Abstract
Cell fate decisions are closely linked to changes in metabolic activity. Over recent years this connection has been implicated in mechanisms underpinning embryonic development, reprogramming and disease pathogenesis. In addition to being important for supporting the energy demands of different cell types, metabolic switching from aerobic glycolysis to oxidative phosphorylation plays a critical role in controlling biosynthetic processes, intracellular redox state, epigenetic status and reactive oxygen species levels. These processes extend beyond ATP synthesis by impacting cell proliferation, differentiation, enzymatic activity, ageing and genomic integrity. This review will focus on how metabolic switching impacts decisions made by multipotent cells and discusses mechanisms by which this occurs.
INTRODUCTION
Energy generation through ATP synthesis is critical for driving biochemical processes. Different cell types, however, adopt alternate strategies for energy generation and biosynthesis with glucose, ketogenic amino acids and fatty acids being the major carbon sources that drive ATP-generating catabolic pathways. Glycolytic-dependent energy generation can occur in two general contexts. In many cell types glucose oxidation generates pyruvate and then acetyl coenzyme A (acetyl-CoA), which is fed into the tricarboxylic acid (TCA) cycle. Reducing equivalents generated by glycolysis and the TCA cycle then serve as an electron source to drive the electron transport chain (ETC) and protons for coupled ATP synthesis, known as oxidative phosphorylation (OxPhos) [1]. In some cells however, glycolysis proceeds at an elevated rate in the absence of OxPhos, producing lactate from pyruvate in preference to acetyl-CoA. This is seen in muscle cells, under anaerobic conditions when the electron transport chain is inactive [2] This mode of metabolism is frequently seen in tumor cells under aerobic conditions and generally referred to as the ‘Warburg effect’ or, ‘aerobic glycolysis’ [3]. Glutamine-dependent energy generation involves its conversion to α-ketoglutarate, which then feeds into the TCA cycle to drive energy generation [4,5].
Energy-generating pathways are highly dynamic and metabolic fluxes vary dramatically across different cell types and tissues in response to developmental signals [6], nutritional status [7], environmental signals [8] and disease pathogenesis [9]. Metabolic flux is finely tuned to maximize function in different cell types and is linked to cell identity just as gene expression, epigenetics and morphology are. Whether to produce signaling molecules such as insulin in pancreatic β-cells or dopamine in neurons, packaging of lipids into vesicles in the liver, or to generate ATP for motor function in skeletal muscle; regulating metabolism is integral for maintenance of cell identity and function. This review will summarize recent developments linking metabolic activity and cell identity with a focus on multipotent stem cells.
Metabolic regulation in adult stem cells
Numerous populations of multipotent stem cells undergo aerobic glycolysis in the stem cell niche to sustain their energy demands [10]. Examples include hematopoietic stem cells in the bone marrow [11], intestinal crypt stem cells [12] and hair follicle stem cells [13]. Muscle satellite stem cells (MuSCs) illustrate how dynamic metabolic regulation can be under different physiological conditions. After postnatal growth muscle MuSCs undergo a metabolic switch from aerobic glycolysis to OxPhos coinciding with exit from the cell cycle [14,15]. Upon injury cues quiescent MuSCs then re-enter the cell cycle to proliferate for muscle repair/regeneration. As part of this mechanism, key rate-limiting enzymes associated with aerobic glycolysis such as lactate dehydrogenase A (LDHA) and pyruvate kinase muscle splice variant 2 (PKM2) are induced during MuSC activation [16]. Curiously, while establishment of elevated glycolytic flux is a requirement of MuSC activation, OxPhos is not reduced, implying that the induction of glycolysis is not related to increased energy production. Ryall et al [15] showed that this metabolic switch functions by adjusting the epigenetic status of stem cells via modulation of the redox state. Induced aerobic glycolysis during MuSC activation lowers the intracellular NAD+:NADH ratio leading to reduction in NAD+-dependent SIRT1 histone deacetylase activity. This causes an increase in global H4K16 acetylation, localized decondensation of chromatin and activation of myogenic genes MYOD, MYOG, MYKL9, and H19 [15]. Knockdown of SIRT1 under quiescent conditions is sufficient to activate MuSCs without metabolic switching, suggesting that the role of metabolic regulation is solely to regulate SIRT1 activity. This study provides a clear link between metabolic switching, redox status, epigenetic regulation and cell fate decisions.
