Abstract
Hodgkin lymphoma (HL) is a unique hematopoietic neoplasm characterized by cancerous Reed-Sternberg cells in an inflammatory background. Patients are commonly diagnosed when in their 20s-30s, present with supra-diaphragmatic lymphadenopathy, often with systemic B symptoms. Even in advanced stage disease, HL is highly curable with combination chemotherapy, radiation or combined modality treatment. Although the same ABVD chemotherapeutic regimen has been the mainstay of therapy for over last 30 years, risk adapted approaches have helped de-escalate therapy in low risk patients while intensifying treatment for higher risk patients. Even patients who are not cured with initial therapy can often be salvaged with alternate chemotherapy combinations, the novel antibody-drug conjugate Brentuximab, high dose autologous or allogeneic hematopoietic stem cell transplant (allo-HCT). The Programmed death-1 (PD-1) inhibitors Nivolumab and Pembrolizumab have both demonstrated high response rates and durable remissions in relapse/refractory HL. Alternate donor sources and reduced intensity conditioning have made allo-HCT a viable option for more HL patients. Future research will look to integrate novel strategies into earlier lines of therapy to improve the HL cure rate and minimize long term treatment toxicities.
Keywords: Hodgkin lymphoma, PD-1 inhibitor, antibody-drug conjugate, Brentuximab, Immunotherapy, PET-adapted therapy, allogeneic stem cell transplant
Introduction
The first descriptions of what came to be known as Hodgkin disease date back to 1832 when the eminent British pathologist Thomas Hodgkin described an autopsy case series of patients with lymphadenopathy and splenic enlargement1. It was not till the late 1990s that our understanding of the entity as a malignancy arising from germinal center or post-germinal center B cells led to the term ‘Hodgkin lymphoma’ (HL) gaining favor2. Characteristically, the cancer cells form a minority of the tumor and are surrounded by a reactive inflammatory milieu comprising lymphocytes, eosinophils, neutrophils, histiocytes and plasma cells. These malignant cells can be pathognomonic multinucleate giant cells or large mononuclear cells and are together referred to as Hodgkin and Reed–Sternberg (HRS) cells.
Hodgkin lymphoma is estimated to account for about 10% of cases of newly diagnosed lymphoma in the United States (8,260 of 80,500), the remainder being Non-Hodgkin lymphoma. Of 21,210 estimated deaths yearly due to lymphoma, about 1,070 (or 5%) are from Hodgkin lymphoma. It accounts for about 0.5% of newly diagnosed cases of cancer in the United States and about 0.2% of all cancer deaths. However lymphoma is the most common cancer diagnosed in adolescents (aged 15 to 19 years) accounting for 21% of new diagnoses, almost two-thirds of which is Hodgkin lymphoma3.
Epidemiology and pathogenesis
Males are expected to comprise about 56% of patients newly diagnosed with HL in 20173. The median age of diagnosis is 39 years; HL is most frequently seen in the 20 – 34 year age group, which makes up almost a third of new diagnoses. The incidence rates do not seem to vary between white and black Americans (3.1 new cases per 100,000 males) but are about half as much in Asian/pacific islanders (1.6 new cases per 100,000 males) and American Indians/Alaskan natives. Incidence rates are also lower in Hispanic Americans (2.6 new cases per 100,000 males) compared to white/black populations. Incidence rates of HL have stayed flat since the mid-1970s, but mortality rates have steadily declined from 1.3 cases per 100,000 in 1975 to 0.3 cases per 100,000 in 2014. Across all stages of diagnosis, the relative 5 year survival of patients with HL has improved from 70% to 85% in the same time period4.
The etiology of HL is not well understood. Epstein-Barr virus (EBV) is a ubiquitous gammaherpesvirus spread mainly through saliva and is the causative agent for infectious mononucleosis. EBV-encoded small RNAs (EBERs) are noncoding RNAs expressed abundantly in latently EBV-infected cells and can be detected by in situ hybridization (ISH). EBV is detected in HRS cells in a majority of HL specimens from the developing world, but much less frequently in the industrialized countries of North America and Western Europe5,6. The risk of developing EBV-positive HL is significantly increased (relative risk, 4.0; 95% CI 3.4 to 4.5) after an episode of infectious mononucleosis, with an estimated median incubation time of 4.1 years (95% CI 1.8 to 8.3). However, the absolute risk of developing HL after infectious mononucleosis remains small at approximately 1 in 10007. Plasma EBV-DNA positivity is an independent predictor of treatment failure, both at diagnosis and on 6 month follow-up on therapy8.
Immunosuppression in a variety of medical conditions increases the risk of HL. The incidence of HL is significantly higher in the HIV-infected population than in the general population (standardized rate ratio [SRR] 14.7 in a US study). The advent of HAART (Highly Active Anti-Retroviral Therapy) has indirectly led to the increase in rates of HL in HIV-infected patients, SRR increasing from 11.7 (1992-1995) to 17.9 (2000-2003)9. Most cases are EBV positive and can occur in patients with normal CD4 counts with a more aggressive histological phenotype, but survival in HIV-associated HL has improved significantly in the post-HAART period10,11. The incidence of HL also increases after solid organ transplantation and in patients with a history of autoimmune conditions such as rheumatoid arthritis (Odds Ratio [OR] = 2.7), systemic lupus erythematosus (OR = 5.8) and sarcoidosis (OR = 14.1)12.
Classification
Hodgkin lymphoma is subdivided into classical Hodgkin lymphoma (cHL) and nodular lymphocyte predominant (NLPHL) based on morphology and immunohistochemistry13,14. Over 90% of cases are of cHL, which behaves as an aggressive neoplasm while LPHL has an indolent biology in most instances. The major focus of this review is cHL, which itself is subdivided into four histologic subtypes based on morphology, abundance of the HRS cells and the background infiltrate. The malignant HRS cell in all subtypes of cHL exhibits a characteristic immunophenotypic pattern of CD15 +, CD30 + and CD45 −.
Nodular sclerosis
Nodular sclerosis cHL (NSCHL) is the most common subtype, accounting for about 70% of cHL cases in the developed world and characterized by neoplastic lacunar type HRS cells in an inflammatory background of band-forming sclerosis. Mediastinal adenopathy is seen in 80% of cases and bulky nodes (>10cm in diameter) are present in about half the patients15. Association with Epstein-Barr virus is less frequent and NSCHL has a better prognosis overall than other types of cHL13.
Mixed Cellularity
Mixed cellularity classical Hodgkin lymphoma (MCCHL) comprises 20-25% of cHL in the United States, but is more frequent in patients with HIV infection and in developing countries. The HRS cells are scattered in a diffuse mixed inflammatory background without sclerosing fibrosis. Epstein-Barr encoded latent membrane protein 1 (LMP1) and EBV small nuclear RNA transcripts (EBER) are expressed much more frequently (approximately 75% of cases) than in nodular sclerosing cHL16.
Lymphocyte rich
Lymphocyte rich classical Hodgkin lymphoma (LRCHL) comprises about 5% of all cHL; specimens have scattered HRS cells within a nodular or diffuse cellular background of small lymphocytes and without neutrophils or eosinophils. Patients tend to have peripheral adenopathy without bulky mediastinal involvement and usually present with early stage disease. Treatment outcomes are excellent using modern combination chemotherapy regimens with rare treatment failures17,18.
Lymphocyte depleted
Lymphocyte-depleted classical Hodgkin lymphoma (LDCHL) is the rarest cHL subtype in the developed world accounting for <1% of cases. Tumor specimens are diffusely infiltrated by HRS cells and without a significant reactive inflammatory infiltrate. It is often seen in association with HIV infection and has a more aggressive disease course compared to the other cHL subtypes.
Overall patients who have LDCHL and MCCHL have a significantly worse prognosis compared to patients who have NSCHL, whereas patients who have LRCHL have the best prognosis19. In addition to subtyping, grading systems incorporating tumor characteristics such as extent of infiltration by the malignant HRS cells, eosinophilia and lymphocyte depletion have been developed and are prognostic of outcomes, but are not frequently used20,21.
Clinical presentation and initial workup
HL is most commonly diagnosed in the 20-34y age group, accounting for 31% of new cases but can be seen across the age spectrum from adolescents to the elderly. Painless lymphadenopathy enlarging over months is a common mode of presentation. The three commonest sites of disease presentation - mediastinal involvement or left neck nodal enlargement or right neck nodal enlargement are each seen in about 60% of patients (not mutually exclusive). Other sites include splenic, axillary, abdominal, hilar or inguino-femoral in descending order of frequency22. Mediastinal masses can grow quite large before a diagnosis is made; bulky disease is defined by transverse diameter of the tumor mass exceeding 10 cm and confers a poorer prognosis in early stage patients. B symptoms – fevers, chills, night sweats or unexplained weight loss >10% of body weight are frequent in patients with advanced stage or bulky disease and are prognostic, thereby included in the staging system. Severe unremitting pruritus without obvious skin pathology on exam can be resistant to topical and systemic agents and can be an early clue to the presence of clinically occult HL23.
Inflammatory markers, such as the erythrocyte sedimentation rate (ESR) can be elevated at diagnosis and can serve as a useful lab marker of disease response. Similarly leukocytosis/neutrophilia and anemia can be seen in patients with extensive disease and portend a poorer prognosis. Excisional biopsy of the involved node (or less commonly an involved extranodal site or bone) is preferred to establish a definitive diagnosis. Occasionally, core biopsies are adequate but the smaller biopsy specimens are often inadequate for definitive diagnosis insofar as HRS cells may be missed in these specimens and assessment of architecture suboptimal. Whereas core biopsies in some circumstances establish a diagnosis, fine needle aspirates never reveal architecture and since the malignant cells of HL are not detected by flow cytometry, fine needle aspirate is never sufficient for a new diagnosis.
