Abstract
Purpose
Posthepatectomy liver failure is a serious complication and considered to be caused by increased portal pressure and flow. Splanchnic vasoactive agents and propranolol are known to decrease portal pressure. The aim of this study was to identify optimal candidates with potential for clinical use among somatostatin, terlipressin, and propranolol using rats with 90% hepatectomy.
Methods
Rats were divided into 5 groups: sham operation (n = 6), control (n = 20), propranolol (n = 20), somatostatin (n = 20), and terlipressin group (n = 20). Seven-day survival rates and portal pressure change were measured, and biochemical, histologic, and molecular analyses were performed.
Results
Portal pressure was significantly decreased in all 3 treatment groups compared to control. All treatment groups showed a tendency of decreased liver injury markers, and somatostatin showed the most prominent effect at 24 hours postoperatively. Histologic liver injury at 24 hours was significantly decreased in propranolol and terlipressin groups (P = 0.016, respectively) and somatostatin group showed borderline significance (P = 0.056). Hepatocyte proliferation was significantly increased after 24 hours in all treatment groups. Median survival was significantly increased in terlipressin group compared to control group (P < 0.01).
Conclusion
Terlipressin is considered as the best candidate, while somatostatin has good potential for clinical use, considering their effects on portal pressure and subsequent decrease in liver injury and increase in liver regeneration.
Keywords: Hepatectomy, Liver failure, Liver regeneration, Somatostatin, Terlipressin
INTRODUCTION
Small remnant liver after liver resection or small graft relative to recipient's body weight or metabolic demand after partial liver transplantation may lead to similar clinical consequences such as prolonged jaundice, coagulopathy, massive ascites, acidosis, or gastrointestinal bleeding, eventually leading to liver failure [1,2]. Although the risk of death in those patients increases significantly, specific treatment is not available other than avoiding surgery beforehand and only supportive measurements are being performed in clinical practice [3]. Those phenomena have been known as posthepatectomy liver failure (PHLF) and small-for-size syndrome (SFSS) in the setting of liver resection and liver transplantation, respectively, which is considered as one of the most significant and urgent unmet needs in the field of liver surgery.
Recently, there have been studies suggesting that PHLF and SFSS share similar pathophysiologic processes [4]. Excessive portal pressure and mesenteric blood flow to small remnant liver or partial liver graft cause sinusoidal endothelial and Kupffer cell injury with release of cytokines [5], which subsequently leads to failure of both regeneration and functional recovery of the liver.
Splanchnic vasoconstrictor agents such as somatostatin and terlipressin exert their vasoactive effects by binding to receptors mainly on splanchnic vasculatures shifting splanchnic blood volume to systemic circulation [6]. Also, propranolol as a nonselective beta-blocker has also been known to decrease portal pressure in cirrhotic patients although the mode of action is quite different. Those agents have been in clinical use in the setting of variceal bleeding or hepatorenal syndrome due to their preferential effects on splanchnic blood flow, and their favorable impacts on clinical outcomes have been reported in many clinical trials with acceptable complication profiles [7,8]. However, studies regarding the effects of splanchnic vasoconstrictors in the setting of PHLF or SFSS are limited. Although there have been several studies evaluating the effects of somatostatin or terlipressin separately using animal models [9,10], comparative studies using both agents have not been reported. Considering the patients' critical condition in these clinical settings, it might be very difficult to perform a randomized clinical trial.
Our aim in this study was to identify optimal candidates with potential for clinical use in the future by comparing the effects of somatostatin and terlipressin along with propranolol on portal pressure and survival after massive hepatectomy in animal models. Using a 90% hepatectomy model in rats, we also compared their effects on changes in histologic findings, biochemical parameters, gene expression profiles related to liver injury, and regeneration.
METHODS
Study design
This study was approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC-2011-161) and followed the Animal Research: Reporting In Vivo Experiments guidelines. Animal handling and care were in accordance with the National guidelines for ethical animal research.
We used 8-week-old male Sprague-Dawley rats that weighed 250–300 g at the time of operation. Rats were housed in a temperature- and humidity-controlled room with a 12:12 hour dark-light cycle. Rats were not fed for 6 hours before the operation. All procedures were performed in clean conditions. Rats were allowed to drink a 20% glucose solution for a day postoperatively.
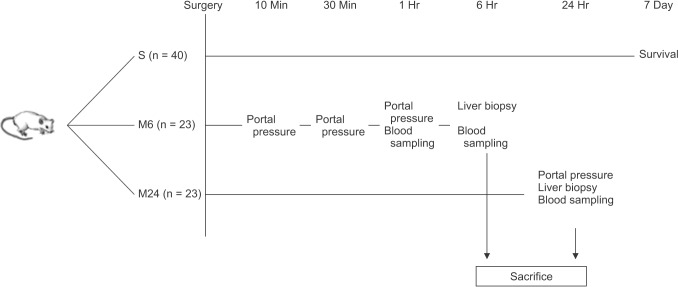
A total of 86 rats were used. Rats were divided into 5 groups: sham operation (n = 6), control (n = 20), propranolol (60 mg/kg/day, orally dissolved in water, started 3 days before the operation, Indenol, Dong Gwang Pharmaceutical, Seoul, Korea; n = 20), somatostatin (20 µg/kg in 1-mL saline, intravenous [IV], Somatostatin, Ferring, Saint-Prex, Switzerland; n = 20), and terlipressin (50 µg/kg in 1-mL saline, IV, Glypressin, Ferring, Switzerland; n = 20). Each group was subdivided into S (n = 10), M6 (n = 5), and M24 (n = 5) subgroups. Subgroup S was used for 7-day survival analysis. Subgroup M6 was used for blood sampling at 1 and 6 hours postoperatively, portal pressure measurement at 10, 30, 60 minutes, and 6 hours postoperatively, and liver biopsy at 6 hours postoperatively. Subgroup M24 was used for blood sampling, portal pressure measurement, and liver biopsy at 24 hours postoperatively (Fig. 1).
