Abstract
Rationale
Complement plays a role in both hepatic ischemia reperfusion (IR) injury (IRI) and liver regeneration, but it is not clear how complement is activated in either process. We investigated the role of self-reactive IgM antibodies in activating complement after hepatic IR and liver resection.
Main Results
Natural IgM antibodies that recognize danger associated molecular patterns (neoepitopes) activate complement following both hepatic IR and liver resection. Antibody-deficient Rag1−/− mice were protected from hepatic IRI, but had increased hepatic injury and an impaired regenerative response after 70% partial hepatectomy (PHx). We identified two IgM mAbs that specifically reversed the effect of Rag1 deficiency in both models; B4 (recognizes annexin IV) and C2 (recognizes subset of phospholipids). Focusing on the B4-specific response, we demonstrated sinusoidal colocalization of IgM and C3d in Rag1−/− mice that were reconstituted with B4 mAb, and further that the annexin IV neoepitope is specifically and similarly expressed after both hepatic IR and PHx in wild-type mice. A single-chain Ab construct (scFv) derived from B4 mAb blocked IgM binding and reduced injury after IR in wild-type mice, although interestingly B4scFv did not alter regeneration following PHx, indicating that anti-annexin IV antibodies are sufficient, but not necessary for the regenerative response in the context of an entire natural antibody repertoire. We also demonstrated expression of the B4 neoepitope in post-ischemic human liver samples obtained after transplantation, and a corollary depletion in IgM recognizing the B4 and C2 neoepitopes in patient sera following liver transplantation.
Conclusion
These data indicate an important role for IgM in hepatic IRI and regeneration, with a similar cross-species injury-specific recognition system that has implications for the design of neoepitope targeted therapeutics.
Keywords: complement, damage-associated molecular patterns, antibodies, resection
Introduction
Activation of the complement cascade is an integral part of both injury and recovery following a variety of hepatic insults, including hepatic ischemia reperfusion (IR) injury (IRI) and resection. However, it is not clear how complement is activated in the setting of either hepatic IR or resection. Seminal studies demonstrated that natural IgM antibodies trigger complement activation upon ischemia and reperfusion of intestine (1, 2) and hindlimb (3, 4). These natural antibodies bind to neoepitopes exposed on post-ischemic endothelium and lead to complement-dependent IRI. There is little known about the role natural IgM antibodies play in hepatic injury and recovery, and indeed with regard to their role in hepatic IRI there is contradictory data (see discussion). With regard liver regeneration, although complement activation is essential for initiating the regenerative process, little is known concerning the complement activation trigger and whether IgM functions in a similar paradigm to IRI. It is known that IgM antibodies bind in the liver following partial hepatectomy (PHx), implicating them in the regenerative process (5), but it has also been reported that antibody-deficient Rag1−/− mice have similar regeneration and injury profiles as WT mice following PHx (6).
Pathogenic self-reactive antibody specificities have been identified using single clone IgM antibody reconstitution studies done in antibody-deficient Rag1−/− mice. CM22 mAb, which binds to non-muscle myosin and glycogen phosphorylase, was the first antibody identified to reconstitute IR injury in Rag1−/− in a model of mesenteric IR (2). Recently other antibody specificities have been identified that can also reconstitute IRI in Rag1−/−. These include B4 mAb, which binds to annexin IV, an early marker of apoptotic cell death (1), and C2 mAb, which recognizes a subset of phospholipids (7). Importantly, it has been shown that blocking these antibodies by using antibody specific peptides or proteins results in protection from IRI in WT mice, demonstrating that blockade of a single specificity is protective in the context of an entire natural antibody repertoire (1, 3, 7, 8). These studies indicate the importance of these self-reactive antibodies in causing tissue injury, and mark them as potential targets for therapeutic intervention. To this end, however, further research is needed to determine the extent of homology in antigen expression between organs, as well as similarities in antigen expression and antibody repertoires between mice and humans. With this in mind, the aim of the current study is to investigate the role of self-reactive Abs in both liver IRI and regeneration. The two IgM mAbs, B4 and C2, that we use in this study, and the neoepitopes that they recognize, have not been investigated in the context of liver injury and regeneration, and with regard to liver regeneration, the role of natural self-reactive IgM has not been investigated in general.
Materials and Methods
Information of animals, surgical procedures and liver functional assay are provided online in the supporting information.
IgM and scFv purification and treatment
C2 and B4 IgM mAbs were derived from hybridomas created by the fusion of C57BL/6 splenocytes with SP2/0-AG14 myeloma cells, and the resulting hybridomas were screened for binding to apoptotic cells (1). Those with positivity to apoptotic cells were further characterized to determine their exact specificities. The previous characterization of the binding specificity of theI gM mAbs by ELISA demonstrated that C2 was specific for a subset of phospholipids (7) and that B4 was specific for post-translationally modified annexin IV, respectively, as previously described (1). F632 IgM mAb, used as isotype control, was expressed by a hybridoma isolated from mice immunized with 4-hydroxy-3-nitrophenylacetyl (NP)-KLH and screened against NP-BSA. This control mAb was characterized previously in a cardiac transplant model (9). Purification of mAbs was performed as described previously (7). For reconstitution studies, Rag1−/− mice were injected i.v. with mAbs (25 μg) immediately following either IR or PHx. Cloning and expression of B4 scFv in CHO cells was previously as described (9). For therapeutic studies, wild type (WT) mice were injected i.v. with B4 scFv (20 μg) 5 minutes before reperfusion or PHx.