Staging Hodgkin Lymphoma
The Ann Arbor staging system with Cotswolds modification has been in use since 1989, but incorporated somewhat antiquated procedures such as liver biopsy, laparotomy and bone marrow trephine for initial staging24. Fluorodeoxyglucose (FDG) positron emission tomography (PET)–computed tomography (CT) has very high sensitivity and specificity in HL – an international workshop in Deauville, France helped establish a consensus on simple, reproducible criteria for PET interpretation in HL. The resultant 5-point ‘Deauville scale’ helped pave the way for risk adapted therapy based on interim PET findings and to later establish the role of PET-CT at initial staging and at the end of treatment25. The Lugano classification in 2014 modernized staging for lymphomas; fluorodeoxyglucose (FDG) positron emission tomography (PET)–computed tomography (CT) was formally incorporated into standard staging. A modification of the Ann Arbor descriptive terminology is used for anatomic distribution of disease extent, but treatment is based on classifying patients as having limited (stages I and II, non-bulky) or advanced (stage III or IV) disease, with stage II bulky disease considered as limited or advanced disease based on histology and other prognostic factors26,27. The Lugano staging and response assessment is fairly new and not yet universally accepted as most major studies have used the Deauville scale.
FDG PET-CT has also supplanted the utility of bone marrow biopsy in HL; in a large retrospective study, no patients with marrow involvement were assessed as having limited stage disease on PET-CT. Although there were a few cases where patients were upstaged from Stage III on PET-CT to Stage IV on the bone marrow, management did not change in any as advanced stage HL is treated similarly regardless of stage28.
Treatment of newly diagnosed Hodgkin Lymphoma
Chemotherapy and radiation are the mainstays of cHL treatment, unlike some types of indolent non-Hodgkin lymphoma where observation is an option. Before the advent of combination chemotherapy, this was a relentless and invariably fatal malignancy with a 5 year survival of less than 10%30. Advances in understanding the biology of the disease and improvement in modalities of chemotherapy and radiotherapy have improved survival across the board in every stage of cHl. It is a highly curable malignancy - the 5-year relative survival for patients diagnosed with cHL at age 0-19 yrs. is 96.4% and 89.8% for those diagnosed between the age of 20-64 yrs. (2007-2013 SEER data)4.
Radiotherapy had been used to treat Hodgkin lymphoma (then termed Hodgkin’s disease) since the early 1900s, but it took several decades for a better understanding of the patterns of spread and the fields and doses of radiation that would be required to turn a palliative measure into a potentially curative treatment. Development of the high-energy linear accelerator at Stanford in the 1950s allowed for more precise fields and accurate dose delivery. Since, in most cases, cHL spreads to contiguous nodal sites, fields involved by tumor and adjacent fields could be irradiated allowing cure of many patients with early, and in some cases, advanced stage disease31,32. Involved field radiation (treating only sites of gross disease) was replaced by extended-field radiation, where regions adjacent to known sites of disease were also treated. Mantle field radiation (covering neck, axillae, mediastinum and hilar regions) along with the inverted Y field to treat the abdomen and spleen, together formed ‘total nodal irradiation’. A Stanford study showed an 80% long term freedom from progression for patients treated with total nodal irradiation, but came with a high risk of long term radiation related toxicities33,34. Involved-site and Involved-node radiation fields were developed in the 3 dimensional and PET-directed radiotherapy era to minimize toxicities of radiotherapy.
Early trials with single agent cytotoxic chemotherapeutics like mechlorethamine, chlorambucil and cyclophosphamide showed promising response rates of 50% but none of these responses were durable despite prolonged courses of maintenance chemotherapy30. Discovery of novel drugs like the vinca alkaloids and procarbazine led to the advent of combination chemotherapy. MOPP (nitrogen mustard [Meclorethamine], vincristine, procarbazine and prednisone) was developed at the National Cancer Institute by Vincent Devita’s group; 6 months of MOPP resulted in 81% of patients with newly diagnosed advanced cHL achieving a complete remission. Treatment paradigms shifted from continuous therapy to a defined endpoint as about half the complete responders were disease free 4 years after completion35.
MOPP was superior to extended-field radiation therapy in treatment of patients with Stages IB, IIA, IIB, or IIIA cHL– although complete remission rates were similar in both arms (96%), relapses were fewer with MOPP (13% vs 35%). The projected 10-year disease-free survival of patients randomized to receive radiation was 60% vs 86% (p=.009) for those who received MOPP36.
Simultaneously to the development of MOPP at the NCI, the Italian Istituto Nazionale Tumori under Gianni Bonnadonna developed a combination regimen with the newly discovered chemotherapeutic drugs Adriamycin i.e. doxorubicin, Bleomycin, Vinblastine and Dacarbazine (ABVD) based on their individual anti-lymphoma activity and non-overlapping toxicities. When compared, the two regimens showed comparable results - six cycles of either MOPP or ABVD yielded similar complete remission rates of about 75% in advanced cHL37. Despite their non-cross-resistant chemo sensitivity profiles, alternating monthly cycles of MOPP and ABVD over 12 months did not improve upon the long term cure rates of either regimen when given alone over 6-8 months. In a large multi-center trial of patients with advanced cHL, complete response rate was 67 percent in the MOPP group, 82 percent in the ABVD group, and 83 percent in the MOPP-ABVD group (P = 0.006 for the comparison of MOPP with the other two regimens).
Overall survival at five years was 66 percent for MOPP, 73 percent for ABVD, and 75 percent for MOPP-ABVD (P = 0.28 for the comparison of MOPP with the doxorubicin regimens)38. These results showing a failure-free survival advantage but no overall survival difference for ABVD over MOPP did not change after a median follow-up of 14 years39. Due to its better toxicity profile compared to MOPP (lesser bone marrow suppression, long term myelotoxicity and minimal effect on fertility) ABVD has become the de-facto standard chemotherapeutic regimen used to treat cHL in the United States of America.
Early stage classical Hodgkin lymphoma
Patients with early stage cHL have an excellent prognosis with very high cure rates of >90%. In past series, these patients were more likely to die from long-term treatment related complications than from lymphoma itself. Therefore the focus of more recent trials has been to minimize the long term risks of curative intent chemotherapy and radiation. Higher doses of radiation and extended fields of treatment were associated with long term cardio-pulmonary toxicities and increased rates of breast cancer in women. The goal of combined modality therapy (incorporating chemotherapy with radiation) has been to minimize doses of radiotherapy and substitute with combination chemotherapy, thereby preserving efficacy while minimizing toxicity.
Early trials of combined modality therapy used the MOPP regimen – a Danish study in supradiaphragmatic stage I and II Hodgkin lymphoma patients compared total nodal irradiation with mantle field radiation followed by six cycles of MOPP and showed a significant reduction in treatment failures with the addition of combination chemotherapy40. The 1500 patient EORTC-GELA (European Organization for Research and Treatment of Cancer- Groupe d’Études des Lymphomes de l’Adulte) H8-F trial compared three cycles of MOPP combined with doxorubicin, bleomycin, and vinblastine (ABV) plus involved-field radiotherapy vs. subtotal nodal radiotherapy alone - the 5 year event-free survival rate was significantly higher for the combined modality arm at 98% vs. 74%. The 10-year overall survival estimates were 97% and 92%, respectively (P=0.001), definitively establishing combination therapy as standard of care in early stage favorable risk patients41.
The German HD10 trial looked into further de-escalation of treatment in favorable risk patients, comparing four treatment groups of a combination chemotherapy regimen of two different intensities followed by involved-field radiation therapy at two different dose levels. 1370 patients with newly diagnosed early-stage cHL with a favorable prognosis were randomized to receive either 2 or 4 cycles of ABVD followed by 20 or 30 Gray (Gy) of involved-field radiation therapy. There was no significant difference between any of the four groups in terms of treatment failure or overall survival, while adverse events and acute toxic effects of treatment were most common in the patients who received four cycles of ABVD and 30 Gy of radiation. Therefore two cycles of ABVD followed by 20 Gy of involved-field radiation is currently the optimal approach to deliver combined modality treatment in early stage favorable risk cHL42. Attempts to further de-escalate chemotherapy in favorable risk patients by omitting dacarbazine and/or bleomycin have not been successful. The German HD13 trial showed dacarbazine could not be omitted from ABVD without a substantial loss of efficacy and dropping bleomycin did not meet a predefined non-inferiority margin43.
The NCIC CTG (National Cancer Institute of Canada Clinical Trials Group)HD.6 trial compared 4-6 cycles of ABVD with subtotal nodal radiation therapy in early stage cHL patients. In the favorable risk cohort, both treatment approaches showed identical freedom from disease progression and overall survival (98%) at the 12-yr mark44. A radiation free approach is therefore feasible in early stage favorable risk patients who have disease at sites vulnerable to late radiation toxicities such as near the breast and heart.
More modern approaches have attempted to use interim-PET to define subgroups of patients who would benefit from the omission or radiotherapy after short duration ABVD. The EORTC (European Organization for Research and Treatment of Cancer) H10 trial looked at omission of involved-node radiotherapy vs combined modality therapy in patients attaining a negative PET scan after two cycles of ABVD, but had to be stopped early due to increased rates of progression in the chemotherapy only arm45. The RAPID trial randomized patients with negative PET after 3 cycles of ABVD to consolidation involved-field radiotherapy or no further treatment. This was a non-inferiority study looking to show a less than 7% 3-year progression-free survival difference between the two groups, but could not with an absolute risk difference of −3.8 percentage points (95% CI, −8.8 to 1.3)46. The ABVD only group had a 3-year progression free survival of 90.8%, therefore the contribution of radiotherapy was small but statistically ‘not-insignificant’ in PET negative patients. Therefore optimal therapy in this group of patients with an overall excellent prognosis should be individualized keeping in mind the patient’s age, sex and co-morbidities.
Early stage cHL with unfavorable prognostic factors
Unfavorable risk factors are defined differently across the world by various cooperative groups. This has made comparisons across countries (and continents) challenging but they incorporate criteria such as an elevated ESR, presence of B symptoms, increased number of involved nodal sites and tumor bulk (outlined in Table 1). The EORTC classifies age ≥50y as unfavorable while the German Hodgkin study group (GHSG) classifies any extra nodal extension from an involved node as poor risk.