Mesenchymal stem cells (MSCs) are another multipotent cell type where metabolic activity impacts biological function beyond energy generation. MSCs are isolated from numerous anatomical locations including the bone marrow, skeletal muscle, white adipose tissue and the placenta [17]. Under most conditions, MSCs utilize aerobic glycolysis for energy production [18,19] through a mechanism regulated by HIF1α [20,21]. During both osteogenic and adipogenic differentiations of MSCs, HIF1α is down-regulated, resulting in a loss of aerobic glycolysis accompanied by increased mitogenesis and elevated OxPhos [20,22]. Increased levels of reactive oxygen species, predominately produced by the ETC, induce adipogenic differentiation within MSCs which can be blocked by antioxidant treatment [10,23]. These observations provide a link between differentiation status and metabolic activity. ROS scavengers such as catalase and superoxide dismutase are down-regulated as MSCs transition to an adipose cell fate [10,22]. However, ROS generation inhibits osteogenic differentiations through the canonical Wnt signaling pathway [17,24]. This provides interesting connections between metabolic products such as ROS, cell signaling pathways and cell fate decisions.
Metabolic switching also plays a key role in directing cell fate in the central nervous system. Neural stem cells (NSCs) in adult brain tissue that differentiate into neuronal and glial lineages are an interesting example. Here, glycolysis is the predominant form of metabolism [25] but, as angiogenesis proceeds within the cerebral cortex, NSCs transition to specialized cell fates due to changes in oxygen tension and oxidative metabolism [26]. Lange and colleagues have shown that increased oxygen tension in the NSC niche inactivates HIF1α, resulting in differentiation to neuronal and glial fates [26]. Not surprisingly, embryonic neural progenitor cells (NPCs) utilize aerobic glycolysis and switch to OxPhos during neuronal differentiation [27,28]. This correlates with up-regulation of PGC1α which induces mitochondrial biogenesis and the establishment of OxPhos [28]. As adult neural stem cells differentiate in vitro to neurons they also decrease aerobic glycolysis though a HIF1α-independent mechanism [25]. Interestingly pre-neural cells that retain an elevated number of mitochondria have a neuronal stem cell differentiation defect [29], emphasizing the developmental link between metabolic flux and cell fate decisions.
Pluripotent stem cells and metabolic remodeling during cell fate specification
Elevated rates of aerobic glycolysis and absence of OxPhos are defining features of human embryonic stem cells (hESCs) and is required for maintenance of their pluripotency. In support of this, reduced aerobic glycolysis arising from reduced lactate transporter [30] or hexokinase activity [31] results in the spontaneous differentiation of hESCs. Regulation of epigenetic modifying enzymes [32] or the availability of metabolites for use as epigenetic modification substrates [31] has been investigated, and for a detailed review of epigenetic regulation of pluripotent stem cell fate see Ryall et al. [16] and Harvey et al. [33]
Pluripotent stem cells however, can exist in two distinct states. First, the ‘primed’ pluripotent state in which cells are developmentally equivalent to the primitive ectoderm of embryonic epiblasts [34]. The second class of pluripotent cells are referred to as ‘naïve’ PSCs and they represent cells in the inner cell mass (ICM) of pre-implantation stage embryos [34]. Human embryonic stem cells (hESCs) and mouse epiblast-like stem cells (mEpiSCs) exhibit characteristics of the primed state and utilize elevated aerobic glycolysis for energy generation [31,35,36]. In contrast, naïve murine PSCs utilize OxPhos and lower glycolytic flux [37]. Although little is known about metabolic switching during the transition between different PSC states, a role for the RNA-binding protein LIN28 has recently been proposed [37]. In this report, up-regulation of LIN28 during the naïve to primed PSC transition was shown to be required for establishing aerobic glycolysis by increasing expression of glycolytic genes and repressing OxPhos through inhibition of ETC complex 1 transcripts NDUFB3 and NDUFB10 [37]. Recently, methods to convert primed hESCs to a naïve state have been established [34,38–41] and naïve hESCs have been directly derived from the ICM of human embryos [42]. However initial reports contradict whether naïve hESCs utilize aerobic glycolysis, just as their human primed counterparts [30], or switch to OxPhos [41] like murine naïve and primed PSCs [35,37]. Determining whether this contradiction marks a distinct developmental property in humans, or other primates, or is a consequence of cell culture will be of great interest.