Fig. 1. Schematic drawing of experimental flow. S represents a subgroup for survival analysis. Ten rats were used for control, propranolol, somatostatin, and terlipressin group, respectively. Animals were followed up for 7 days after surgery. M6 and M24 represent subgroups for measurement of portal pressure, sampling of blood and liver tissue at 6 and 24 hours after surgery, respectively. Each subgroup was comprised of sham (n = 3), control (n = 5), propranolol (n = 5), somatostatin (n = 5), and terlipressin group (n = 5).
Ninety percent hepatectomy in rats
To create a small-for-size liver, 90% partial hepatectomy was performed. Ketamine (60 mg/kg, Ketamine HCL, Huons, Seongnam, Korea) and xylazine (10 mg/kg, Rompun, Bayer, Leverkusen, Germany) were injected intraperitoneally for anesthesia. After a midline incision was created, the liver was exposed using a retractor. The falciform ligament, ligamentum venosum, and other perihepatic ligaments were dissected, and liver resection was performed in the order of left lobe, median lobe, right inferior lobe, and right superior lobe using the vessel-oriented technique described by Kubota et al. [11] Each hepatic artery and portal vein were ligated with 6-0 polypropylene (Prolene, Ethicon, Somerville, NJ, USA) followed by piercing sutures for liver parenchymal resection and hepatic vein ligation, simultaneously.
Rats in the sham operation group were prepared in the same manner preoperatively and anesthetized with the same agents and doses. After incision and exposure, the abdominal wall was closed without any procedure in the sham group. Somatostatin or terlipressin was administered through the inferior vena cava (IVC) 5 minutes postoperatively in each group, and the same amount of normal saline was administered in the control group. Terlipressin and somatostatin were injected subcutaneously in subgroup S once daily, and propranolol was administered orally. Rats in all groups were injected subcutaneously with 5 mL of 10% glucose solution postoperatively and were allowed to drink 20% glucose solution on the day following the operation.
Seven-day survival
Ten rats in each group (subgroup S) were used for survival analysis. Survival time (hour after operation) was observed in all rats.
Portal pressure measurement
Six rats in the sham group and 10 rats in each of the other groups (n = 40) were used to measure portal pressure. Portal pressure was measured at 10, 30, and 60 minutes postoperatively in subgroup M6 and at 24 hours postoperatively in subgroup M24. Portal pressure was measured by direct puncture of the portal vein using a 26-G needle (BD Precisionglide, Becton Dickinson, Franklin Lakes, NJ, USA) with an invasive intravascular pressure monitoring device (Vigileo Monitor, Edwards Lifesciences, Irvine, CA, USA).
Biochemical analysis
AST, ALT, and total bilirubin were checked for the degree of liver damage and function. Six rats in the sham group and 10 rats in each of the other groups (n = 40) were used. Blood sampling was done at 1 and 6 hours postoperatively in subgroup M6 and at 24 hours postoperatively in subgroup M24. Blood sampling was done via the IVC, and a biochemical analyzer (TBA-200FR NEO, Toshiba Medical Systems Corp., Otawara, Japan) was used.
Histologic scoring
Rats were sacrificed for liver biopsy at 6 and 24 hours postoperatively in subgroup M6 (n = 20), subgroup M24 (n = 20), and the sham group (n = 6), respectively. Tissue was prepared and stained with hematoxylin and eosin for histologic examination and scored based on a previous study by Dahmen et al. [12].
Ki-67 immunohistochemical staining
Formalin-fixed paraffin-embedded tissue blocks were sectioned to a thickness of 4 µm. Sections were deparaffinized for 5 minutes 3 times in xylene and rehydrated for 5 minutes per session. For antigen retrieval, 10 mM citrate buffer (pH, 6.0; DAKO, Glostrup, Denmark) was heated in a microwave for 15 minutes. To reduce nonspecific background staining, slides were incubated in a hydrogen peroxide block (Polink-2 detection kit, GBI, Bothell, WA, USA) for 10 minutes. The slides were washed 3 times in Tris-buffered saline (TBS; pH, 7.6) for 5 minutes and incubated with a block (Polink-2 detection kit) at room temperature for 5 minutes. We then used anti-Ki-67 antibodies (1:100, Diagnostic BioSystems, Pleasanton, CA, USA). A primary antibody amplifier (Polink-2 detection kit) was applied and incubated for 10 minutes. Subsequently, a secondary antibody reaction was achieved with a horseradish peroxidase polymer (Polink-2 detection kit). After washing with TBS, the samples were stained with a 3,3′-diaminobenzidine chromogenic reaction and counter-stained with Mayer's hematoxylin (Scytec, Logan, UT, USA). Liver tissue was obtained at 6 hours postoperatively in subgroup M6 (n = 20) and at 24 hours postoperatively in subgroup M24 (n = 20). The mean number of stain-positive hepatocytes of four high-power fields was calculated in each specimen.