Table I.
Staging Hodgkin lymphoma and risk factors
| Stage | Involved sites | A or B suffix to stage | |
|---|---|---|---|
| Limited stage favorable | Limited stage unfavorable | ||
| I | One node or a group of adjacent nodes | Limited stage with additional risk factors *
|
Absence or presence of B symptoms (below) qualifies any stage as A or B respectively
|
| II | Two or more nodal groups on the same side of the diaphragm | ||
| Advanced stage | |||
| II bulky | Single nodal mass, in contrast to multiple smaller nodes, of 10 cm or greater than a third of the transthoracic diameter at any level of thoracic vertebrae | ||
| III | Nodes on both sides of the diaphragm; nodes above the diaphragm with spleen involvement | ||
| IV | Additional noncontiguous extra lymphatic involvement (for e.g. lung, liver or skeletal metastases) | ||
Risk factors are defined differently by various study groups
The EORTC-GELA H8-U trial showed identical 5-year event free survival and 10 year overall survival for 3 approaches of combined modality therapy intensity – 4 cycles of MOPP-ABV chemotherapy and involved-field radiotherapy were equivalent to 6 cycles of chemotherapy and subtotal nodal radiotherapy41. The shorter and less toxic regimen (4 cycles of chemotherapy with involved-field radiotherapy) has since become the standard of care for patients with unfavorable risk cHL.
The GHSG conducted HD11 in a 2×2 design to compare 4 cycles of ABVD vs a more intense regimen BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) in combination with 20 Gy vs 30 Gy of involved field radiotherapy. BEACOPP did not improve outcomes over ABVD, but the trial did help establish the importance of radiation dose (30 Gy was superior to 20 Gy) when used in combination with ABVD in unfavorable risk patients47. Further chemotherapy dose-intensification using 2 cycles of escalated BEACOPP in the HD14 trial improved upon the 5-year progression free survival compared to combined modality therapy with 4 cycles of ABVD by 6.2%, but was associated with more acute toxicities and no difference in overall survival48.
Radiation sparing approaches have been studied less, but may have a role in patients who have early stage non-bulky disease. The NCIC CTG-ECOG (Eastern Cooperative Oncology Group) HD.6 trial compared 4-6 cycles (depending on rapidity of response) of ABVD with 2 cycles of ABVD and extended field radiotherapy. In non-bulky unfavorable risk patients, 12-year survival was superior in the chemotherapy alone arm (94% vs 87%; hazard ratio= .05; p= 0.04); despite a lower 12-year freedom from progressive disease (87% vs 92%; HR=1.91; P=.05). The poorer OS in the combination arm despite higher cHL cure rates was attributed to higher treatment related deaths on the combined modality arm44.
However trials such as HD.6 in early stage unfavorable risk cHL have not been optimal, using older radiation techniques with extended fields rather than modern involved-site radiation therapy which greatly limits exposure to surrounding normal tissues, the major cause of long term toxicity. In select patients with non-bulky disease, skipping radiation likely results in short term loss of benefit but might be beneficial by limiting long term toxicities.
Advanced cHL
Advanced cHL is treated mainly with combination chemotherapy. The evolution of modern cytotoxic combination regimens have been outlined in the introduction to this section and established ABVD as the primary regimen to treat advanced cHl.
The group at Stanford University developed a combined-modality approach – the Stanford V regimen using reduced doses of doxorubicin and bleomycin (aimed at minimizing cardiac and pulmonary toxicity) and delivered over a shorter course of 12 weeks, but required the addition of irradiation to sites of disease >5cm in size at diagnosis and for macroscopic splenic involvement. Multiple randomized trials have shown mostly equivalent outcomes in response rates, progression-free survival and overall survival when Stanford V has been compared to ABVD49,50. It remains an option where limiting the duration of chemotherapy or reducing anthracycline/bleomycin exposure takes precedence over the potential additive toxicity from irradiation.
The German Hodgkin lymphoma study group pioneered an intensified seven drug combination of eBEACOPP (escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) to try to improve upon ABVD. Multiple trials have compared eBEACOPP with ABVD and mostly shown an improved response rate and progression-free survival benefit without leading to significant overall survival benefit51,52.
In an Italian trial comparing escalated BEACOPP with ABVD with salvage therapy planned upon treatment failure, the estimated 7-year rate of freedom from first progression was 85% in the eBEACOPP group, as compared with 73% in the ABVD group (p=0.004). Severe toxicities were much lower in the ABVD group compared to the eBEACOPP group (43% vs. 81% with hematologic toxic effects, P<0.001; and 7% vs. 19% with non-hematologic toxic effects including severe infections and mucositis, P=0.001). Overall survival was no different in the two groups due to effective second-line salvage treatment comprising an ifosfamide-containing combination for reinduction and high-dose BEAM for consolidation. Escalated BEACOPP exposes 7 out of 8 patients to an unnecessarily higher risk of toxicities, as they would likely be cured with ABVD53.
A recently updated Cochrane meta-analysis in 2017 included five large randomized controlled trials comparing eBEACOPP with ABVD for patients with early unfavorable or advanced stage cHl and showed a significant PFS benefit (HR 0.54, 95% CI 0.45 - 0.64) and a small but statistically significant overall survival benefit (HR 0.74, 95% CI 0.57 - 0.97)54. This comes at the cost of increased toxicity; of particular concern to the young demographic of patients developing cHL, is the 50% infertility rate in women and 90% azoospermia in men treated with escalated BEACOPP55,56. Therefore the adoption of escalated BEACOPP has been limited outside of Germany. ABVD is the most commonly used regimen for advanced cHL in the United States of America.
The field of advanced CHL treatment has moved from using higher doses and intensity of cytotoxic agents to a risk-adapted approach. Gallamini et al showed that an interim FDG PET-CT done after 2 cycles of chemotherapy was highly prognostic – patients with a positive PET had a progression free survival of 12.8% at the 2 year mark, whereas those with a complete response had a 95% progression free survival57. This finding shifted the focus to de-escalating therapy for interim-PET negative patients and dose intensification for those with positive scans.
The RATHL (Risk adapted therapy in Hodgkin lymphoma) study enrolled 1200 patients with advanced cHL, all of whom received 2 cycles of ABVD followed by an interim PET/CT scan. Those who had a negative interim-PET (84% of patients enrolled) were randomized to either continue ABVD or omit bleomycin for cycles 3-6 (AVD group). The 3 year progression free survival (85%) and overall survival (97%) were identical in both groups, and patients on the AVD arm had lower rates of febrile neutropenia and lung toxicity.
In contrast, the 16% of patients who had a positive interim PET had dose intensification of therapy with a BEACOPP based regimen; most of them (74.4%) were able to achieve PET negativity on subsequent imaging. Patients in this poor risk group had a 3 year progression free survival of 67.5%, much higher than what was seen in past experiences with continuation of ABVD after a positive interim-PET. This strategy spares patients who have an excellent prognosis from excess treatment toxicities, while reserving more intensive chemotherapy regimens for patients who are likely to benefit58.
32.1% of patients enrolled in the study had bulky disease at presentation and this finding was not prognostic in patients who had a negative interim PET. Only 6.5% of patients in this trial received consolidative radiation to sites of bulky disease suggesting that omitting radiotherapy would be reasonable in those with a negative interim PET.
Treatment of relapsed/refractory classical Hodgkin Lymphoma
Most patients with cHL are cured by first line therapy, but a significant percentage of patients (especially those with advanced cHL) relapse or have primary refractory disease despite advances in combination chemo-radiotherapy and risk-adapted treatment escalation53,58. Patients with primary refractory disease (i.e. those who do not achieve a remission at the end of treatment) and patients who relapse less than 1 year from primary treatment have a worse prognosis in this group59.
Salvage high dose combination chemotherapy followed by autologous hematopoietic stem-cell transplantation(HSCT) in patients who are responding to treatment has shown the best long term outcomes and could potentially cure about 50% of patients with relapsed cHL. In a 141 patient retrospective analysis of patients with relapsed/refractory cHL treated with consolidative auto HSCT, with a median follow-up of 6.3 years (range, 1–20 years), the probability of PFS at 5 and 10 years was 48% (95% CI, 39%–57%) and 45% (95% CI, 36%–54%) and that of OS was 53% (95% CI, 44%–62%) and 47% (95% CI, 37%–57%), respectively60.
Two separate randomized trials have shown the benefit of high dose therapy with autologous stem cell rescue compared to chemotherapy alone. The British National Lymphoma Investigation (BNLI) group compared high-dose chemotherapy (BEAM = carmustine, etoposide, cytarabine, and melphalan) plus autologous bone marrow transplantation with the same drugs at lower doses not requiring bone-marrow rescue (mini-BEAM) in patients with relapsed Hodgkin lymphoma61. Progression-free survival showed significant difference in favor of BEAM plus ABMT (p = 0.005). A similar study by the GHSG started salvage with two cycles of Dexa-BEAM (dexamethasone and carmustine, etoposide, cytarabine, and melphalan) and either two further courses of Dexa-BEAM or high-dose BEAM and autologous stem cell rescue. Among patients with chemo-sensitive disease (complete or partial responders), freedom from treatment failure at 3 years was significantly better for patients given BEAM-HSCT (55%) than for those on Dexa-BEAM (34%; p=0·019)62. A 2013 Cochrane review showed a significant PFS benefit for the addition of auto HSCT to conventional chemotherapy but only non-significantly positive difference regarding overall survival63.
Other combination chemotherapeutic salvage regimens are more favorable to outpatient administration with a gentler toxicity profile and include drugs that have not been used as a part of frontline therapy such as Ifosfamide (ICE – Ifosfamide, carboplatin and etoposide) or Gemcitabine (GDP - Gemcitabine, dexamethasone and cisplatin)64,65. Patients with relapsed cHL who do not respond to salvage chemotherapy (chemo resistant) have significantly worse outcomes and should be candidates for novel approaches using antibody-drug conjugates or immunotherapy.