As pluripotent cells pass through the primed state and commit to one of the three embryonic germ layers, glycolytic flux decreases and OxPhos becomes important for energy generation [30,36]. This also corresponds to increased production of ROS, which is generally linked to DNA damage and compromised genomic integrity. This is a critical issue for stem cells that must minimize ROS production to limit the transmission of damaged DNA to progeny cells. Glutamine-dependent ROS activity is also important for modulating the oxidation status of OCT4, a transcription factor required for maintaining the pluripotent state [43]. Elevated ROS levels following glutamine withdrawal or chemical treatment induces the oxidation of cysteine bridges within OCT4 resulting in decreased transcriptional activity and loss of pluripotency. This led to the hypothesis that ROS and glutathione levels modulate pluripotency by regulating the post-translational modification of pluripotency factors. Although hESCs are dependent on glutamine for maintenance of pluripotency, naïve mESCs cultured with leukemia inhibitory factor and inhibitors CHIR-99021 and PD0325901, can self-renew in the absence of glutamine [32]. This underscores the concept that distinct metabolic strategies are used by primed and naïve PSCs and raises the possibility of cross-species differences.
Establishment of aerobic glycolysis during reprogramming
Reports describing aerobic glycolysis in primed PSCs suggested that some form of metabolic remodeling may be required for establishment and maintenance of induced pluripotent stem cells (iPSCs). This was first indicated by reports showing that glycolytic activators and OxPhos inhibitors increase reprograming efficiencies [44–46]. Further analysis showed that glycolytic activation and decreased OxPhos occurs at an early stage of reprogramming and prior to the expression of endogenous pluripotency factors [44,46–49]. The implication that metabolic remodeling precedes expression of pluripotency markers suggests a ‘cause’ rather than ‘effect’ mechanism and has generated considerable interest. This led to work showing that HIF1α and HIF2α are required to induce early metabolic remodeling required for iPSC formation under normoxic conditions [50], however HIF2α must be down-regulated later in reprograming to allow for caspase 3 activity. Hawkins et. al [51] provided a mechanistic basis for this by showing that an OxPhos-driven metabolic burst, in response to Yamanaka factor expression, increased ROS levels leading to HIF stabilization. Elevated ROS activity signals through proteins such as KEAP1 and NRF2 and ultimately leads to increased HIF1α levels [51–53]. Transient ROS signaling during early reprograming demethylates NANOG’s promoter and induces its expression [54]. This is in contrast to elevated ROS generation during MSC differentiation, as increased ROS production during early reprogramming promotes the establishment of aerobic glycolysis and iPSC generation.
Kida and colleagues also observed an oxidative burst during the early phase of reprogramming and went on to define an additional pathway, upstream of the one utilizing HIF1α, that is required for establishment of aerobic glycolysis during reprogramming [55]. This report showed that the OxPhos burst is initiated through the temporal expression of EERα and PGC1α/β, which are induced by reprogramming factors SOX2, KLF4 and MYC [55]. Besides performing a critical role in cell proliferation, MYC’s role in reprogramming is also tied to its induction of aerobic glycolysis. LIN28 can substitute for MYC during reprogramming and functions by promoting glycolytic gene expression [56] and repression of ETC genes [37]. Furthermore, MYCs role in reprogramming can be entirely replaced by ectopic expression of LDHA or PKM2; two genes that encode rate-limiting enzymes for glycolysis [49]. Taken together, these reports show that a key role of reprogramming factors during iPSC formation is to establish aerobic glycolysis. The transition from an OxPhos mode of metabolism to aerobic glycolysis signifies a metabolic switch that is important for maintenance and establishment of the pluripotent state.