Levels of natural IgM and albumin in human and mouse serum
ELISAs for phosphatidylethanolamine (PE) (Sigma-Aldrich), phosphatidylcholine (PC) (Biosearch Technologies, Inc., Novato, CA), and annexin IV (purified as described (1)) were used to determine the levels of specific natural IgM antibodies in human and mouse serum. Human serum was collected from 13 patients undergoing a liver transplantation at three time points; just prior to reperfusion, 1 hour post reperfusion, and 24 hours post reperfusion. Mouse serum was collected from sham or IR operated mice 6 hours post reperfusion. Phospholipid and annexin IV ELISAs were performed as previously described (7). Total IgM in human and mouse serum was measured by ELISA according to manufactures instructions (Bethyl Laboratories, Montgomery, TX). Serum levels of albumin were measured using a colorimetric assay according to manufactures instructions (Pointe Scientific INC).
Histological analysis
For histological examination, tissue blocks were placed in 10% buffered formaldehyde solution for 48 hours before being embedded in paraffin. Liver histology was assessed by light microscopy (Olympus BH -2) of H&E-stained 4-μm sections in a blinded fashion on a semi-quantitative scale. Ten random fields on each slide were assessed for necrosis of the parenchymal area by standard morphologic criteria (loss of architecture, vacuolization, karyolysis, increased eosinophilia), and the extent of necrosis was semiquantitatively estimated by assigning a severity score on a scale of 0–4 as previously described (10) (absent, 0; mild, 1; moderate, 2; severe, 3; and total necrotic destruction of the liver, 4).
Assessment of liver regeneration
Mice were injected with BrdU (50 mg/kg i.p.) 2 hours prior to sacrifice, and incorporation of BrdU was visualized by immunohistochemistry using an anti-BrdU antibody (AbCam) as described (11). Restitution of liver weight is expressed as percentage of regenerated liver mass relative to total liver weight. Residual liver weight was calculated with the following equation: ([B (C A)]/B)*100, where A is the weight of the resected liver (30% of total liver), B is the estimated total liver weight based on the weight of the resected liver (B = A/0.7), and C is the final liver weight at the time of sacrifice. There was no significant difference in the liver to body weight ratios in WT vs. Rag1−/− mice used in the studies (liver/body weight for WT = 0.51 +/− 0.02, Rag1−/− = 0.049 +/− 0.03). Mitotic index was calculated by tallying the percentage of hepatocytes undergoing mitosis in 10 HPF on tissue sections stained with H&E. For reconstitution of Rag1−/− mice with C5a (CompTech, Tyler, TX), the peptide was administered by i.p. injection at 15 ug/mouse/injection, with one dose given immediately before PHx, and again at 6 and 12 h after PHx, as described previously (12).
Immunohistochemistry and immunofluorescence
Paraffin or frozen sections were cut at 4μm for IHC and IF respectively. IHC sections were deparaffinized and antigen retrieval was performed using proteinase K (Vector Labs, Burlingame, CA). Frozen sections were fixed in acetone for 6 minutes and then air dried and equilibrated in PBS. C3d deposition was detected using a goat anti-mouse C3d (1:20, R&D Systems, Minneapolis, MN), and IgM binding in mouse and human tissues was detected using a goat anti-mouse IgM (1:50, Sigma-Aldrich) and rabbit anti-human IgM (1:1000, Sigma-Aldrich) or anti-mouse IgM-FITC for IF (1:50, Millipore), human endothelial cells were detected using anti-human CD31-FITC (1:25, Abcam), annexin IV on human liver biopsies was detected using mouse B4 mAb (5 μg/ml, prepared as described above). For IHC primary antibodies were detected using goat-IMMpress or rabbit-IMMpress kits (Vector Labs). For IF primary antibodies were detected using anti-goat IgG AlexaFluor-555 conjugate (1:200, Invitrogen, Carlsbad, CA) or anti-mouse IgM AlexaFluor-555 conjugate (1:200, Invitrogen). Quantification of IHC staining was accomplished using automated image filters to extract DAB signal using appropriate negative and positive controls. Signal intensity was then computed per pixel, and average intensity per pixel was computed. All analyses were done using automatic scripts in MATLAB, and all images were analyzed as a batch using the same settings. Quantification of fluorescence for C3d and IgM deposition in human samples was done using an automated procedure that measured the average signal intensity per pixel on 40× fields using NIH ImageJ. Data is reported as intensity per pixel.
Human sample analysis
All tissue samples and sera were collected as part of an approved institutional IRB. For analysis of B4 mAb binding to human samples, liver biopsies were obtained from human ischemic donor livers prior to transplantation and post-reperfusion, and “normal” liver samples were taken from areas of normal pathology following resection of hepatic hemangioma. Samples taken pre and post-reperfusion were also analyzed for IgM and C3d deposition, as described above. Information on donors, ischemia time and sample analysis are provided in supplementary table 1. “All samples were stored at −80 °C until analyzed. Samples were cut into 8 μM sections, washed and permeabilized prior to blocking with serum-free protein block. Sections were then incubated with B4 mAb or control F632 mAb (5 ng/ul), washed, and incubated with HRP-conjugated anti-mouse IgM (1:200, Sigma-Aldrich). Signal was developed using DAB. All sections were imaged on an Olympus BX61 Microscope Light microscope with Visiopharm Acquisition Software. IgM and C3d deposition was analyzed by IF and quantified, as described above. Serum samples were analyzed as described below. Data on donor and recipient organs were part of our transplant and resection databases, and analysis was a part of our institutional approved IRB.