RNA extraction and amplification
Total RNA from liver tissues was extracted using a PicoPure RNA isolation kit (Arcturus) (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's recommendations. After deoxyribonuclease treatment (Invitrogen, Carlsbad, CA, USA), RNA was eluted and stored at −80℃ until use. All total RNA samples were first tested for quality on an Agilent Bioanalyzer 2100B using an RNA Pico LabChip Kit (Agilent Technologies, Palo Alto, CA, USA) and subsequently were amplified with the RiboAmp OA RNA Amplification Kit (Arcturus). The quality of the amplified RNA was again evaluated on an RNA Pico LabChip Kit (Agilent Technologies).
Real-time quantitative reverse-transcription polymerase chain reaction analysis
Approximately 2 µg total RNA from the rat liver was reverse transcribed using the StrataScript first-strand synthesis system (Stratagene, La Jolla, CA, USA). Complementary DNA (cDNA) was amplified with endothelin-1 (ET-1), endothelial nitric oxide synthase (eNOS), hepatocyte growth factor (HGF), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers and SYBR Green polymerase chain reaction (PCR) master mix (Applied Biosystems, Foster City, CA, USA) by PCR with an iCycler real-time PCR detection system (Bio-Rad, Hercules, CA, USA) for 40 cycles. Relative RNA levels were calculated using the iCycler software and a standard equation (Applied Biosystems). ET-1 (Rn00561129_m1), eNOS (Rn02132634_s1), and HGF (Rn00566673_m1) (Applied Biosystems) were used as probe and primer. PCR was repeated 4 times with one sample and normalization was done with GAPDH. C(t), which is the threshold cycle number at which the initial amplification becomes detectable by fluorescence (defined as_Rn_0.1 in our experiments), was determined. A standard curve was established in C(t) versus a copy number of ssDNA (equivalent to cDNA after RT), and the copy number of cDNA was determined for each RT sample as an approximation of mRNA copies. All analyses were standard procedures of the 7700 detection system. For GAPDH PCR, a 2-pg equivalent of total RNA after RT was used because of its great abundance. Quantification of ET-1, eNOS, and HGF mRNA was expressed as copy numbers per nanogram of total RNA and also as the ratio of ET-1, eNOS, and HGF to GAPDH. The value for each sample was an average of three independent PCR measurements. Intraexperimental variation (standard deviation/mean) of a sample was within 10%, and interexperimental variation (standard deviation/mean) was within 20%.
Data analysis
All quantitative values are expressed as the median and 25%–75% interquartile range, unless specified otherwise. Seven-day survival rates were compared using Kaplan-Meier survival analysis. Gene expression analysis was done with a nested analysis of variance (ANOVA) using the least square mean after log transformation. Other data were analyzed by ANOVA, Kruskal-Wallis test, and Mann-Whitney mean analysis. Statistical significance was defined as a P-value < 0.05, and all statistical analyses were performed using Predictive Analytics SoftWare Statistics 18.0 (IBM, Somers, NY, USA).
RESULTS
Portal pressure measurement
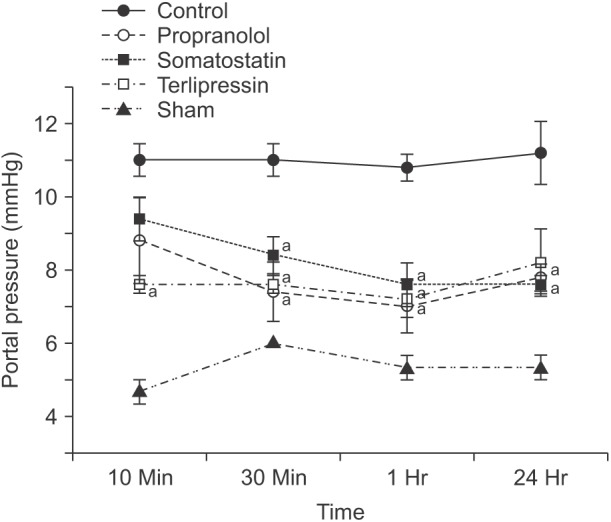
Portal pressures were measured at 10, 30, 60 minutes, and 24 hours postoperatively in all groups (Fig. 2). Portal pressures of the control group were significantly increased compared to sham group at each time point (P = 0.036). Overall, in the treatment groups, portal pressures were measured higher than those of sham group but lower than those of control group. In the propranolol group, pressure difference was significant at 30, 60 minutes, and 24 hours compared with that of the control group (P = 0.016, P = 0.008, and P = 0.008, respectively). Portal pressure of somatostatin group also showed a significant decrease at 30, 60 minutes, and 24 hours compared with that of the control group (P = 0.016, P = 0.008, and P = 0.008, respectively). Similarly, portal pressure in the terlipressin group was significantly decreased at 10, 30, and 60 minute compared to that in the control group (P = 0.008, P = 0.008, and P = 0.008, respectively).