CONCLUSIONS
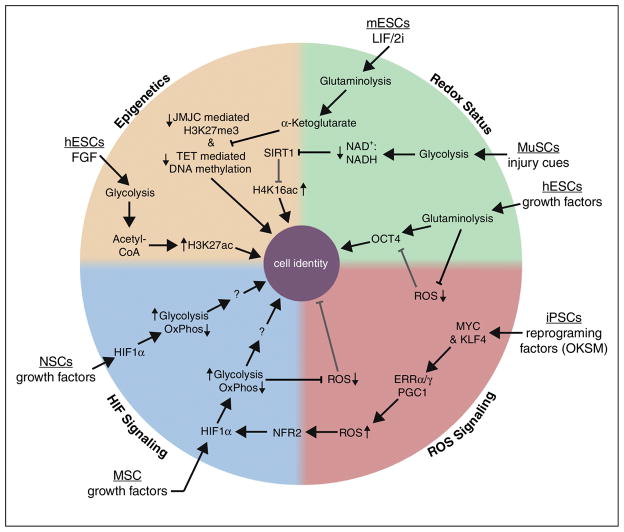
Aerobic glycolysis is required for maintenance of the primed pluripotent state and its establishment is an early event during reprogramming. Moreover, many adult stem cell populations utilize aerobic glycolysis while actively dividing. Regulation of aerobic glycolysis and OxPhos can occur though multiple mechanisms in a context-dependent manner. HIF1α signaling in NSCs and MSCs, growth factor regulation of epigenetic pathways in pluripotent cells, redox regulation of transcription factor and epigenetic networks in muscle and pluripotent stem cells and modulation of reactive oxygen species during reprogramming are all examples where metabolic regulation is coupled to cell identity and/or fate (see Figure). Research into metabolic regulation of mammalian cells is undergoing a renaissance because it is now clear that it impacts cellular process far beyond its known functions in biosynthesis and energy generation. This renaissance is sure to continue over the next ten years and beyond because of the growing interest in the link between metabolism and cellular decisions in development and disease.
Figure. Mechanisms for the metabolic regulation of cell identity in multipotent cells.
Extracellular signaling pathways, generated in human and mouse embryonic stem cells (hPSCs and mPSCs, respectively), muscle satellite stem cells (MuSCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem cells (NSCs) modulate intracellular metabolic pathways. Metabolic flux then regulates cell identity through one or more of four main pathways: epigenetics [orange], redox status [green], reactive oxygen signaling (ROS) signaling [red], hypoxia induced factor (HIF) signaling [blue]. Large arrows with open arrowheads represent activating signals while repressive signals are marked by barred lines. Grey lines represent pathways that are repressed by aerobic glycolysis or glutaminolysis. Small arrows with closed arrowheads indicate whether that adjacent pathway, metabolite, signaling molecule, epigenetic mark, or metabolite ratio is increased or decreased. Abbreviations: mouse embryonic stem cells (mESCs), leukemia inhibitory factor (LIF), PD0325901 and CHIR99021 inhibitors (2i), Jumonji C domain-containing proteins (JMJC), ten eleven translocation enzymes (TET), human embryonic stem cells (hESCs), fibroblast growth factor (FGF), muscle satellite stem cells (MuSC), ratio of oxidized to reduced nicotinamide adenine dinucleotide (NAD+:NADH), NAD-dependent deacetylase sirtuin 1 (SIRT1), octamer-binding transcription factor 4 (OCT4), Kruppel like factor 4 (KLF4), reprograming factors: OCT4, KLF4, Sex determining region Y-box 2, MYC (OKSM), estrogen-related receptor α and γ (ERRα/γ), nuclear factor, erythroid 2 like 2 (NFR2), oxidative phosphorylation (OxPhos), mesenchymal stem cells (MSCs), neural stem cells (NSCs)
HIGHLIGHTS.