Antibody-drug conjugate
A characteristic of cHL is the universal expression of the receptor CD30 on the Hodgkin Reed-Sternberg (HRS) cell. Monoclonal antibodies (mAb) such as the anti-CD20 antibody Rituximab, have played a significant role in increasing cure rates for patients with Non-Hodgkin lymphoma. Arming antibodies with highly potent toxic agents for selective intracellular release provides both targeting and delivery of doses of cytotoxic therapy that would not be possible if given systemically. Brentuximab vedotin is an antibody-drug conjugate (ADC) containing the potent antimitotic drug, monomethylauristatin E (MMAE), linked to an anti-CD30 monoclonal antibody through a cleavable dipeptide linker. The linker undergoes proteolysis in lysosomes inside the CD30 positive cell and free MMAE is released. Intracellular concentrations of released drug are high over a prolonged time period, yet the amount of effluxed drug is sufficient to exert bystander activity on surrounding CD30 antigen-negative cells also66.
In a pivotal phase 2 trial in patients with relapsed/refractory cHL after failed hematopoietic autologous stem cell transplantation, Brentuximab vedotin (BV) had an overall response rate of 75% with a complete response rate of 34%. Five year follow-up showed an overall survival of 41% and progression-free survival rate of 22%, but patients who achieved a complete response (CR) to BV (n=34) had estimated OS and PFS rates of 64% (95% CI: 48-80%) and 52% (95% CI: 34-69%), respectively. Of the 34 CR patients, 6 underwent a consolidative allo-SCT with estimated 5-year PFS and OS rates of 67% and 83%. The other 28 non-transplant CR patients had estimated 5-year PFS and OS rates of 48% (95% CI: 28-68) and 60% (95%CI: 41-78) respectively. Overall 9% of all enrolled patients remained in sustained CR without receiving any further anticancer therapy after treatment with Brentuximab vedotin (given by intravenous infusion once every 3 weeks, for up to 16 cycles) indicating that a limited course of treatment with BV could be curative even in a very hard to treat population of patients with relapsed/refractory cHL67. For elderly patients and those with medical comorbidities who are not candidates for multi-agent salvage chemotherapy and autologous stem cell transplant, BV can be an effective yet well tolerated therapy.
Neuropathy is the main non-hematological toxicity of BV; it is managed by dose reduction or drug holiday. Most patients experience either resolution or improvement in symptoms a year after completion of therapy and improvement can take several months67.
About half of patients with relapsed cHL after first line treatment can be cured with salvage chemotherapy and autologous stem-cell transplantation, but those who have primary refractory cHL (not achieving remission) and patients who relapse early after completion of first line therapy do much worse. The AETHERA trial aimed to see if early BV consolidation after autologous stem-cell transplantation could prevent relapse. Patients with unfavorable-risk relapsed or primary refractory classical Hodgkin lymphoma who had undergone autologous stem-cell transplantation were randomly assigned to receive 16 cycles of BV or placebo. Median progression-free survival was significantly improved for patients in the BV group at 42·9 months compared with those in the placebo group at 24·1 months, with a PFS hazard ratio of 0·57 (95% CI 0.40-0.81)68. Overall survival was no different between the two arms, likely confounded by 85% of patients in the placebo group receiving BV after progression. Across both arms, the 3-year rate of overall survival was >80%, a testament to the activity of BV both as consolidation and salvage therapy in patients at high risk of relapse after autologous stem-cell transplantation.
BV is being investigated in earlier lines of therapy for cHL. A phase 1 study in patients with advanced cHL getting first line therapy compared BV in combination with standard (ABVD) or a modified-standard (AVD) regimen showed unacceptably high pulmonary toxicity rates (44%) in the BV plus ABVD arm. The lung injury rates were much higher compared to past experiences with ABVD; BV is thought to potentiate bleomycin mediated lung damage and they should not be used together69. BV with AVD was well tolerated without unexpected side effects and no pulmonary toxicities, making this a promising regimen for earlier lines of therapy, especially for patients with pre-existing pulmonary compromise. A frontline trial in advanced cHL patients comparing BV + AVD with ABVD has been completed and is awaiting publication.
Immunotherapy
Combination chemotherapy is able to cure most patients with cHL, but for those with disease that did not respond to treatment (refractory cHL) or returned soon after completion of therapy (relapsed cHL), immunotherapy has drastically changed the vista. CHL tumors are almost unique in being composed of a tiny fraction of cancer cells (Hodgkin Reed Sternberg cells) in a sea of dysfunctional reactive immunologic cells (lymphocytes, plasma cells, macrophages, etc.) that comprise most of the tumor mass. The neoplastic Hodgkin Reed Sternberg (HRS) cells secrete a variety of cytokines and chemokines to manipulate the microenvironment and evade immune attack70. One of the pathways involved in functional impairment of T cells seen in tumor immune evasion is the Programmed death-1 (PD-1)-PD-1 ligand (PD-L1) signaling system. Tumor cells expressing PD-1 ligand on their surface engage the PD-1 receptor on T cells and inhibit T cell activation and proliferation.
PD-1 expression is markedly elevated in tumor-infiltrating T cells of cHL and PD-L1 expression is high in the malignant HRS cells71. Epstein-Barr virus (EBV) infection also induces PD-L1 expression in cHL72. Most cHL specimens show chromosome 9p24.1 alterations (56% show copy gain; 5% polysomy and 36% amplification) which result in overexpression of the PD-1 ligands and promote their induction through Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling73. All these factors made PD-1/PD-L1 a promising pathway to target therapeutically with immune checkpoint blockade.
In a heavily pre-treated population of patients with cHL (78% of whom had relapsed after autologous stem-cell transplantation and 78% after BV) an objective response to the novel PD-1 antibody Nivolumab was seen in 87% of patients including 17% complete remissions. Responses were also durable with a progression free survival rate at 24 weeks of 86%74. In a similar cohort of heavily pre-treated patients with cHL, Nivolumab demonstrated an objective response in 53 (66·3%, 95% CI 54·8-76·4) of 80 patients. In addition to being effective at shrinking cHL tumors, Nivolumab was well tolerated with the most common drug-related adverse events including fatigue, infusion-related reactions and rash75.
Pembrolizumab a PD-1 inhibitor was studied in a large trial of 210 adult cHL patients with refractory or relapsed disease after autologous stem cell transplantation (ASCT; 129 patients) and/or BV (175 patients), and received a median of four prior systemic therapies. The overall response rate was 69% (95% CI: 62, 75); partial responses were seen in 47% of patients and complete responses in 22%. The estimated median response duration was 11.1 months76.
Checkpoint inhibition is not associated with the toxicities of traditional cytotoxic therapy such as nausea, vomiting, hair loss, etc. but comes with a risk of several autoimmune side effects. These adverse reactions are related to a hyperactive T-cell response, resulting in the generation of high levels of CD4 T-helper cell cytokines or increased migration of cytolytic CD8 T cells within normal tissues77. Skin rash is the most common immune mediated side effect of checkpoint inhibition and presents most commonly with a maculopapular rash. More severe reactions including Sweet’s syndrome, Stevens Johnson syndrome and toxic epidermal necrolysis have been reported78. Pneumonitis affected about 5% of lung cancer patients treated with PD-1 inhibitors, but data in patients with cHL is lacking79. Fulminant myocarditis has been reported with combination immune checkpoint blockade and both PD-1 blockers discussed above80-83. Diarrhea/colitis and endocrine toxicities such as hypophysitis, hypothyroidism, hyperthyroidism, thyroiditis, and adrenal insufficiency have been widely described with checkpoint blockade84. Clinically significant immune related adverse events are managed by withholding anti PD-1 treatment and may require steroids and other immunosuppressive medications when severe.
Checkpoint inhibitors may be associated with imaging findings during treatment suggestive of progressive disease despite evidence of clinical benefit. Immune mediated ‘tumor flare’ or pseudo-progression can lead to patients being taken off treatment too early. To address this issue in the context of lymphoma immunomodulatory therapy, modified response criteria have been developed including a “indeterminate response” to identify lesions until confirmed as pseudo-progression or true progressive disease on subsequent imaging85.
Allogeneic hematopoietic stem cell transplantation (allo-HCT) can be curative in relapsed/refractory cHL but relapse rates remain as high as 40% even with alternate donor sources such as HLA-haploidentical allo-HCT86. Early trials of nivolumab and pembrolizumab in cHL excluded patients who had a previous history of allo-HCT due to concern about potentiation or reactivation of graft versus host disease (GVHD) by PD-1 inhibition. A French study retrospectively assessed the efficacy and toxicity of nivolumab in 20 patients with cHL who had relapsed after allo-HCT. The overall response rate was an impressive 95% with 42% complete responses and at a median follow-up of a year, the median progression free survival and overall survival had not been reached87.
Nivolumab-induced GVHD occurred in 6 patients (30%) within 1 week of the first dose, prompting discontinuation after a single infusion. All these patients had a history of prior acute GVHD. No nivolumab-induced GVHD was seen in patients with a prior history of chronic GVHD but no prior acute GVHD. The time between allo-HCT and nivolumab treatment was significantly shorter in patients who presented with nivolumab-induced GVHD (median, 8.5 months [range, 2-19 months] vs median, 28.5 months [range, 7-111 months]; p=.0082)87. Another US multicenter retrospective study found a 77% response rate for PD-1 blockade post allo-HCT, but just over half (55% of patients) developed treatment-emergent GVHD after initiation of anti–PD-1, usually after 1-2 doses of checkpoint blockade. In conclusion, PD-1 blockade in relapsed cHL allo-HCT patients appears to be highly efficacious but frequently complicated by rapid onset of severe and treatment-refractory GVHD88. Therefore, checkpoint inhibition might be an alternative to donor lymphocyte infusion for select patients with relapsed/refractory cHL post allo-HSCT who need a tumor response, but is complicated by high rates of GVHD.
