balloon catheters, interventional radiology, and thoracic surgery should be readily available. Rigid bronchoscopy was considered for all 3 bronchoscopies described herein. In Case 1, severe hypoxemia precluded rigid bronchoscopy, but ability to wedge the flexible bronchoscope in the segmental bronchus proximal to the lesion during ablation would have afforded some protection from emergent hemorrhage had any occurred. Rigid bronchoscopy was immediately available during both Case 2 bronchoscopies but was ultimately not required, in part because the large laryngectomy stoma permitted passage of instruments which usually require a rigid bronchscope, such as the 3 mm suction catheter utilized in the second bronchoscopy.
In conclusion, we present here 2 cases involving 3 Dieulafoy lesions of the bronchus, unique in their comorbid associations and the use of Nd:YAP laser for attempted ablation, which was successful in one case.
5. Author contributions
All authors participated in all aspects of the production of this manuscript, including study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All authors have provided final approval to submit this version of the manuscript and agree to be accountable for all aspects of this work.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgment
Robert J. Lentz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Abbreviations: CT = computed tomography, FiO2 = fraction of inspired oxygen, LB6 = superior segment of the left lower lobe, LLL = left lower lobe, Nd:YAP = Neodymium: Yttrium-Aluminum-Perovskite, W = watt, YAG = yttrium aluminum garnet.
Funding: This work was not supported by independent funding.
Notation of prior publication/presentation: An abridged version of Case #1 was presented at the CHEST 2016 Annual Meeting (on October 25, 2016).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Dieulafoy G-P. Exulceratio simplex. L’intervention chirurgicale dans les hématémèses foudroyantes consécutives à l’exulcération simple de l’estomac. Bull Acad Med 1898;39:49–84. [Google Scholar]
- [2].van der Werf TS, Timmer A, Zijlstra JG. Fatal haemorrhage from Dieulafoy's disease of the bronchus. Thorax 1999;54:184–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parrot A, Antoine M, Khalil A, et al. Approach to diagnosis and pathological examination in bronchial Dieulafoy disease: a case series. Respir Res 2008;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pomplun S, Sheaff MT. Dieulafoy's disease of the bronchus: an uncommon entity. Histopathology 2005;46:598–9. [DOI] [PubMed] [Google Scholar]
- [5].Yang R, Li J, Liu J, et al. [Dieulafoy disease of the bronchus: 3 cases report with literature review]. Zhonghua Jie He He Hu Xi Za Zhi 2013;36:577–80. [PubMed] [Google Scholar]
- [6].Fang Y, Wu Q, Wang B. Dieulafoy's disease of the bronchus: report of a case and review of the literature. J Cardiothorac Surg 2014;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stoopen E, Baquera-Heredia J, Cortes D, et al. Dieulafoy's disease of the bronchus in association with a paravertebral neurilemoma. Chest 2001;119:292–4. [DOI] [PubMed] [Google Scholar]
- [8].Dalar L, Sokucu SN, Ozdemir C, et al. Endobronchial argon plasma coagulation for treatment of Dieulafoy disease. Respir Care 2015;60:e11–3. [DOI] [PubMed] [Google Scholar]
- [9].Madan K, Dhungana A, Hadda V, et al. Flexible bronchoscopic argon plasma coagulation for management of massive hemoptysis in bronchial Dieulafoy's disease. Lung India 2017;34:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Padilla-Serrano A, Estrella-Palomares V, Martínez-Palacios B, et al. A case of massive hemoptysis related to a smoking-history: an acquired form of the Dieulafoy's disease? Rev Port Pneumol Engl Ed 2015;21:276–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