metabolic regulation is highly-dynamic, cell-type specific and involved in cell fate determination
aerobic glycolysis is a feature of ‘primed’ pluripotent cells and widely seen in adult stem cells
elevated rates of aerobic glycolysis are required for maintenance of the pluripotent state
metabolic switching is required for cellular reprogramming and establishment of pluripotency
aerobic glycolysis in stem cells is dependent on epigenetic control, redox regulation and signal regulated transcription0factor networks
Acknowledgments
This work was supported by the National Institutes of Health, Institute of General Medical Sciences (P01 GM75334).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan T, Xu Z, Liu J, Wu W, Wang Y. Lkb1 regulation of skeletal muscle development, metabolism and muscle progenitor cell homeostasis. Journal of cellular physiology. 2017 doi: 10.1002/jcp.25786. [DOI] [PubMed] [Google Scholar]
- 3.Jones W, Bianchi K. Aerobic glycolysis: beyond proliferation. Front Immunol. 2015;6:227. doi: 10.3389/fimmu.2015.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to Coupling of the Warburg Effect with Glutamine Catabolism in Cancer Cells. Cell Reports. 2016;17:821–836. doi: 10.1016/j.celrep.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner DK, Harvey AJ. Blastocyst metabolism. Reprod Fertil Dev. 2015;27:638–654. doi: 10.1071/RD14421. [DOI] [PubMed] [Google Scholar]
- 7.Li K, Wahlqvist ML, Li D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients. 2016:8. doi: 10.3390/nu8110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etchegaray J-P, Mostoslavsky R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Molecular cell. 2016;62:695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rani V, Deep G, Singh RK, Palle K, Yadav UCS. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hu C, Fan L, Cen P, Chen E, Jiang Z, Li L. Energy Metabolism Plays a Critical Role in Stem Cell Maintenance and Differentiation. Int J Mol Sci. 2016;17:253. doi: 10.3390/ijms17020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi: 10.1126/science.aaa2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi X, Alves T, Zeng X-L, Kibbey R, Estes M, Guan X. GLP-2 reprograms glucose metabolism in intestinal stem cells. The FASEB Journal. 2015;29:851–854. [Google Scholar]
- 13.Tang Y, Luo B, Deng Z, Wang B, Liu F, Li J, Shi W, Xie H, Hu X, Li J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ. 2016;4:e1821. doi: 10.7717/peerj.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. The EMBO journal. 2014;33:2782–2797. doi: 10.15252/embj.201488278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, et al. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. During MuSC activation metabolic reprograming initiates myogenic genes by lowering the NAD+/NADH ratio, which inhibits the deacetylase SIRT1 and therefore increases H4K16 acetylation, which induce the expression of proliferative genes. This study elucidates a novel pathway linking metabolic regulation to redox control of epigenetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell. 2015;17:651–662. doi: 10.1016/j.stem.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells and Development. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C-T, Shih Y-RV, Kuo TK, Lee OK, Wei Y-H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 19.Fillmore N, Huqi A, Jaswal JS, Mori J, Paulin R, Haromy A, Onay-Besikci A, Ionescu L, Thébaud B, Michelakis E, et al. Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival. PLoS ONE. 2015;10:e0120257. doi: 10.1371/journal.pone.0120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shum LC, White NS, Mills BN, Bentley KL, de M, Eliseev RA. Energy Metabolism in Mesenchymal Stem Cells During Osteogenic Differentiation. Stem Cells and Development. 2016;25:114–122. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31:1902–1909. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE. 2013;8:e77077. doi: 10.1371/journal.pone.0077077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metabolism. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 25.Candelario KM, Shuttleworth CW, Cunningham LA. Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. J Neurochem. 2013;125:420–429. doi: 10.1111/jnc.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, Boon R, Zhao H, Boeckx B, Chang J, et al. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. The EMBO journal. 2016;35:924–941. doi: 10.15252/embj.201592372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. 2016:5. doi: 10.7554/eLife.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien LC, Keeney PM, Bennett JP. Differentiation of Human Neural Stem Cells into Motor Neurons Stimulates Mitochondrial Biogenesis and Decreases Glycolytic Flux. Stem Cells and Development. 2015;24:1984–1994. doi: 10.1089/scd.2015.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masotti A, Celluzzi A, Petrini S, Bertini E, Zanni G, Compagnucci C. Aged iPSCs display an uncommon mitochondrial appearance and fail to undergo in vitro neurogenesis. Aging (Albany NY) 2014;6:1094–1108. doi: 10.18632/aging.100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR. Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell. 2016;19:476–490. doi: 10.1016/j.stem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, et al. Glycolysis-Mediated Changes in Acetyl-CoA and Histone Acetylation Control the Early Differentiation of Embryonic Stem Cells. Cell Metabolism. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 32•.Carey BW, Finley LWS, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. Thompson and colleges describe the requirement of elevated levels of α-ketoglutarate in mESCs to activate tet-dependent demethylation of DNA and decreased H3K27me3 to promote pluripotency. This work was also the first to discover that naïve mESCs are not dependent on exogenous glutamine for proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey AJ, Rathjen J, Gardner DK. Metaboloepigenetic Regulation of Pluripotent Stem Cells. Stem Cells International. 2016;2016:1816525. doi: 10.1155/2016/1816525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, et al. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell. 2016;19:502–515. doi: 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, et al. HIF1a induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. The EMBO journal. 2012;31:2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, Ramalho-Santos J, Van Houten B, Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M, et al. LIN28 Regulates Stem Cell Metabolism and Conversion to Primed Pluripotency. Cell Stem Cell. 2016;19:66–80. doi: 10.1016/j.stem.2016.05.009. Zhang et al is the first paper to demonstrate the inhibition of OxPhos by LIN28, through a let-7 independent mechanism, by directly binding the mRNA mitochondrial proteins and repressing their expression. They also uncover the essential role of LIN28 in glycolytic and epigenetic regulation of the transition of mESCs from the naïve to primed state. [DOI] [PubMed] [Google Scholar]
- 38.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 39.Chan Y-S, Göke J, Ng J-H, Lu X, Gonzales KAU, Tan C-P, Tng W-Q, Hong Z-Z, Lim Y-S, Ng H-H. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al. Derivation of naive human embryonic stem cells. Proceedings of the National Academy of Sciences. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo G, Meyenn von F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J. Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem cell reports. 2016;6:437–446. doi: 10.1016/j.stemcr.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Marsboom G, Zhang G-F, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J. Glutamine Metabolism Regulates the Pluripotency Transcription Factor OCT4. Cell Reports. 2016;16:323–332. doi: 10.1016/j.celrep.2016.05.089. This paper establishes a regulatory cascade in which elevated levels of glutathione, synthesized from extracellular glutamine, maintains a reduced environment that is required for OCT4 stability and DNA binding. The first report to describe that glutamine metabolic flux is required for the activity and maintenance of a core pluripotency factor, and therefore pluripotency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folmes CDL, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metabolism. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of Human Primary Somatic Cells by OCT4 and Chemical Compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T, et al. HIF1α Modulates Cell Fate Reprogramming Through Early Glycolytic Shift and Upregulation of PDK1-3 and PKM2. Stem Cells. 2014;32:364–376. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folmes CDL, Martinez-Fernandez A, Faustino RS, Yamada S, Perez-Terzic C, Nelson TJ, Terzic A. Nuclear reprogramming with c-Myc potentiates glycolytic capacity of derived induced pluripotent stem cells. J Cardiovasc Transl Res. 2013;6:10–21. doi: 10.1007/s12265-012-9431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, Borkent M, Apostolou E, Alaei S, Cloutier J, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y, Guo WT, Tian S, He X, Wang XW, Liu X, Gu KL, Ma X, Huang D, Hu L, et al. miR-290/371-Mbd2-Myc circuit regulates glycolytic metabolism to promote pluripotency. The EMBO journal. 2015;34:609–623. doi: 10.15252/embj.201490441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Hawkins KE, Joy S, Delhove JMKM, Kotiadis VN, Fernandez E, Fitzpatrick LM, Whiteford JR, King PJ, Bolanos JP, Duchen MR, et al. NRF2 Orchestrates the Metabolic Shift during Induced Pluripotent Stem Cell Reprogramming. Cell Reports. 2016;14:1883–1891. doi: 10.1016/j.celrep.2016.02.003. This study was the first to demonstrate that elevated levels of ROS are required in early IPSC formation to establish an oxidative state that allows for NRF2 to initiate the aerobic glycolytic program through HIF1α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baird L, Llères D, Swift S, Dinkova-Kostova AT. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proceedings of the National Academy of Sciences. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 54.Ying Z, Chen K, Zheng L, Wu Y, Li L, Wang R, Long Q, Yang L, Guo J, Yao D, et al. Transient Activation of Mitoflashes Modulates Nanog at the Early Phase of Somatic Cell Reprogramming. Cell Metabolism. 2016;23:220–226. doi: 10.1016/j.cmet.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 55••.Kida YS, Kawamura T, Wei Z, Sogo T, Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR, et al. ERRs Mediate a Metabolic Switch Required for Somatic Cell Reprogramming to Pluripotency. Cell Stem Cell. 2015;16:547–555. doi: 10.1016/j.stem.2015.03.001. The authors document a burst of oxidative phosphorylation, caused by PGC-1 and EERα/γ, within SCA1-/CD34- cells as the earliest described required event of reprograming, and this burst induces the establishment of aerobic glycolysis in IPSC formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shyh-Chang N, Daley GQ. Lin28: Primal Regulator of Growth and Metabolism in Stem Cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]