Biodistribution study
A B4 scFv biodistribution study was performed as we have previously described (13). Briefly, B4 scFv was radiolabeled with 125Iodine (New England Nuclear Corp.) using Peirce Iodination Reagent according to manufactures instructions (Thermo Scientific), and 5 mCi was used to label 100 μg B4scFv. Rag1−/− mice were injected via the tail vein with 2 μg radiolabeled protein immediately following IR, 70% Phx or sham surgery, and animals sacrificed 6 h post surgery. Blood was removed by cardiac puncture and the animals were perfused with PBS before the heart, brain, liver, intestine, lung, kidney, and spleen were removed. Tissues were rinsed with PBS, shredded, weighed and then radioactivity was measured with a Hewlett-Packard 5780 γ counter. Results were recorded as μCi/g of tissue.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0. Statistical significance between multiple groups was assessed using ANOVA. Student’s T-test or Mann-Whitney test was used to determine statistical significance between any two groups. A p value < 0.05 was considered significant.
Results
IgM monoclonal antibodies reconstitute hepatic IRI in Rag1−/− mice
Compared to WT mice, Rag1−/− mice subjected to 30 minutes of total warm hepatic ischemia followed by 6 hours of reperfusion were protected from hepatic IRI, as demonstrated by significantly lower serum ALT levels and histology scores (Fig. 1, supplementary Fig. 1). To confirm the role of natural self-reactive IgM in propagating hepatic IRI, antibody-deficient Rag1−/− mice were reconstituted with one of two self-reactive IgM mAbs. These mAbs, isolated from unmanipulated WT C57BL/6 mice and termed B4 and C2, have been previously characterized and shown to recognize modified annexin IV (B4) (1) and a subset of phospholipids (C2) (7). Both B4 and C2 mAb restored injury levels in Rag1−/− mice close to that seen in WT mice. Reconstitution of Rag1−/− mice with control F632 IgM mAb had no effect on hepatic IRI (Fig. 1). Thus, single IgM mAbs of different specificities are sufficient to propagate hepatic IRI in antibody-deficient mice.
Fig. 1.
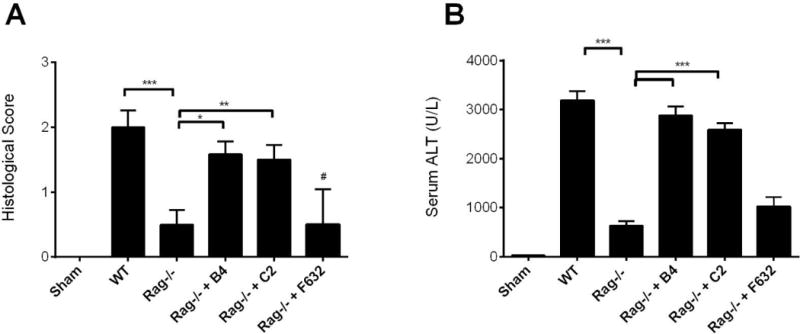
IgM antibodies reconstitute hepatic ischemia reperfusion injury in Rag1−/− mice. Hepatic IRI was significantly reduced in Rag1−/− mice compared to WT mice. Reconstitution of Rag1−/− mice with B4 mAb or C2 mAb, but not control mAb F632, restored injury in Rag1−/− mice. Antibodies were given i.v. immediately after reperfusion and all measurements were taken at 6 h post reperfusion. (A) Histological quantification of necrosis and injury, determined on a scale of 0–4. Representative H&E stained sections are shown in supplementary Figure 1. (B) Serum ALT levels. Results expressed as mean ± SEM, n = 6. *** p<0.001, **p<0.01, *p<0.05.
Self-reactive IgM antibodies also play an important role in liver regeneration
Rag1−/− mice have a diminished regenerative response following partial hepatectomy (14) for incompletely understood reasons. To investigate the role of natural IgM Abs in liver regeneration, we reconstituted Rag1−/− mice with either B4 or C2 mAb after 70% PHx. Rag1−/− mice had significantly increased injury and a significantly decreased regenerative response following 70% PHx, and reconstitution of Rag1−/− mice with either B4 or C2 mAb, but not control F632 mAb, protected the liver from injury as determined by histology score and serum ALT (Fig. 2A,B, supplementary Fig. 2). Both B4 and C2 mAbs also restored regeneration to levels not significantly different to that seen in WT mice, as determined by BrdU incorporation, mitotic index and liver weight restitution (Fig. 2C–D). Thus, single IgM mAbs of different specificities are sufficient to stimulate the regenerative response following hepatic resection. Since there was a trend for increased restoration of injury/protection with B4 mAb compared to C2 mAb, we focused on the B4 mAb in subsequent studies.