Fig. 2. Portal pressure was measured at 10, 30, 60 minutes, and 24 hours after completion of 90% hepatectomy. Dots indicate the mean and whiskers the standard error of the mean. aP < 0.05 vs. control in the same time point.
Biochemical analysis
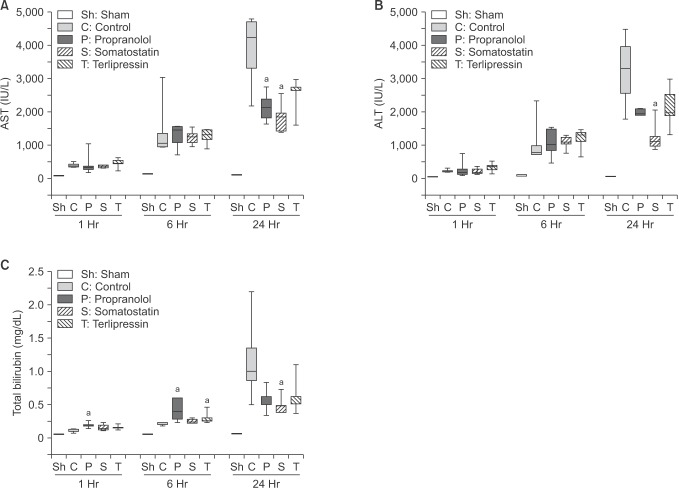
AST, ALT, and total bilirubin were measured in each group at 1, 6, and 24 hours postoperatively (Fig. 3). Overall, median values of each measurement increased over time in every group except sham group suggesting aggravation of liver injury and hepatic function after massive hepatectomy. Compared to control group at each time point, AST level was significantly decreased at 24 hours in propranolol and somatostatin groups (P = 0.032 and P = 0.016, respectively). ALT level showed a similar trend, but only somatostatin group showed a significant decrease at 24 hours compared to control group (P = 0.016). Finally, bilirubin was significantly higher than that of control group in propranolol group at 1 and 6 hours (P = 0.008 and P = 0.016, respectively) and in terlipressin group at 6 hours (P = 0.016). However, at 24 hours, median values of all treatment groups were lower than that of control group (P = 0.056, P = 0.016, and P = 0.222 in propranolol, somatostatin, and terlipressin groups, respectively).
Fig. 3. Postoperative evolutions of AST (A), ALT (B), and total bilirubin (C) among sham, control, and each treatment group. Data are expressed as the median, with the 25%–75% percentiles in boxes and the 5%–95% percentiles as whiskers. aP < 0.05 vs. control group in the same time point.
Gene expression
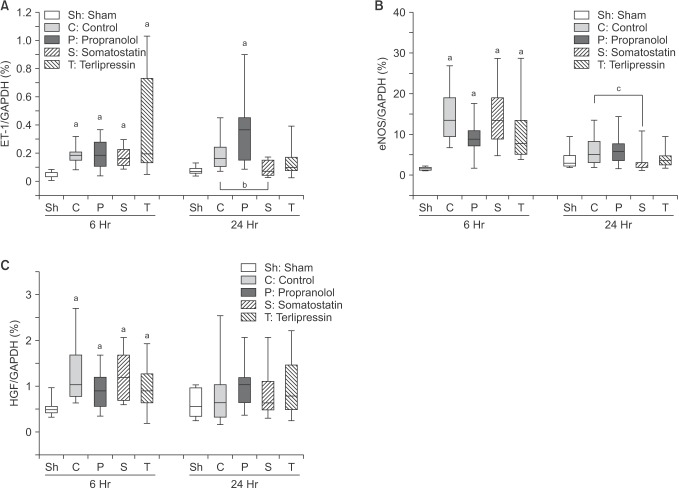
Relative expression differences were calculated with C(t) values of each gene in all groups (Fig. 4). At 6 hours postoperatively, when compared to sham group, ET-1, eNOS, and HGF expressions were significantly increased in all groups including control group. However, when compared to the control group, no treatment group showed significant difference suggesting that those increases are the result of the hepatectomy, not from the medications, and this time point may be too early to show significant effects of medications at molecular levels.
Fig. 4. Level of mRNA expression of endothelin-1 (ET-1) (A), endothelial nitric oxide synthase (eNOS) (B), and hepatocyte growth factor (HGF) (C) was measured from liver tissue obtained at 6 and 24 hours after 90% hepatectomy using real-time quantitative polymerase chain reaction. Data are expressed as the median, with the 25%–75% percentiles in boxes and the 5%–95% percentiles as whiskers. aP < 0.05 vs. sham group; bP = 0.068 control vs. somatostatin; cP = 0.058 control vs. somatostatin. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
At 24 hours postoperatively, all measurements did not show significant difference compared to sham group, except ET-1 in propranolol group. When compared to control group, somatostatin group showed decreased expression of ET-1 and eNOS with borderline significance (P = 0.068 and P = 0.058, respectively), which corresponds to the results of AST, ALT, and total bilirubin measurements.