Another subject of active debate is the safety of allo-HCT in patients after PD-1 blockade therapy. An international retrospective analysis of 39 patients with lymphoma who received prior treatment with a PD-1 inhibitor before allo-HCT showed 1-year cumulative incidences of grade 2-4 and grade 3-4 acute GVHD of 44% and 23% respectively, whereas the 1-year incidence of chronic GVHD was 41%. Those treated concurrently with Ipilimumab, an anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) monoclonal Ab and a PD-1 inhibitor all developed acute GVHD including a fatal case of grade 4 acute GVHD. An atypical noninfectious febrile syndrome was seen in 7 patients; this developed shortly post- transplant and required prolonged courses of steroid therapy. Despite these hurdles the one-year overall and progression-free survival rates were 89% (95% CI, 74-96) and 76% (95% CI, 56-87) respectively indicating that allo-HSCT is feasible in patients who have received prior PD-1 blockade89.
The excellent tolerability, high response rate and potential durability of response to Immunotherapy in cHL holds great promise. Newer trials are looking to advance immuno-oncologics into earlier lines of treatment and in novel combinations with BV for a ‘chemo-free’ approach to treating relapsed/refractory cHL.
Allogeneic stem cell transplantation
Allogeneic stem cell transplantation (allo-HCT) can produce long term disease control via the ‘graft vs. lymphoma effect’ but its use was limited in the past due to lack of suitable donors and the acute morbidity and mortality in the peri-transplant period90. Major changes in transplant technology in the last 15 years have helped drive improvement in outcomes with allo-HCT. A meta-analysis of allo-HCT studies of 1850 patients treated for HL showed 3-year relapse free survival of 31 (25-37)% and OS of 50 (41-58)%. Accrual initiation year in 2000 or later was associated with 5-10% lower non-relapse mortality and relapse rates, and 15-20% higher relapse free and overall survival91.
1. Advent of Reduced Intensity Conditioning (RIC)
Myeloablative preparative regimens use high doses of chemotherapy and radiation pre-transplant to get maximum tumor kill and induce immunosuppression that enables engraftment of donor hematopoietic stem cells. However, myeloablation is associated with higher short-term toxicities and worse non-relapse mortality. Non-myeloablative or RIC regimens use lower doses of chemo-radiotherapy, have lower early post-transplant morbidity and mortality and rely mainly on the immunological properties of the graft to combat lymphoma. The graft versus lymphoma effect, which is responsible for long-term disease control does not depend on the intensity of the preparative regimen90,92.
A retrospective European study in patients with relapsed/refractory HL treated with allo-HCT found nonrelapse mortality (HR 2.85; 95% CI, 1.62 to 5.02) and overall survival improved (HR, 2.05; 95% CI, 1.27 to 3.29; P = .04) in patients treated with RIC regimens compared to myeloablative conditioning93.
2. Use of alternate donor sources
Less than a third of patients have matched sibling donors for allo-HCT and a search for a suitable HLA-matched unrelated donor through the National Marrow Donor Program can take several months. Alternate donor sources such as umbilical cord blood and HLA haplo-identical donors have overcome most problems related to donor availability in allo-HCT.
A retrospective case-series of RIC allo-HCT found equivalent outcomes using either unrelated umbilical cord blood (UCB) donors or matched-sibling donors (MSD) for patients with advanced HL with comparable 2-year progression free survival rates of 25% for UCB and 20% for MSD respectively94. A single unit of umbilical cord blood might not have adequate hematopoietic progenitors leading to slower engraftment and higher risk of graft failure. Using two UCB units together (double UCB transplants) in RIC allo-HSCT can mitigate some of these risks and has been shown to be feasible and efficacious (5-year PFS of 31.3%) in a heavily pretreated cohort of patients with HL95.
Almost all patients have a first-degree relative (for e.g. either parent or progeny) identical for one HLA haplotype who could serve as a haploidentical (haplo) donor. Modern immunosuppressive regimens using post-transplant cyclophosphamide are able to effectively minimize the rates of Graft versus host disease (GVHD) in patients receive a haplo-identical allo-HSCT. A multi-center retrospective review of RIC allo-HCST for 90 patients with relapsed or refractory HL compared outcomes of HLA-matched related (n=38), unrelated (n=24) or HLA-haploidentical related (n= 28) donors. Two-year OS, PFS were 53%, 23% (HLA-matched related), 58%, 29% (unrelated), and 58%, 51% (HLA-haploidentical related), respectively. The risks of relapse were lower in the HLA-haploidentical recipients compared to the other two groups and neither acute nor chronic graft versus host disease rates were increased86.
Nodular LP Hodgkin lymphoma
Nodular lymphocyte predominant HL (NLPHL) cases comprise a small percentage (about 5%) of total number of patients diagnosed with HL. It is generally a much more indolent lymphoma, usually asymptomatic and is always negative for EBV. Unlike cHL (where HRS cells are CD15 +, CD30 + and CD45 -), the malignant cells in NLPHL are CD15 -, CD30 -, CD45+ germinal center B cells and invariably express CD20. The malignant cells show a nodular growth pattern and popcorn like lymphocytic-histiocytic malignant cells without much fibrosis. There is a striking predilection for males, who make up about 3/4th of all patients diagnosed with the disease and a strong familial risk (the standardized incidence ratio in first-degree relatives of patients was 19 in a Finnish study)96,97. Almost 80% patients present with Stage I-II lymphadenopathy at diagnosis and have an excellent long-term prognosis98.
In contrast to cHL, the treatment of NLPHL is not clearly defined; older studies are confounded by NLPHL being included with cHL despite the natural history of both diseases being very different. There are no randomized control trials of NLPHL treatment and treatment recommendations are based mostly on case series.
Early Stage NLPHL
Complete surgical excision without adjuvant therapy has shown excellent efficacy in pediatric patients with early stage NLPHL – a Children’s Oncology Group study showed a 5 year event free survival of over 75%99,100.
Radiation therapy alone is potentially curative in early stage NLPHL. An Australian study showed 15 year freedom from progression of 82% and overall survival of 83% in patients treated with local radiation101. Stage IA NLPHL patients treated within GHSG studies between 1988 and 2009 received combined-modality treatment (n = 72), extended-field radiotherapy (n = 49), involved-field radiotherapy (IF-RT, n = 108), or four weekly standard doses of rituximab (n = 27). At 8 years, progression-free survival and overall survival rates were 91.9% and 99.0% for IF-RT with identical tumor control in all the treatment arms including radiation102. Current guidelines from the International Lymphoma Radiation Oncology group recommend involved node or involved site radiation therapy using 30-36 Gy to treat early stage LPHL103.
Advanced stage NLPHL
Patient with advanced stage (III/IV) NLPHL have a worse prognosis than those with early stage NLPHL with systemic symptoms and relapses over an extended period. The prognosis for advanced NLPHL is also significantly worse than advanced cHL and often resembles the natural history of low-grade NHL104. A multicenter retrospective analysis in the 1990s (before the advent of monoclonal antibody based therapy) reported virtually all patients responding to first line chemotherapy but about 38% of Stage III and 76% of patients with Stage IV disease relapsed over a 8 year period post completion of chemotherapy105.
A particular concern in NLPHL is the increased risk of transformation to aggressive diffuse large B-cell lymphoma (DLBCL), a type of aggressive NHL. A retrospective study at the Mayo Clinic found a 7.6% transformation rate after a median follow-up of 16 years (transformation rate of 0.74 per 100 patient-years), but the British Columbia cancer agency reported higher actuarial risk of transformation of 7% and 30% at 10 and 20 years respectively106,107. Both studies identified splenic involvement as a significant risk factor for transformation.
Rituximab is a monoclonal antibody targeting CD20, which has revolutionized treatment of CD20-positive B-cell NHL. It has a favorable adverse effect profile without standard toxicities associated with cytotoxic agents. Patients with newly diagnosed or relapsed NLPHL treated with a once weekly, 4 week course of rituximab had a 100% response rate (complete response, 67%; partial response, 33%) but most patients relapsed (estimated 5 year PFS of 39.1 %). When followed by maintenance Rituximab (once every 6 months for 2 years) the estimated 5-year PFS was extended to 58.9%108. These results are similar to other reports of the efficacy of Rituximab in this disease109,110.
Multi-agent combinations like ABVD and CHOP (Cyclophosphamide, Doxorubicin, Vincristine and Prednisone) in combination with Rituximab are the most commonly used chemotherapeutic regimens used to treat symptomatic patients with advanced LPHL in the United States. A retrospective review from MD Anderson cancer center of 59 patients with advanced NLPHL treated with Rituximab and CHOP combination showed a complete response rate of 89%, estimated 5- and 10-year progression free survival of 89% and 60% respectively without any transformation111. Although prospective data for this approach is limited due to the rarity of patients who present with this disease in advanced stage, we follow the National Comprehensive Cancer Network (NCCN) consensus guidelines in treating this disease with rituximab-based combination chemotherapy, a strategy with extensive data in patients with NHL112.
Survivorship
With current treatment advancements, about 90% of all patients diagnosed with HL will be long-term survivors. HL is also a disease of the young, most frequently seen in the 20 – 34 year age group; therefore patients could require decades of monitoring before the full spectrum of treatment toxicity could potentially manifest as a competing cause of morbidity and mortality. A Dutch study of long-term cause-specific mortality of patients treated for HL found a continuing increase in the relative risk of death from all causes other than HL was 6.8 times that of the general population, and still amounted to 5.1 after more than 30 years113. The available long term toxicity data is not necessarily directly applicable to patients undergoing HL treatment now – older regimens such as MOPP chemotherapy and mantle field radiation, subtotal nodal radiation are rarely, if ever used anymore but can be useful in assessing potential side effects of modern chemo-radiotherapy regimens.