Fig. 2.
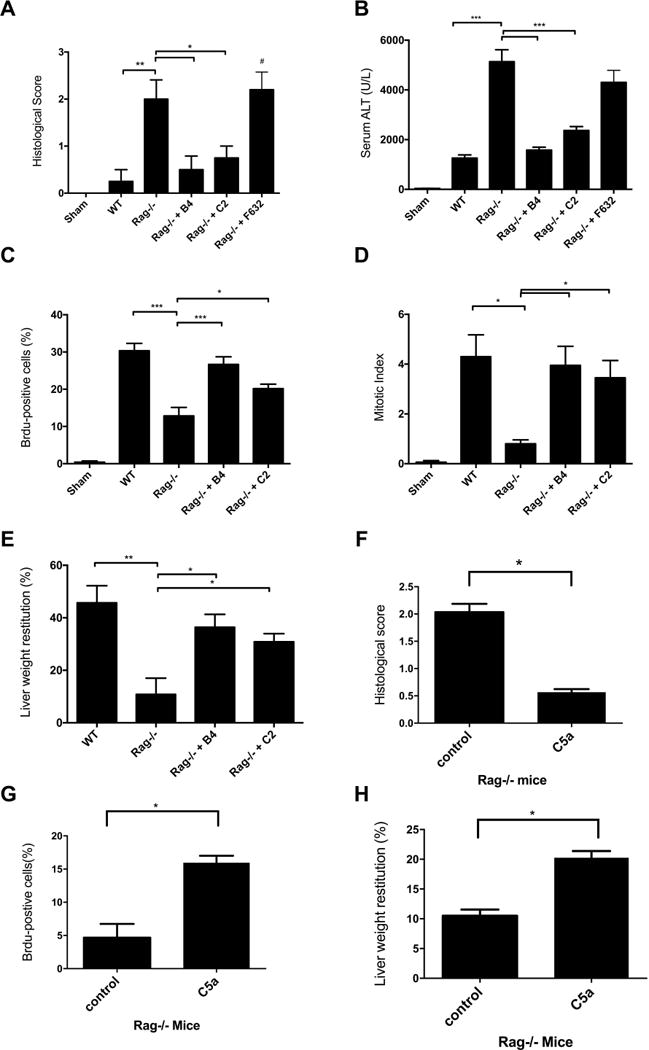
IgM antibodies are sufficient to stimulate regeneration in Rag1−/− following 70% partial hepatectomy. Compared to WT mice, Rag1−/− mice have increased injury and decreased regeneration following 70% PHx. Reconstitution of Rag1−/− mice with B4 or C2 mAbs, but not control F632 mAb, was protective and restored regeneration to levels seen in WT mice. Antibodies were given i.v. immediately following 70% PHx, and injury and regeneration was assessed at 48hrs post surgery. Hepatic injury was decreased in B4 and C2 reconstituted Rag1−/− mice as determined by (A) Histological quantification of necrosis and injury, determined on a scale of 0–4. Representative H&E stained sections are shown in supplementary Figure 2 (B) Serum ALT. Reconstitution of Rag1−/− mice with B4 or C2 mAb reconstituted increased the regenerative response as measured by (C) BrdU incorporation, detected immunohistologically and expressed as % positives cell counted in 10 hpf, (D) mitotic index, evaluated by calculating the percentage of hepatocytes undergoing mitosis in H&E stained sections and, (E) Liver weight restitution. Reconstitution of Rag1−/− mice that underwent 70% PHx with C5a peptide decreased liver injury as determined by (F) Histological injury (representative H&E stained sections are shown in supplementary Figure 3, (G) BrdU incorporation, detected immunohistologically and expressed as % positives cell counted in 10 hpf, and (H) Liver weight restitution. Results expressed as mean ± SEM, n = 5–6. *** p<0.001, **p<0.01, *p<0.05.
The complement activation products C3a and/or C5a play a key role in in liver regeneration via their effect on cell signaling processes involved in hepatocyte proliferation (12). And although not previously studied in the context of hepatic IR or resection, IRI in other organs is promoted by IgM-mediated activation of complement (reviewed in (15)). We therefore investigated whether the basis for the impaired regenerative response after PHx in Rag1−/− mice may be due to the lack of IgM-mediated complement activation, as opposed to the result of tissue injury induced by PHx in Rag1−/− mice. Reconstitution of Rag1−/− mice after PHx with C5a peptide reduced injury and enhanced the regenerative response (Fig. 2F–G, supplementary Fig. 3). These data, together with additional data on complement activation described below, indicate that an important component of the impaired regenerative response in Rag1−/− mice is due to lack of complement activation and anaphylatoxin (C3a/C5a) generation.