Scoring of histologic change
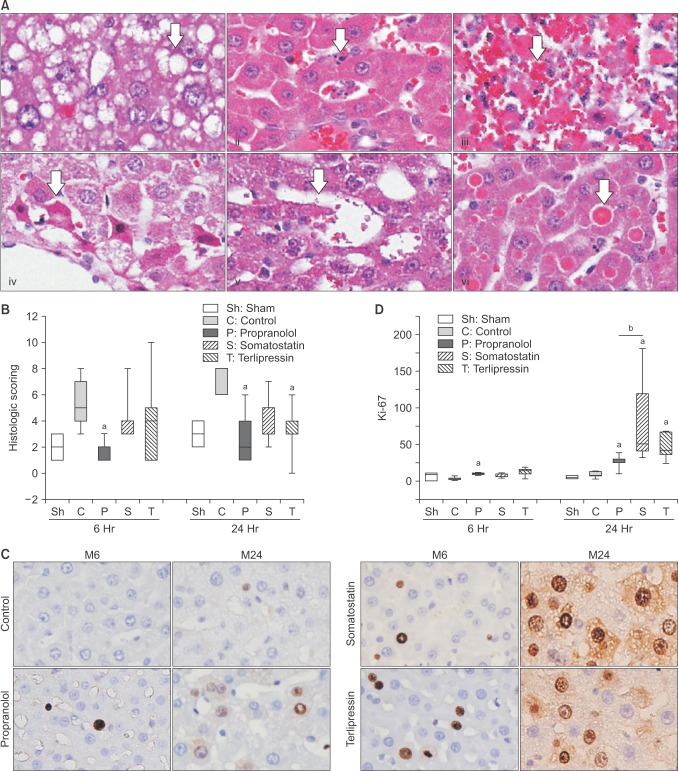
Histologic scoring of H&E stained tissue obtained at 6 and 24 hours postoperatively was performed in each group (Fig. 5). Representative images of findings used for scoring are shown in Fig. 5A. Histologic scoring at 6 hours after hepatectomy was 2 (1.5–2.5), 5 (4–7), 2 (1–2), 4 (3–4), and 4 (1–5) in sham, control, propranolol, somatostatin, and terlipressin group, respectively (Fig. 5B). Propranolol group showed significantly lower scores compared to that of control group (P = 0.008). Histologic scores at 24 hours after hepatectomy were 3 (2.5–3.5), 6 (6–8), 2 (1–4), 3 (3–5), and 3 (3–4), respectively. Propranolol and terlipressin group showed significantly lower scores compared to that of control group (P = 0.016 and P = 0.016, respectively). Somatostatin group showed borderline significance (P = 0.056).
Fig. 5. (A) Representative images of microscopic findings after 90% partial hepatectomy (H&E, ×1,000). White arrow in each image indicates small vacuolar transformation of the hepatocyte cytoplasm (i), activated Kupffer cell (ii), confluent necrosis (iii), small cell necrosis (iv), sinusoidal dilatation (v), and eosinophilic globuli (vi) in the hepatocyte cytoplasm. (B) Results of histologic scoring at 6 and 24 hours after partial hepatectomy. (C) Representative images of Ki-67 immunohistochemical staining positive hepatocytes in each group. M6 and M24 represent tissues obtained at 6 and 24 hours after hepatectomy, respectively. (D) Count of Ki-67 immunohistochemical stain (×1,000) positive hepatocytes for evaluation of cellular proliferation. Number of Ki-67 positive hepatocyte was increased in all treatment groups at 24 hours postoperatively. Somatostatin group showed the most pronounced increase, which was significantly higher than propranolol group. Data are expressed as the median, with the 25%–75% percentiles in boxes and the 5%–95% percentiles as whiskers. aP < 0.05 vs. control group in the same time point, bP < 0.05 propranolol vs. somatostatin group.
Ki-67 immunohistochemical staining
Ki-67 immunohistochemical staining was used to compare the degree of hepatocyte proliferation in each group (Fig. 5C, D). At 6 hours after hepatectomy, there was no significant difference among groups. However, at 24 hours after hepatectomy, all treatment groups showed a significant increase in number of Ki-67 positive hepatocytes compared with that of control group (P = 0.032, P = 0.008, and P = 0.008 in propranolol, somatostatin, and terlipressin group, respectively). Especially, somatostatin group showed a significant increase when compared to that of propranolol group (P = 0.016).
Seven-day survival
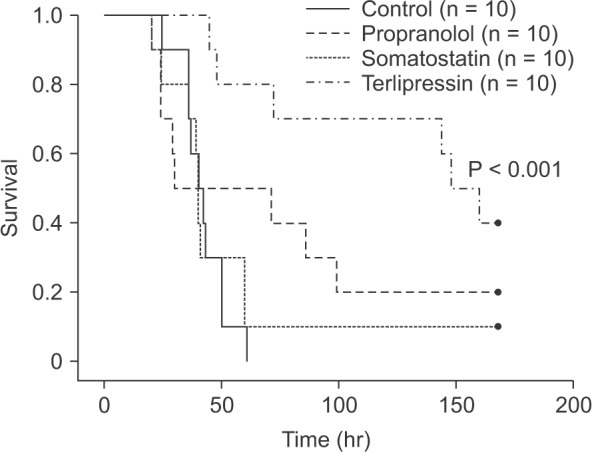
Seven-day survival rates were 17.5% for all groups and 0%, 20%, 10%, and 40% for the control, propranolol, somatostatin, and terlipressin groups, respectively. Median survival time of each group was 41 (36–50), 30 (24–99), 40 (36–60), and 148 hours (72–168 hours) for control, propranolol, somatostatin, and terlipressin group, respectively. Kaplan-Meier survival curves are shown in Fig. 6. Survival rate of terlipressin group showed statistically significant improvement compared to control and somatostatin group (P < 0.001 and P = 0.007, respectively).