A prime example of having to balance out cure versus the risks of delayed treatment toxicity is the NCIC CTG-ECOG HD.6 trial of early stage unfavorable risk patients - long term survival was superior in the chemotherapy alone arm (94% vs 87%) despite a lower freedom from progressive disease (87% vs 92%) in the combined modality arm primarily due to higher delayed combined modality treatment related deaths44.
Second Malignancies
Multiple studies have shown an increased risk for second (and multiple) cancers after treatment for HL- both hematological neoplasms and solid tumors together form the largest cause of mortality in long term survivors of HL113,114. Rates of acute myeloid leukemia (AML) declined significantly starting in the mid-1980s with ABVD replacing the alkylator-heavy MOPP chemotherapy regimen but remain much higher than in the general population. HL survivors are also at 13 times higher risk for developing a second primary NHL115,116.
Second primary solid tumors form the major burden of secondary cancers with increased long term risk for cancers of the oropharynx, esophagus, stomach, colon, pancreas, lung, mesothelioma, melanoma, sarcoma, breast, urinary bladder, and thyroid. A Dutch study with 40 year follow-up estimated the standardized incidence ratio for any solid cancer was 4.2 with an absolute excess risk of 100 cancers/10,000 person-year and a 30-year cumulative incidence of 29%. Despite therapeutic advances in HL treatment, this increased cumulative incidence of second solid cancers did not differ between patients treated in the 1960s and the 1990s116. Lung cancer accounts for a substantial number of these cases (25 cases/10,000 person-yrs.) and HL survivors experience significantly inferior stage-specific overall survival compared to patients with de novo lung cancer117.
The risk of female breast cancer secondary to radiotherapy is particularly high in those who received radiation while younger than 30 years of age and remains increased for decades after completion of radiotherapy. In a study of women who received mantle irradiation for HL the relative risk of breast carcinoma was 56 for those 19 years or younger at the time of treatment, 7.0 for those age 20-29 years, and 0.9 for those 30 years and older118. Other studies have reproduced similar results confirming that breast tissue is highly susceptible to ionizing radiation in younger females necessitating long term surveillance119. With newer radiation techniques and lower dose exposure to breast tissue, the rates of breast cancer are hypothesized to be lower than with mantle irradiation120. The American Cancer Society and other organizational guidelines recommend screening MRI as a useful adjunct to routine mammography for women treated with mediastinal radiation who have a 20-25% or greater lifetime risk of breast cancer121,122.
Nonmalignant systemic side effects
HL patients are susceptible to non-ischemic cardiomyopathy from anthracycline chemotherapy mediated by oxidative stress and apoptosis. Anthracyclines like doxorubicin form the backbone of almost all modern chemotherapeutic regimens for HL and can cause a dose-dependent systolic dysfunction, especially once the cumulative dose exceeds 400 mg/m2. Delayed late cardio toxicity can present as overt heart failure or asymptomatic left ventricular dysfunction several years after completion of chemotherapy123.
HL survivors are also at risk for coronary heart disease (CHD) from radiation exposure. A Dutch study revealed that the risk of coronary disease increased linearly with increasing mean heart dose (excess relative risk per Gray, 7.4%) with a median interval of 19 years between diagnosis of HL and CHD124. However most patients on this retrospective study were treated with older radiation techniques; contemporary radiotherapy minimizes critical organ exposure with radiation dose reduction, radiation field/volume reduction, and use of modern RT planning and delivery125. HL survivors are also at-risk for valvular dysfunction and congestive heart failure from cumulative effects of combined modality therapy with anthracyclines and radiation126.
Pulmonary toxicities can arise acutely and sub acutely during treatment (bleomycin or radiation-induced pneumonitis) and lead to chronic respiratory impairment. Patients with prior bleomycin exposure are advised against high inspired supplemental oxygen concentrations due to anecdotal reports of late lung toxicity. Patients whose thyroids are irradiated as a part of their HL treatment are at 50% risk for thyroid disease at 20 years – mostly hypothyroidism and Graves disease127.
Infertility
ABVD carries little to no excess risk of premature ovarian failure compared to alkylating chemotherapy (MOPP and BEACOPP) which can impair gonadal function and fertility recovery post chemotherapy128. In patients with relapsed disease receiving salvage chemotherapy/high dose therapy or allo-HCT, preservation of ovarian function and fertility is unlikely. Sperm banking, embryo cryopreservation, oocyte freezing can potentially be used before initiation of therapy in HL patients desiring fertility preservation129.
Conclusions
Treatment for Hodgkin lymphoma has improved significantly since the ABVD chemotherapeutic combination was invented over 30 years ago. Despite using the same ABVD regimen in most patients treated first line, we now have a much better understanding of disease biology, late side effects of therapy and have moved towards a personalized risk adapted approach. This approach promises to deliver low toxicities and high cure rates for lower risk patients while reserving aggressive regimens for those high risk patients who really need it. For those minority of patients who fail first line therapy, novel drugs like the antibody-drug conjugate BV and immunotherapies Nivolumab and Pembrolizumab have shown high response rates and durability of benefit. Further research is needed to see if these novel drugs could make life better for both HL patients undergoing treatment and for the growing cohort of HL survivors.
Figure I.
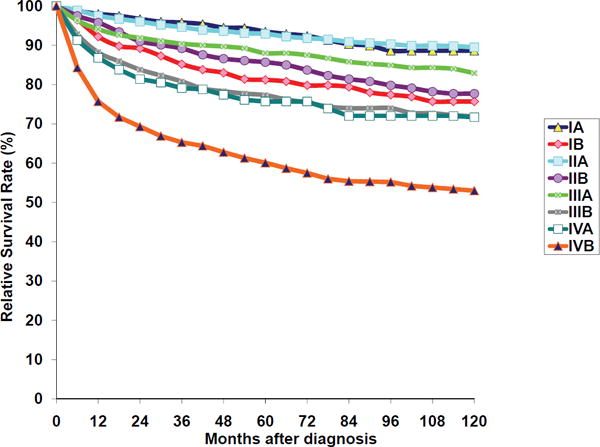
Hodgkin Lymphoma: Relative Survival Rates (%) by Stage and B-Symptoms, Ages 15+, 12 SEER Areas, 1988-2001
Figure II.
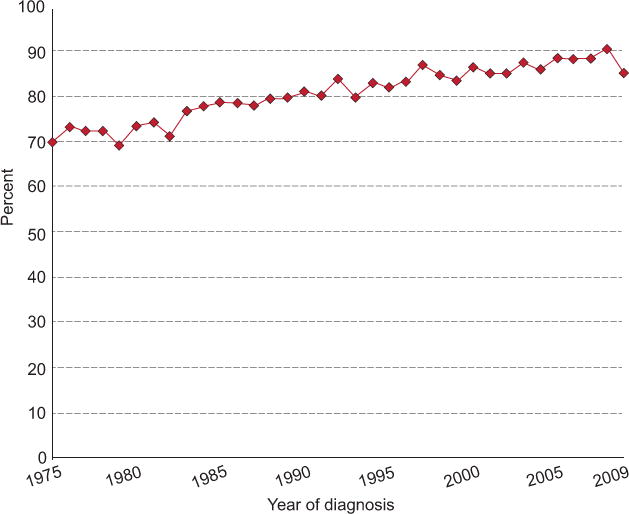
5-year relative survival by year of diagnosis in HL patients 1975 -2013
Figure III.
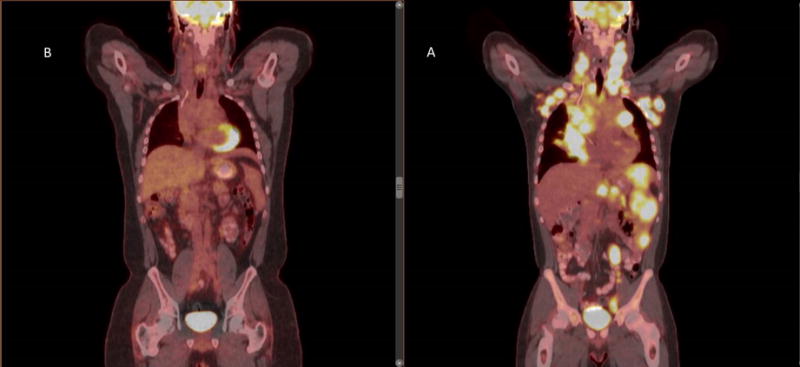
Pre-treatment PET/CT scan (a) of a patient with stage IVB HL showing bulky mediastinal disease, neck, axillary, abdominal adenopathy and splenic involvement. After 6 months of chemotherapy the post-treatment PET/CT (b) shows complete metabolic remission with resolution of all PET-avidity and adenopathy.
Figure IV.
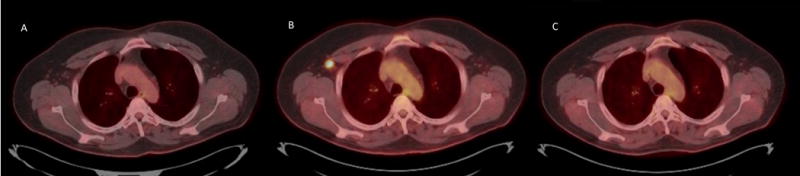
Pseudo-progression on PET/CT in a patient with relapsed cHL on immunotherapy. (A) PET-CT after 6 months of immunotherapy showing complete remission. 3 months later new PET-avid 1.8 cm adenopathy was seen (B) which resolved on the subsequent scan while continuing the same treatment (C).
Figure V.