Serum IgM antibodies and B4 mAb localize to injured tissue and activate complement
Hepatic deposition of IgM occurs following hepatic IRI (16, 17) and resection (5). To investigate the relationship between IgM deposition and complement activation, and in particular a role for post-ischemia and post-resection expression and recognition of the B4 (annexin IV) neoepitope, we analysed IgM and complement activation (indicated by C3d deposition) after either hepatic IR or 70% PHx. Following hepatic IR, IgM and C3d deposition was observed in an overlapping sinusoidal pattern in livers from WT mice and Rag1−/− mice reconstituted with B4 mAb, but noti in control treated Rag−/− mice (Fig. 3A). Similarly, following PHx, sinusoidal staining of both IgM and C3d was observed in WT mice and in Rag1−/− mice reconstituted with B4, but not in control treated Rag1−/− mice (Fig. 3B). The similar staining patterns and the fact that no C3d deposition occurred in control treated Rag1−/− mice is indicative of IgM activating complement following both IR and PHx. There was little or no IgM or C3d detected in Rag1−/− mice reconstituted with control IgM mAb F632, demonstrating a clonally specific response to a post-ischemic and post-resection neoepitope. Thus, the annexin IV neoepitope recognized by B4 mAb may represent a therapeutic target for the design of biologically based pharmacuticals aimed at either inhibiting hepatic IRI or to promoting liver regeneration.
Fig. 3.
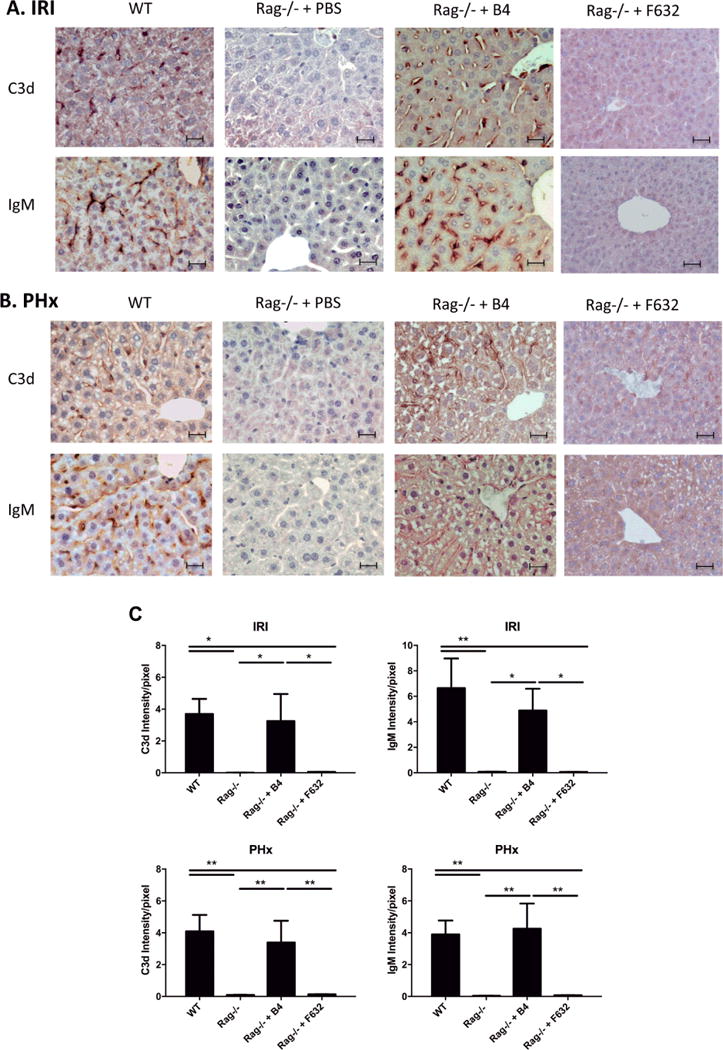
IgM binding and C3d deposition following hepatic ischemia and reperfusion and 70% partial hepatectomy. IgM deposition and complement activation (C3d deposition) was visualized following hepatic IR or 70% PHx in WT mice, Rag1−/− mice, and Rag1−/− mice reconstituted with B4 mAb. (A) Immunohistochemical staining for IgM and C3d at 6 h post IR showed IgM and C3d staining in a sinusoidal pattern in WT mice and Rag1−/− mice reconstituted with B4 mAb. No staining was seen in Rag1−/− mice treated with PBS. (B) Immunohistochemical staining of IgM and C3d at 48 h post PHx showed IgM and C3d staining in a sinusoidal pattern in WT and Rag1−/− mice reconstituted with B4 mAb. No staining was seen in Rag1−/− mice treated with PBS. Representative images from 3 animals per group. Scale bars represent 20 uM. (C) Quantification of C3d and IgM staining, Mean +/− SEM, n = 3, **p<0.01, *p<0.05.
Characterization of a single chain Ab derived from B4 IgM mAb
Although the data above indicate an important role for IgM antibodies in both hepatic IRI and liver regeneration, other studies done using Rag1−/− mice have indicated a key role for T cells in hepatic IRI and regeneration (6, 14, 17–19). Given the potential confounders and limitation of studies done in genetically engineered mice we sought to confirm the role of natural Abs, and specifically annexin IV specific antibodies, using a WT mouse model and a B4scFv shown previously to competitively inhibit B4 mAb binding (9).