Fig. 6. Kaplan-Meier survival analysis after 90% hepatectomy in rats. Terlipressin group showed a significantly increased 7-day survival rate (P < 0.001 vs. control, P = 0.007 vs. somatostatin group). Propranolol and somatostatin groups did not show significant survival improvement compared to control group.
DISCUSSION
With the development of operative techniques, major hepatectomy and various liver transplantations have been performed widely. However, PHLF or SFSS resulting from small remnant liver or graft can result in life-threatening complications and have been recognized as a major challenge to overcome, influencing the whole process of surgical treatment of liver disease—not just limited to patient selection or treatment outcomes [3,4]. To lower the risk of PHLF, it is important to preserve at least 25% of liver volume and probably more for diseased livers or elderly patients. Similarly, with SFSS, approximately 30%–35% standard liver volume or >0.8% of graft-to-recipient weight ratio should remain. Increased shear stress in the hepatic sinusoids resulting from portal hypertension is considered to cause SFSS [9]. Increased portal pressure after hepatectomy results in shear stress in the hepatic sinusoids, and modest amounts of shear stress promote liver regeneration [13]. However, excessive shear stress resulting from massive hepatectomy brings endothelial injury to the sinusoids followed by microcirculatory impairment, causing sinusoidal congestion, space of Disse destruction, and impaired regeneration in the end [14]. To prevent or manage PHLF or SFSS, several procedures such as portal vein banding, splenic artery embolization, mesocaval or portocaval shunts, and splenectomy have been introduced [15]. However, these procedures are invasive and often times irreversible. When the liver requires more portal flow after successful regeneration, another invasive procedure may be required to reverse relative portal insufficiency. Complications such as prolonged shunts or postsplenectomy sepsis after those invasive procedures have also been reported [16]. If we can apply medication instead of invasive procedures to reduce the risk of PHLF or SFSS, the risk of potential complications may be avoided or at least minimized, and treatment itself can be readily reversible depending on the patient's condition.
Propranolol is a nonselective beta-adrenergic antagonist. It decreases cardiac output and blocks adrenergic dilatory tone in splanchnic arterioles, leaving alpha-adrenergic mediated vasoconstriction, which in turn decreases portal flow and pressure. Although somatostatin and terlipressin share some characteristics in their biologic activities, they basically bind to different receptors, which implies potential differences in detailed action mechanisms or even final outcomes in the setting of PHLF or SFSS. In the treatment of acute variceal bleeding, somatostatin selectively decreases splanchnic blood flow and portal flow. It is also known to decrease portal pressure by diminishing sinusoidal pressure independent of nitric oxide and to prevent ischemia-reperfusion injury by lessening oxidative stress. Also, Hessheimer et al. [17] suggested that somatostatin exerts some direct cytoprotective effect on hepatic stellate cells, which express somatostatin receptors. On the other hand, terlipressin decreases portal pressure by direct and strong splanchnic vasoconstriction. It was shown to reduce intrahepatic vascular resistance, resulting in a concomitant increase in hepatic arterial blood flow. Additionally, its short-term use improves hepatic hyperdynamic state without influencing sodium excretion and renal function.
Considering proven efficacies and favorable complication profiles from accumulated clinical experiences in variceal bleeding and hepatorenal syndrome, those agents may be considered as sources readily applicable to clinical use in the setting of PHLF or SFSS. However, for various reasons, reports from clinical use or trial have been extremely rare in this setting. Only anecdotal experiences have been reported so far [18,19].
Various hepatectomy models have been developed since Higgins et al. [20] performed a 70% hepatectomy. In this study, we used 90% hepatectomy model with the vessel-oriented technique described by Kubota et al. [11], which seemed appropriate to establish a PHLF setting. Madrahimov et al. [21] reported 7-day survival rates of 100% after 90% hepatectomy, but in the experiment of Myronovych et al. [22], all rats died within 30 hours postoperatively. Makino et al. [23] showed a 7-day survival rate of 0% and average survival time of 20.6 hours. Gaub and Iversen [24] administered a glucose solution postoperatively, which increased the survival rate by supplying regenerative factors such as insulin and preventing hypoglycemia.
In previous studies of portal pressure in rats, Debbaut et al. [25] reported that mean portal pressure after 90% partial hepatectomy was 10.92 mmHg and 12.5 mmHg in the experiment of Dahmen et al. [12], which were consistent with results in our experiments. In our study, mean portal pressures at 30 and 60 minutes and 24 hours were all decreased significantly along with a decrease in biochemical parameters, especially in somatostatin group at 24 hours postoperatively, which showed that portal pressure control in the immediate postoperative period was important, and there was a significant decrease in portal pressure for 24 hours with one dose of each medication. Although the differences in amount of reduction in portal pressure were not evident among medications, the improvement in biochemical parameters were most evident in somatostatin group. Tissue injury was evident at 6 hours postoperatively in the control group and the severity seemed to worsen over time. In contrast, treatment groups showed lower histologic injury scores compared to control group, especially at 24 hours postoperatively. At the same time, regenerative index from Ki-67 staining showed significant increase in all treatment groups compared to control group at 24 hours postoperatively. Especially, somatostatin group showed the largest increase in regenerative activity.