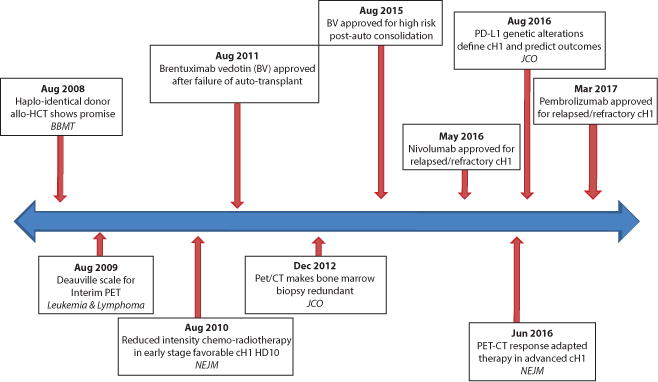
Timeline of landmark developments in Hodgkin Lymphoma (HL) over the last decade. Italicized acronyms indicate journal of publication. BBMT – Biology of blood and marrow transplantation, NEJM – New England journal of medicine, JCO – Journal of clinical oncology
Acknowledgments
Funding sources: NIH grant P30CA006973
Footnotes
Conflict of Interest Disclosure:
Satish Shanbhag – none
Richard Ambinder – support from Bristol Myers Squib, Celgene
Author contributions: Satish Shanbhag created the original draft, both authors are responsible for review, editing and finalizing the manuscript
References
- 1.Hodgkin On some Morbid Appearances of the Absorbent Glands and Spleen. Medico-chirurgical transactions. 1832;17:68–114. doi: 10.1177/095952873201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe ES, H N, Stein H, Vardiman JW. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2001. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, N A, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; Bethesda, MD: based on November 2016 SEER data submission, posted to the SEER web site, April 2017.; https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 5.Ambinder RF, Browning PJ, Lorenzana I, et al. Epstein-Barr virus and childhood Hodgkin’s disease in Honduras and the United States. Blood. 1993;81(2):462–467. [PubMed] [Google Scholar]
- 6.Chang KL, Albujar PF, Chen YY, Johnson RM, Weiss LM. High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of Hodgkin’s disease occurring in Peru. Blood. 1993;81(2):496–501. [PubMed] [Google Scholar]
- 7.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. The New England journal of medicine. 2003;349(14):1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 8.Kanakry JA, Li H, Gellert LL, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013;121(18):3547–3553. doi: 10.1182/blood-2012-09-454694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel P, Hanson DL, Sullivan PS, et al. INcidence of types of cancer among hiv-infected persons compared with the general population in the united states, 1992–2003. Annals of internal medicine. 2008;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 10.Glaser SL, Clarke CA, Gulley ML, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98(2):300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 11.Gerard L, Galicier L, Boulanger E, et al. Improved survival in HIV-related Hodgkin’s lymphoma since the introduction of highly active antiretroviral therapy. AIDS (London, England) 2003;17(1):81–87. doi: 10.1097/00002030-200301030-00011. [DOI] [PubMed] [Google Scholar]
- 12.Landgren O, Engels EA, Pfeiffer RM, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. Journal of the National Cancer Institute. 2006;98(18):1321–1330. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Vol. 2. IARC; 2008. [Google Scholar]
- 14.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–1392. [PubMed] [Google Scholar]
- 15.Butler T. A Clinicopathologic Study of 659 Cases. Cancer. 1981;49:1848–1858. doi: 10.1002/1097-0142(19820501)49:9<1848::aid-cncr2820490918>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Gulley ML, Eagan PA, Quintanilla-Martinez L, et al. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood. 1994;83(6):1595–1602. [PubMed] [Google Scholar]
- 17.Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-Rich Classical Hodgkin’s Lymphoma: Clinical Presentation and Treatment Outcome in 100 Patients Treated Within German Hodgkin’s Study Group Trials. Journal of Clinical Oncology. 2005;23(24):5739–5745. doi: 10.1200/JCO.2005.17.970. [DOI] [PubMed] [Google Scholar]
- 18.Diehl V, Sextro M, Franklin J, et al. Clinical Presentation, Course, and Prognostic Factors in Lymphocyte-Predominant Hodgkin’s Disease and Lymphocyte-Rich Classical Hodgkin’s Disease: Report From the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. Journal of Clinical Oncology. 1999;17(3):776–776. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 19.Allemani C, Sant M, De Angelis R, Marcos-Gragera R, Coebergh JW, the EWG Hodgkin disease survival in Europe and the U.S. Cancer. 2006;107(2):352–360. doi: 10.1002/cncr.21995. [DOI] [PubMed] [Google Scholar]
- 20.von Wasielewski S, Franklin J, Fischer R, et al. Nodular sclerosing Hodgkin disease: new grading predicts prognosis in intermediate and advanced stages. Blood. 2003;101(10):4063–4069. doi: 10.1182/blood-2002-05-1548. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan KA, Bennett MH, Tu A, et al. Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin’s disease. A study of 1659 patients. Cancer. 1989;64(8):1686–1693. doi: 10.1002/1097-0142(19891015)64:8<1686::aid-cncr2820640822>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Mauch PM, Kalish LA, Kadin M, Coleman CN, Osteen R, Hellman S. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer. 1993;71(6):2062–2071. doi: 10.1002/1097-0142(19930315)71:6<2062::aid-cncr2820710622>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Gobbi PG, Cavalli C, Gendarini A, et al. Reevaluation of prognostic significance of symptoms in Hodgkin’s disease. Cancer. 1985;56(12):2874–2880. doi: 10.1002/1097-0142(19851215)56:12<2874::aid-cncr2820561227>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7(11):1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 25.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leukemia & lymphoma. 2009;50(8):1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(27):3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(36):4508–4514. doi: 10.1200/JCO.2012.42.4036. [DOI] [PubMed] [Google Scholar]
- 29.Ries LAG, Y J, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. National Cancer Institute, SEER Program, Bethesda, MD. 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program 1988-2001, Patient and Tumor Characteristics. (NIH Pub. No. 07-6215). [Google Scholar]
- 30.Jacobs EM, Peters FC, Luce JK, Zippin C, Wood DA. Mechlorethamine hcl and cyclophosphamide in the treatment of hodgkin's disease and the lymphomas. JAMA : the journal of the American Medical Association. 1968;203(6):392–398. [PubMed] [Google Scholar]
- 31.Rosenberg SA, Kaplan HS. Evidence for an orderly progression in the spread of Hodgkin’s disease. Cancer research. 1966;26(6):1225–1231. [PubMed] [Google Scholar]
- 32.Kaplan HS. The Radical Radiotherapy of Regionally Localized Hodgkin’s Disease. Radiology. 1962;78(4):553–561. doi: 10.1148/78.4.553. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan HS, Rosenberg SA. Extended-field radical radiotherapy in advanced Hodgkin’s disease: short-term results of 2 randomized clinical trials. Cancer research. 1966;26(6):1268–1276. [PubMed] [Google Scholar]
- 34.Rosenberg SA, Kaplan HS. The evolution and summary results of the Stanford randomized clinical trials of the management of Hodgkin’s disease: 1962-1984. International journal of radiation oncology, biology, physics. 1985;11(1):5–22. doi: 10.1016/0360-3016(85)90357-8. [DOI] [PubMed] [Google Scholar]
- 35.Devita VT, Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Annals of internal medicine. 1970;73(6):881–895. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- 36.Longo DL, Glatstein E, Duffey PL, et al. Radiation therapy versus combination chemotherapy in the treatment of early-stage Hodgkin’s disease: seven-year results of a prospective randomized trial. Journal of Clinical Oncology. 1991;9(6):906–917. doi: 10.1200/JCO.1991.9.6.906. [DOI] [PubMed] [Google Scholar]
- 37.Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36(1):252–259. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. The New England journal of medicine. 1992;327(21):1478–1484. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 39.Canellos GP, Niedzwiecki D. Long-Term Follow-up of Hodgkin’s Disease Trial. New England Journal of Medicine. 2002;346(18):1417–1418. doi: 10.1056/NEJM200205023461821. [DOI] [PubMed] [Google Scholar]
- 40.Nissen NI, Nordentoft AM. Radiotherapy versus combined modality treatment of stage I and II Hodgkin’s disease. Cancer treatment reports. 1982;66(4):799–803. [PubMed] [Google Scholar]
- 41.Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. The New England journal of medicine. 2007;357(19):1916–1927. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 42.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. The New England journal of medicine. 2010;363(7):640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 43.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385(9976):1418–1427. doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 44.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. The New England journal of medicine. 2012;366(5):399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raemaekers JMM, André MPE, Federico M, et al. Omitting Radiotherapy in Early Positron Emission Tomography–Negative Stage I/II Hodgkin Lymphoma Is Associated With an Increased Risk of Early Relapse: Clinical Results of the Preplanned Interim Analysis of the Randomized EORTC/LYSA/FIL H10 Trial. Journal of Clinical Oncology. 2014;32(12):1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 46.Radford J, Illidge T, Counsell N, et al. Results of a Trial of PET-Directed Therapy for Early-Stage Hodgkin’s Lymphoma. New England Journal of Medicine. 2015;372(17):1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 47.Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(27):4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 48.von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(9):907–913. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 49.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(32):5390–5396. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 50.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(6):684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merli F, Luminari S, Gobbi PG, et al. Long-Term Results of the HD2000 Trial Comparing ABVD Versus BEACOPP Versus COPP-EBV-CAD in Untreated Patients With Advanced Hodgkin Lymphoma: A Study by Fondazione Italiana Linfomi. Journal of Clinical Oncology. 2016;34(11):1175–1181. doi: 10.1200/JCO.2015.62.4817. [DOI] [PubMed] [Google Scholar]
- 52.Carde P, Karrasch M, Fortpied C, et al. Eight Cycles of ABVD Versus Four Cycles of BEACOPPescalated Plus Four Cycles of BEACOPPbaseline in Stage III to IV, International Prognostic Score >/= 3, High-Risk Hodgkin Lymphoma: First Results of the Phase III EORTC 20012 Intergroup Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(17):2028–2036. doi: 10.1200/JCO.2015.64.5648. [DOI] [PubMed] [Google Scholar]
- 53.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s Lymphoma When High-Dose Salvage Is Planned. New England Journal of Medicine. 2011;365(3):203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 54.Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. The Cochrane database of systematic reviews. 2017;5:Cd007941. doi: 10.1002/14651858.CD007941.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sieniawski M, Reineke T, Nogova L, et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG) Blood. 2008;111(1):71–76. doi: 10.1182/blood-2007-02-073544. [DOI] [PubMed] [Google Scholar]
- 56.Jurczak W, Krochmalczyk D, Pabian W, et al. Quality of life and fertility in Hodgkin disease patients treated with escalated BEACOPP. Haematologica. 2009;94:35–35. [Google Scholar]