We first confirmed that B4 scFv localizes to the liver following either hepatic IR or 70% PHx by injecting 125I-labeled B4 scFv immediately after surgery and measuring tissue distribution of radiolabel 6 h after injection. Although still present in the circulation, 125I-B4 scFv localized preferentially to the liver following hepatic IR or 70% PHx (Fig. 4). A similar biodistribution after IRI and 70% PHx was obtained with the parent 125I-labeled B4 IgM mAb (not shown). Data above show that reconstitution of Rag1−/− mice with a single IgM mAb was sufficient to restore injury following hepatic IR, and to determine whether interfering with the binding of IgM of single specificity would protect against IRI in the context of an entire natural Ab repertoire, we treated WT mice with B4 scFv following hepatic IR. Compared to PBS treated controls, B4 scFv treatment protected against hepatic IRI as determined by serum ALT and histological score (Fig. 5A,B, supplementary Fig. 4). However, whereas B4 mAb reconstitution in Rag1−/− mice restored regenerative capacity following PhX, treatment of WT mice with B4 scFv following Phx had no effect, and there was no difference in regenerative outcomes between PBS and B4 scFv treated mice (Fig. 5C–D, supplementary Fig. 5). The ability of B4 scFv to protect against IRI without impairing regeneration points toward the therapeutic potential of B4 scFv, or the use of B4 scFv as a targeting vehicle for therapeutics, in clinical settings such as liver resection or transplantation.
Fig. 4.
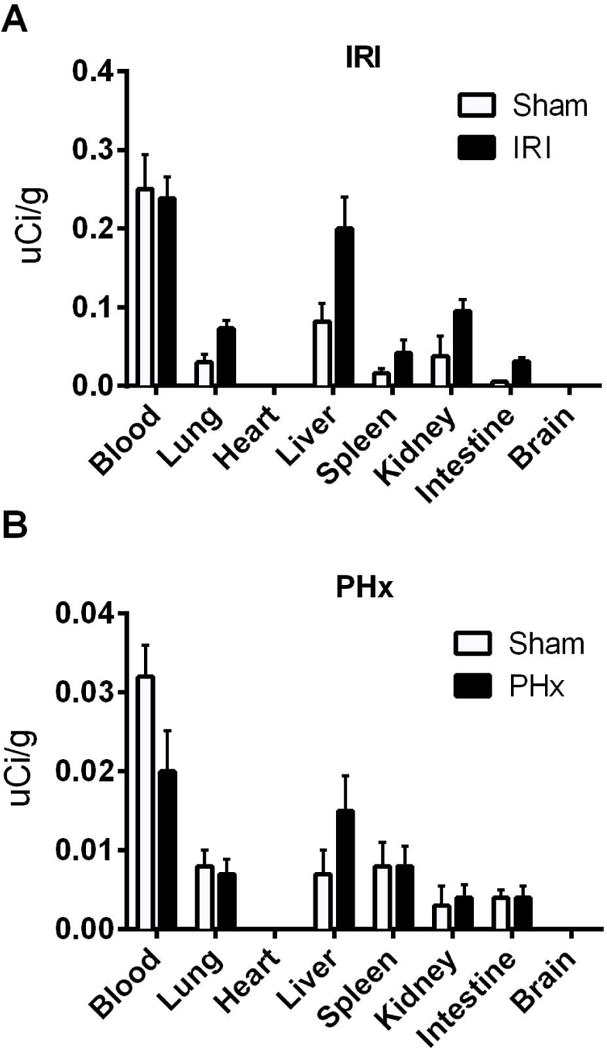
Biodistribution of B4 scFv following hepatic ischemia reperfusion and partial hepatectomy in Rag1−/− mice. Rag1−/− mice were reconstituted with 125I-radiolabelled B4 scFv immediately following IR, PHx or sham surgeries. Tissues were harvested and radioactivity measured at 6 h post surgery. (A) Biodistribution of B4 scFv following hepatic IR. (B) Biodistribution of B4 scFv following 70% PHx. There is preferential localization of B4 scFv to the liver after both types of surgery. Data is representative of 2 independent experiments, n = 2 for each group.
Fig. 5.