ET-1 is a vasoconstrictor produced in the sinusoidal endothelial system and is increased by various liver injuries. ET-1 expression is related to sinusoidal contraction followed by portal hypertension. Xu et al. [9] reported that somatostatin suppressed ET-1 expression and prevented endothelial contraction. We also showed increased ET-1 expression in control and treatment groups compared with the sham group. Specifically, the somatostatin group showed decreased ET-1 expression compared with the control group with borderline significance. Somatostatin seems to have a beneficial effect on the small remnant liver not only by decreasing portal pressure resulting in decreased sinusoidal injury but also by suppressing ET-1 expression and preventing sinusoidal contraction. Endothelial shear stress stimulates nitric oxide production preventing endothelial contraction, and eNOS itself keeps hepatocytes from progressing to apoptosis [26]. eNOS expression was increased at 6 hours postoperatively in all groups compared to sham group but showed decreased expression at 24 hours postoperatively in the somatostatin group compared with the control group, which may suggest an effect of somatostatin on the sinusoidal endothelium. HGF is a main growth factor related to liver regeneration and acts as a mitogenic stimulus. Its plasma concentration increases 1 hour after hepatectomy and it is produced 3 to 6 hours postoperatively by hepatic stellate cells and continues for 24 hours in rats [27]. Increased portal flow is considered as an important triggering factor. Liver regeneration is known to occur in proportion to the increase in portal flow and portal pressure. In an experiment using a swine liver transplantation model, Kelly et al. [28] reported that as the graft size decreased, the regenerative portion increased. Increased portal flow encourages regeneration, but increased pressure damages the hepatic microstructure. Therefore, liver dysfunction shown in PHLF or SFSS is primarily related not to impaired regeneration but to the structural problems caused by increased portal pressure followed by sinusoidal injury. Here, HGF expression in both control and treatment groups were increased compared with that in the sham group, but there was no difference between control and treatment groups at both 6 and 24 hours postoperatively. We suggest that the decreased portal pressure by medication was modest and it might be enough to alleviate structural deterioration but not enough to affect HGF expression.
In our study, the 7-day survival rate was 0% and mean survival time was 39.6 hours in the control group, which was consistent with previous studies by other researchers. The terlipressin group had a significant survival benefit, which seemed to be attributed to decrease in portal pressure and subsequent decrease in liver injury and increase in liver regeneration. Although significant decrease in portal pressure was observed in all 3 treatment groups, it was reflected as improvement in survival only in terlipressin group. Favorable effects of somatostatin, shown from biochemical, histologic, and molecular analyses supporting its protective functions against the high flow state after liver resection, did not translate as significant improvement in survival in this study. A potential explanation for lack of survival improvement in somatostatin group can be due to inefficient drug delivery. Although Xu et al. [9] administered somatostatin once after liver transplantation with the same dose used here and showed survival improvement, somatostatin has a very short half-life of <3 minutes, and it is likely difficult to maintain effective blood concentrations throughout the entire period of the experiment. We injected somatostatin subcutaneously once daily in subgroup S, but blood concentrations might have been too low to be fully effective without continuous infusion, which is unavailable in rat models and can be considered as a limitation of this study. However, this issue may be easily resolved in large animal models or in clinical settings by continuous infusion. On the other hand, the lack of survival improvement with propranolol despite decreased portal pressure can be explained by the hypotensive effect of propranolol, which could aggravate hemodynamic instability after major hepatectomy. Blood volume in rats is just 15–18 mL, and 90% hepatectomy could result in a hypovolemic state postoperatively. Propranolol could worsen hemodynamic instability with its systemic hypotensive effect. In previous studies, Reyes-Salcido et al. [29] reported that propranolol increased thymidine kinase activity and cell proliferation, resulting in enhanced liver regeneration, while Walldorf et al. [30] reported that propranolol instantly diminished lipid accumulation, preventing regeneration after partial hepatectomy in rats. Considering these contrasting results and our findings, propranolol may not be the optimal candidate in the setting of PHLF or SFSS. Meanwhile, terlipressin has a relatively longer half-life and might contribute to hemodynamic stability, considering its pharmacodynamics.
This study has some limitations such as evaluating the effects of hemodynamic agents in small animals, lack of optimal delivery method for somatostatin, and lack of observation after 24 hours postoperatively other than survival rate due to high mortality from 90% hepatectomy, which is a part of the natural course of this disease entity. However, main aspects of medications of interest and their effects on counteracting portal hypertension in the setting of PHLF were able to be evaluated. Based on this study, more studies using large animals or clinical studies would be possible for the management of PHLF or SFSS using these splanchnic vasoactive agents.
In conclusion, terlipressin showed a significant improvement in survival after 90% hepatectomy in rats and is considered the best candidate for further study. Somatostatin also showed favorable responses in various analyses, which seemed to have great potential as a useful candidate for treatment of PHLF or SFSS in clinical settings.