- 57.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(24):3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 58.Johnson P, Federico M, Kirkwood A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. New England Journal of Medicine. 2016;374(25):2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohri A, Barnett M, Fairey RN, et al. Outcome of treatment of first relapse of Hodgkin’s disease after primary chemotherapy: identification of risk factors from the British Columbia experience 1970 to 1988. Blood. 1991;77(10):2292–2298. [PubMed] [Google Scholar]
- 60.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-Term Results of Autologous Stem Cell Transplantation for Primary Refractory or Relapsed Hodgkin’s Lymphoma. Biology of Blood and Marrow Transplantation. 2006;12(10):1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 63.Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. The Cochrane database of systematic reviews. 2013;(6):Cd009411. doi: 10.1002/14651858.CD009411.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–360. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]
- 65.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 66.Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular Activation of SGN-35, a Potent Anti-CD30 Antibody-Drug Conjugate. Clinical Cancer Research. 2010;16(3):888–897. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]
- 67.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 69.Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. The lancet oncology. 14(13):1348–1356. doi: 10.1016/S1470-2045(13)70501-1. [DOI] [PubMed] [Google Scholar]
- 70.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(14):1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 72.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clinical Cancer Research. 2012;18(6):1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(23):2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. The lancet oncology. 2016;17(9):1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of Clinical Oncology. 0(0) doi: 10.1200/JCO.2016.72.1316. JCO.2016.2072.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. Journal of Clinical Oncology. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Postow MA. Managing Immune Checkpoint-Blocking Antibody Side Effects. ASCO 2015 Educational Book. 2015 doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 79.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. Journal of Clinical Oncology. 2017;35(7):709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. New England Journal of Medicine. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Reports. 2016;2016 doi: 10.1136/bcr-2016-216228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for ImmunoTherapy of Cancer. 2015;3(1):11. doi: 10.1186/s40425-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohé C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer. 2016;99:117–119. doi: 10.1016/j.lungcan.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 84.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of Oncology. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–2496. doi: 10.1182/blood-2016-05-718528. [DOI] [PubMed] [Google Scholar]
- 86.Burroughs LM, O’Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(11):1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129(18):2471–2478. doi: 10.1182/blood-2016-11-749556. [DOI] [PubMed] [Google Scholar]
- 88.Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130(2):221–228. doi: 10.1182/blood-2017-01-761346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129(10):1380–1388. doi: 10.1182/blood-2016-09-738385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomson KJ, Peggs KS, Smith P, et al. Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone marrow transplantation. 2008;41(9):765–770. doi: 10.1038/sj.bmt.1705977. [DOI] [PubMed] [Google Scholar]
- 91.Rashidi A, Ebadi M, Cashen AF. Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: a systematic review and meta-analysis. Bone marrow transplantation. 2016;51(4):521–528. doi: 10.1038/bmt.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porter DL, Stadtmauer EA, Lazarus HM. ‘GVHD’: graft-versus-host disease or graft-versus-Hodgkin’s disease? An old acronym with new meaning. Bone marrow transplantation. 2003;31(9):739–746. doi: 10.1038/sj.bmt.1703895. [DOI] [PubMed] [Google Scholar]
- 93.Sureda A, Robinson S, Canals C, et al. Reduced-Intensity Conditioning Compared With Conventional Allogeneic Stem-Cell Transplantation in Relapsed or Refractory Hodgkin’s Lymphoma: An Analysis From the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology. 2008;26(3):455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 94.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107(9):3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 95.Thompson PA, Perera T, Marin D, et al. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leukemia & lymphoma. 2016;57(7):1607–1615. doi: 10.3109/10428194.2015.1105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saarinen S, Pukkala E, Vahteristo P, Makinen MJ, Franssila K, Aaltonen LA. High familial risk in nodular lymphocyte-predominant Hodgkin lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(7):938–943. doi: 10.1200/JCO.2012.43.5958. [DOI] [PubMed] [Google Scholar]
- 98.Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(24):5739–5745. doi: 10.1200/JCO.2005.17.970. [DOI] [PubMed] [Google Scholar]
- 99.Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer. 2007;110(1):179–185. doi: 10.1002/cncr.22762. [DOI] [PubMed] [Google Scholar]
- 100.Appel BE, Chen L, Buxton AB, et al. Minimal Treatment of Low-Risk, Pediatric Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(20):2372–2379. doi: 10.1200/JCO.2015.65.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer. 2005;104(6):1221–1229. doi: 10.1002/cncr.21303. [DOI] [PubMed] [Google Scholar]
- 102.Eichenauer DA, Plütschow A, Fuchs M, et al. Long-Term Course of Patients With Stage IA Nodular Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the German Hodgkin Study Group. Journal of Clinical Oncology. 2015;33(26):2857–2862. doi: 10.1200/JCO.2014.60.4363. [DOI] [PubMed] [Google Scholar]
- 103.Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG) International journal of radiation oncology, biology, physics. 2014;89(4):854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood. 2014;123(23):3567–3573. doi: 10.1182/blood-2013-12-541078. [DOI] [PubMed] [Google Scholar]
- 105.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(3):776–783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 106.Kenderian SS, Habermann TM, Macon WR, et al. Large B-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood. 2016;127(16):1960–1966. doi: 10.1182/blood-2015-08-665505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Mansour M, Connors JM, Gascoyne RD, Skinnider B, Savage KJ. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(5):793–799. doi: 10.1200/JCO.2009.24.9516. [DOI] [PubMed] [Google Scholar]
- 108.Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(9):912–918. doi: 10.1200/JCO.2013.53.2069. [DOI] [PubMed] [Google Scholar]
- 109.Schulz H, Rehwald U, Morschhauser F, et al. Rituximab in relapsed lymphocyte-predominant Hodgkin lymphoma: long-term results of a phase 2 trial by the German Hodgkin Lymphoma Study Group (GHSG) Blood. 2008;111(1):109–111. doi: 10.1182/blood-2007-03-078725. [DOI] [PubMed] [Google Scholar]
- 110.Eichenauer DA, Fuchs M, Pluetschow A, et al. Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood. 2011;118(16):4363–4365. doi: 10.1182/blood-2011-06-361055. [DOI] [PubMed] [Google Scholar]
- 111.Fanale MA, Cheah CY, Rich A, et al. Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood. 2017;130(4):472–477. doi: 10.1182/blood-2017-02-766121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin Lymphoma Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2017;15(5):608–638. doi: 10.6004/jnccn.2017.0064. [DOI] [PubMed] [Google Scholar]
- 113.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(18):3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 114.van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124(3):319–327. doi: 10.1182/blood-2013-10-532184. quiz 466. [DOI] [PubMed] [Google Scholar]
- 115.Schonfeld SJ, Gilbert ES, Dores GM, et al. Acute myeloid leukemia following Hodgkin lymphoma: a population-based study of 35,511 patients. Journal of the National Cancer Institute. 2006;98(3):215–218. doi: 10.1093/jnci/djj017. [DOI] [PubMed] [Google Scholar]
- 116.Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. New England Journal of Medicine. 2015;373(26):2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 117.Milano MT, Li H, Constine LS, Travis LB. Survival after second primary lung cancer: a population-based study of 187 Hodgkin lymphoma patients. Cancer. 2011;117(24):5538–5547. doi: 10.1002/cncr.26257. [DOI] [PubMed] [Google Scholar]
- 118.Aisenberg AC, Finkelstein DM, Doppke KP, Koerner FC, Boivin JF, Willett CG. High risk of breast carcinoma after irradiation of young women with Hodgkin’s disease. Cancer. 1997;79(6):1203–1210. doi: 10.1002/(sici)1097-0142(19970315)79:6<1203::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 119.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin’s disease. Journal of the National Cancer Institute. 1993;85(1):25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 120.Koh ES, Tran TH, Heydarian M, et al. A comparison of mantle versus involved-field radiotherapy for Hodgkin’s lymphoma: reduction in normal tissue dose and second cancer risk. Radiation Oncology (London, England) 2007;2:13. doi: 10.1186/1748-717X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: a cancer journal for clinicians. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 122.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. The lancet oncology. 2013;14(13):e621–629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. International journal of cardiology. 2010;144(1):3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Nimwegen FAV, Schaapveld M, Cutter DJ, et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. Journal of Clinical Oncology. 2016;34(3):235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 125.Maraldo MV, Ng AK. Minimizing Cardiac Risks With Contemporary Radiation Therapy for Hodgkin Lymphoma. Journal of Clinical Oncology. 2016;34(3):208–210. doi: 10.1200/JCO.2015.64.6588. [DOI] [PubMed] [Google Scholar]
- 126.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 127.Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin’s disease. The New England journal of medicine. 1991;325(9):599–605. doi: 10.1056/NEJM199108293250902. [DOI] [PubMed] [Google Scholar]
- 128.van der Kaaij MAE, Heutte N, Meijnders P, et al. Premature Ovarian Failure and Fertility in Long-Term Survivors of Hodgkin’s Lymphoma: A European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d’Étude des Lymphomes de l’Adulte Cohort Study. Journal of Clinical Oncology. 2012;30(3):291–299. doi: 10.1200/JCO.2011.37.1989. [DOI] [PubMed] [Google Scholar]
- 129.van der Kaaij MAE, van Echten-Arends J, Simons AHM, Kluin-Nelemans HC. Fertility preservation after chemotherapy for Hodgkin lymphoma. Hematological oncology. 2010;28(4):168–179. doi: 10.1002/hon.939. [DOI] [PubMed] [Google Scholar]