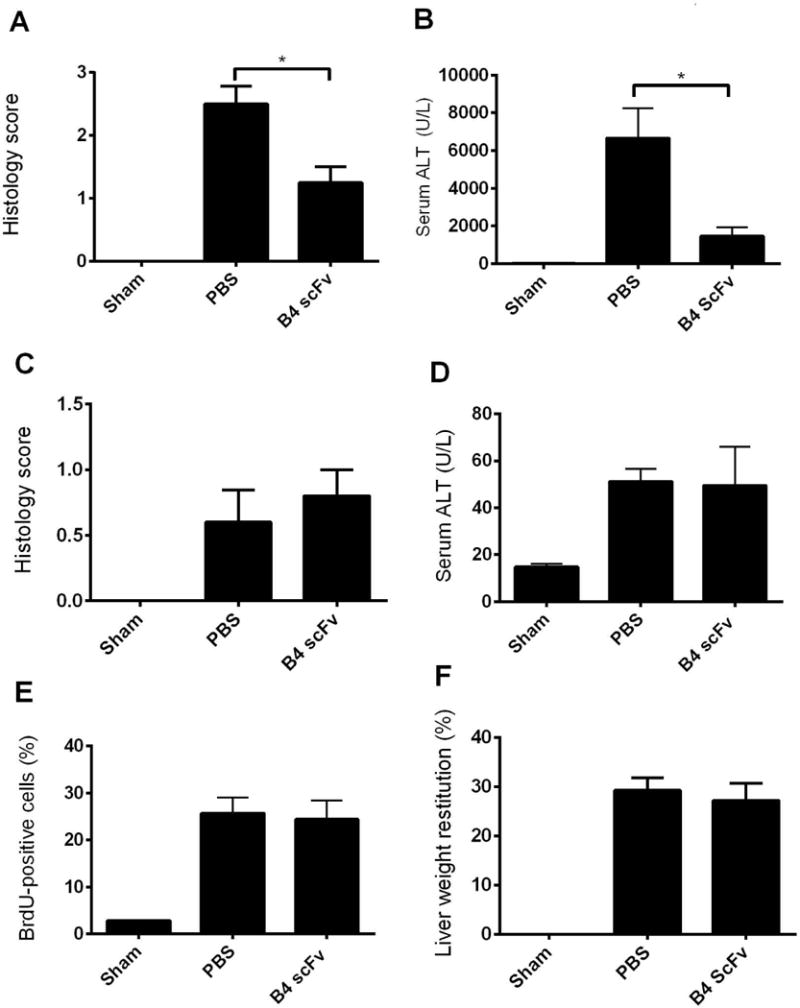
Treatment with B4 scFv limits injury following hepatic ischemia reperfusion injury and does not alter liver recovery or regeneration following 70% partial hepatectomy in wild type mice. WT mice treated with B4 scFv had decreased hepatic injury compared to PBS treated controls. WT mice underwent 30 minutes of ischemia followed by 6 h of reperfusion. B4 scFv (20 μg) and PBS was administered i.p. 5 minutes prior to reperfusion. Hepatic injury was measured by (A) Histological quantification of necrosis and injury, determined on a scale of 0–4. Representative H&E stained sections are shown in supplementary Figure 4. (B) Serum ALT. Results expressed as mean ± SEM, n=4–6. *p < 0.05. Following 70% PHx, WT mice treated with B4 scFv (20 μg) and PBS control treated mice had similar injury and regeneration outcomes. All measures were made at 48 h post PHx. B4 scFv and PBS were administered i.p. 5 minutes prior to PHx surgery. Following 70% PHx, liver injury was determined by (C) Histological quantification of necrosis and injury, determined on a scale of 0–4. Representative H&E stained sections are shown in supplementary Figure 5. (D) Serum ALT. Liver regeneration was measured by (E) BrdU incorporation, detected immunohistologically and expressed as % positives cell counted in 10 hpf and, (F) percent liver weight restitution. Results expressed as mean ± SEM, n = 4–6. No significant differences between PBS treated and B4 scFv treated mice for all measures.
Hepatic human ischemic tissue expresses B4 antigen
To examine the translational potential of B4 scFv or of a B4 scFv targeting strategy, we investigated expression of the B4 epitope in human post-ischemic tissue. Liver biopsies were obtained from ischemic human donor livers prior to transplantation. Sections stained positive for annexin IV expression using B4 mAb, with a similar sinusoidal pattern of deposition to that seen in post-ischemic mouse liver sections (Fig. 6A). The low signal seen with control F632 IgM mAb was similar to that seen with secondary Ab alone (data not shown). B4 mAb did not bind to biopsy sections taken from areas of normal pathology obtained from resections for hepatic hemangioma. These data demonstrate specific binding of B4 mAb to post-ischemic human livers.
Fig. 6.
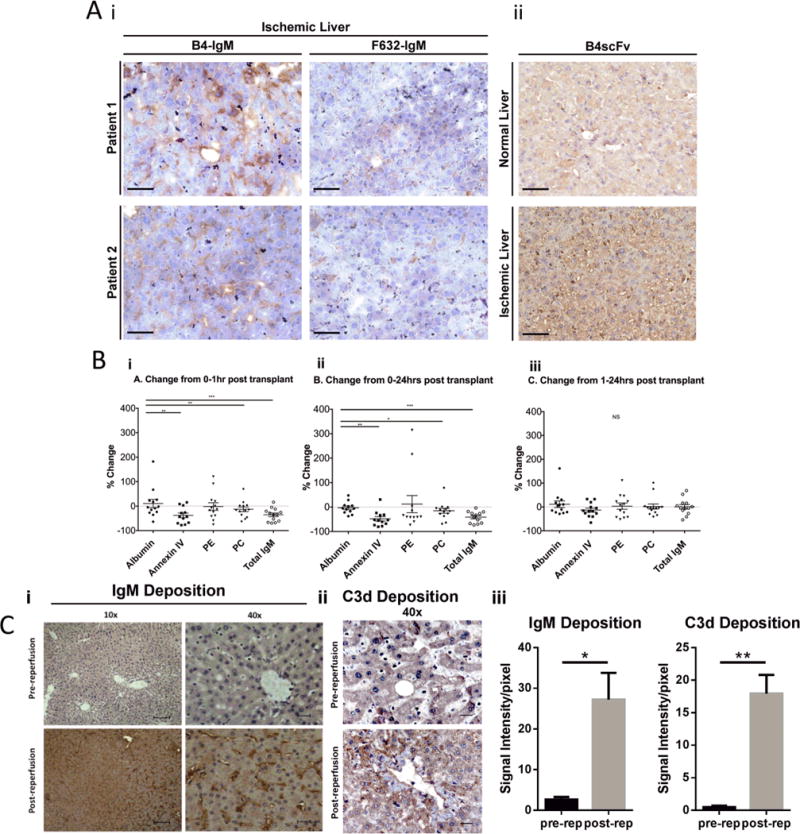
Analysis of clinical samples. (A) B4 antigen is expressed in ischemic but not normal human liver samples. “Ischemic” liver biopsies were obtained from ischemic human donor livers prior to transplantation, and “normal” liver samples were taken from areas of normal pathology following resection of hepatic hemangioma. (A) Shows immunohistochemical staining of sections using (A.i) B4 mAb, control F632 mAb, and (A.ii) B4scFv as indicated. Representative images from 3 samples from 3 patients. Scale bar represents 50 uM. (B) Serum levels of specific pathogenic natural IgM antibodies decrease following human liver transplantation. Serum levels of IgM antibodies against annexin IV, phosphatidylethanolamine (PE), phosphatidylcholine (PC), and total IgM and albumin were measured by ELISA in serial serum samples taken from patients undergoing liver transplantation. Serum was harvested at three time points: just prior to transplantation (baseline= 0 hour), 1 hour, and 24 hours after transplantation. (B.i.) Percent change from 0–1 hour post transplantation. (B.ii) Percent change from 0–24 hours post transplantation. (B.iii) Percent change from 1–24 hours post transplantation. Each data point represents one patient, n = 13. Data representative of 3 separate experiments. *** p<0.001, **p<0.01, *p<0.05. (C) Immunofluorescence staining for IgM and C3d in human livers pre-transplantation and 1 hour post reperfusion. (Ci,ii) Representative images, n = 3 patients. Scale bar = 50 uM (40×). (Ciii) Quantification of fluorescence intensity of IgM and C3d staining. Mean+/− SEM, n = 3, **p<0.01, *p<0.05.
Serum levels of natural IgM are decreased in human liver transplant recipients