ACKNOWLEDGEMENTS
This work was supported by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A1A05006371, 2017R1A2B2005754).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Helling TS. Liver failure following partial hepatectomy. HPB (Oxford) 2006;8:165–174. doi: 10.1080/13651820510035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 3.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, Fard N, et al. Small for Size and Flow (SFSF) syndrome: an alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol. 2016;40:267–275. doi: 10.1016/j.clinre.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–149. doi: 10.1016/s0039-6060(97)90283-x. [DOI] [PubMed] [Google Scholar]
- 6.Mukhtar A, Dabbous H. Modulation of splanchnic circulation: role in perioperative management of liver transplant patients. World J Gastroenterol. 2016;22:1582–1592. doi: 10.3748/wjg.v22.i4.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baik SK, Jeong PH, Ji SW, Yoo BS, Kim HS, Lee DK, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol. 2005;100:631–635. doi: 10.1111/j.1572-0241.2005.41381.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Man K, Zheng SS, Liang TB, Lee TK, Ng KT, et al. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transpl. 2006;12:621–627. doi: 10.1002/lt.20630. [DOI] [PubMed] [Google Scholar]
- 10.Yi NJ, Chang SH, Kwon CH, Cho JY, Yang EL, Suh KS, et al. Terlipressin effect of portal pressure control on liver regeneration in 90% hepatectomized rats. J Korean Surg Soc. 2005;69:157–165. [Google Scholar]
- 11.Kubota T, Takabe K, Yang M, Sekido H, Endo I, Ichikawa Y, et al. Minimum sizes for remnant and transplanted livers in rats. J Hep Bil Pancr Surg. 1997;4:398–404. [Google Scholar]
- 12.Dahmen U, Madrahimov N, Madrahimova F, Ji Y, Schenk A, Dirsch O. Small-for-size syndrome in the rat: does size or technique matter? J Surg Res. 2008;149:15–26. doi: 10.1016/j.jss.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518–526. doi: 10.1007/BF02385805. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka K, Hatano E, Narita M, Kitamura K, Yanagida A, Asechi H, et al. Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transpl. 2011;17:60–69. doi: 10.1002/lt.22189. [DOI] [PubMed] [Google Scholar]
- 15.Gruttadauria S, Pagano D, Luca A, Gridelli B. Small-for-size syndrome in adult-to-adult living-related liver transplantation. World J Gastroenterol. 2010;16:5011–5015. doi: 10.3748/wjg.v16.i40.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez HD, Liu ZW, Cashman S, Fusai GK. Small for size syndrome following living donor and split liver transplantation. World J Gastrointest Surg. 2010;2:389–394. doi: 10.4240/wjgs.v2.i12.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessheimer AJ, Escobar B, Munoz J, Flores E, Gracia-Sancho J, Taura P, et al. Somatostatin therapy protects porcine livers in small-for-size liver transplantation. Am J Transplant. 2014;14:1806–1816. doi: 10.1111/ajt.12758. [DOI] [PubMed] [Google Scholar]
- 18.Ozden I, Kara M, Pinarbasi B, Salmaslioglu A, Yavru A, Kaymakoglu S, et al. Somatostatin and propranolol to treat small-for-size syndrome that occurred despite splenic artery ligation. Exp Clin Transplant. 2007;5:686–689. [PubMed] [Google Scholar]
- 19.Yu YD, Kim DS, Byun GY, Seo SO. Can propranolol be a viable option for the treatment of small-for-size syndrome? Liver Transpl. 2012;18:747–748. doi: 10.1002/lt.23420. [DOI] [PubMed] [Google Scholar]
- 20.Higgins GM, Anderson RM. Experimental pathology of the liver I restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 21.Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, et al. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363–372. doi: 10.1016/j.jhep.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, et al. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171–176. doi: 10.1016/j.jss.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Gaub J, Iversen J. Rat liver regeneration after 90% partial hepatectomy. Hepatology. 1984;4:902–904. doi: 10.1002/hep.1840040519. [DOI] [PubMed] [Google Scholar]
- 25.Debbaut C, De Wilde D, Casteleyn C, Cornillie P, Van Loo D, Van Hoorebeke L, et al. Modeling the impact of partial hepatectomy on the hepatic hemodynamics using a rat model. IEEE Trans Biomed Eng. 2012;59:3293–3303. doi: 10.1109/TBME.2012.2199108. [DOI] [PubMed] [Google Scholar]
- 26.Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001;120:1251–1262. doi: 10.1053/gast.2001.23239. [DOI] [PubMed] [Google Scholar]
- 27.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 28.Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, et al. Porcine partial liver transplantation: a novel model of the “small-for-size” liver graft. Liver Transpl. 2004;10:253–263. doi: 10.1002/lt.20073. [DOI] [PubMed] [Google Scholar]
- 29.Reyes-Salcido V, Villalobos-Molina R. Evidence that dl-propranolol increases thymidine kinase activity, cell mitosis, and beta-adrenoceptors during rat liver regeneration. Arch Med Res. 2003;34:273–275. doi: 10.1016/s0188-4409(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 30.Walldorf J, Hillebrand C, Aurich H, Stock P, Hempel M, Ebensing S, et al. Propranolol impairs liver regeneration after partial hepatectomy in C57Bl/6-mice by transient attenuation of hepatic lipid accumulation and increased apoptosis. Scand J Gastroenterol. 2010;45:468–476. doi: 10.3109/00365520903583848. [DOI] [PubMed] [Google Scholar]