To further investigate whether a similar B4 recognition system occurs in humans, we investigated the level of anti-annexin IV IgM Abs in serum from 13 patients pre and post-liver transplantation. We additionally investigated levels of anti-PC and anti-PE IgM in patient serum, two phospholipids recognized by the C2 mAb (7) that also restores hepatic IRI in Rag1−/− mice (above). Serial serum samples were taken from patients undergoing liver transplantation at three time points: just prior to transplantation (baseline), and at 1 hour and 24 hours after transplantation. Analysis by ELISA revealed that serum levels of IgM specific for annexin IV were significantly decreased at 1 and 24 hours post-transplantation compared to levels of albumin, a serum protein used to control for dilutional effect of infusion of blood products given during surgery. Serum levels of IgM specific for PC were also significantly decreased at 1 and 24 hours post-transplantation. The decrease in anti-PE levels did not reach significance, although the decrease would be significant with the exclusion of data from 2 patients exhibiting an extremely high increase in anti-PE levels (Fig. 6B). Total IgM was also significantly decreased at 1 and 24 hours post transplantation.
Patients received an average of 15 units of packed RBCs (range 3–32), 4 units of platelets (range 0–11), and 22 units of fresh frozen plasma (FFP) (range 4–48). However, it is unlikely that the decrease in serum IgM is due entirely to a dilutional effect for several reasons; most importantly, no correlation was seen between amount of blood products given and percentage change of IgM. If the decrease in serum IgM following transplantation was due to a dilutional effect, a positive correlation between amount of blood products given and precent change in IgM levels would be expected, which was not the case for any of the antigens measured (data not shown). Because of this lack of correlation, as well as no depletion of anti-PE IgM and the unique response seen in each patient (on a patient to patient level the degree of antibody depletion was not consistent across specificities), the data suggest that anti-annexin IV and anti-PC IgM is specifically removed from the circulation following liver transplantation.
To correlate depletion of IgM from human serum with IgM deposition in the post-ischemic liver, we analysed IgM and C3d deposition in transplanted human livers before and after reperfusion. Little to no IgM or C3d was detected in livers prior to reperfusion, but in post-ischemic and reperfused livers, high levels of bound IgM and C3d was observed in a characteristic sinusoidal pattern (Fig. 6C).
To further support the above conclusion, we performed an analogous study using our mouse model of hepatic IRI that lacks many of the confounding conditions that are unavoidable in human liver transplantation, such as the infusion of blood products and genetic variability. Following hepatic IR in mice, serum levels of anti-Annexin IV IgM and total IgM were significantly decreased as compared to sham animals. Of the two C2 mAb antigens examined, levels of anti-PE were significantly decreased, whereas levels of anti-PC were unchanged, which was different to that observed in human serum after liver transplantation (Fig. 7). These data suggest that following both human and murine hepatic IR, IgM specific for annexin IV is depleted from the circulation. The data further suggest that there may be differences in phospholipid subsets that represent post-ischemic neoepitopes expressed in mice and humans, although the C2 mAb, that recognizes both PC and PE (and some additional phospholipids), recognizes post-ischemic neoepitopes from both species (7). Regarding the reduction in levels of total IgM observed after IR, there are several specificities for natural pathogenic self-reactive Abs, other than the two under study here. It has been estimated that the majority of circulating IgM is natural IgM (20), and this likely accounts for the reduction in total circulating IgM following IR.
Fig. 7.
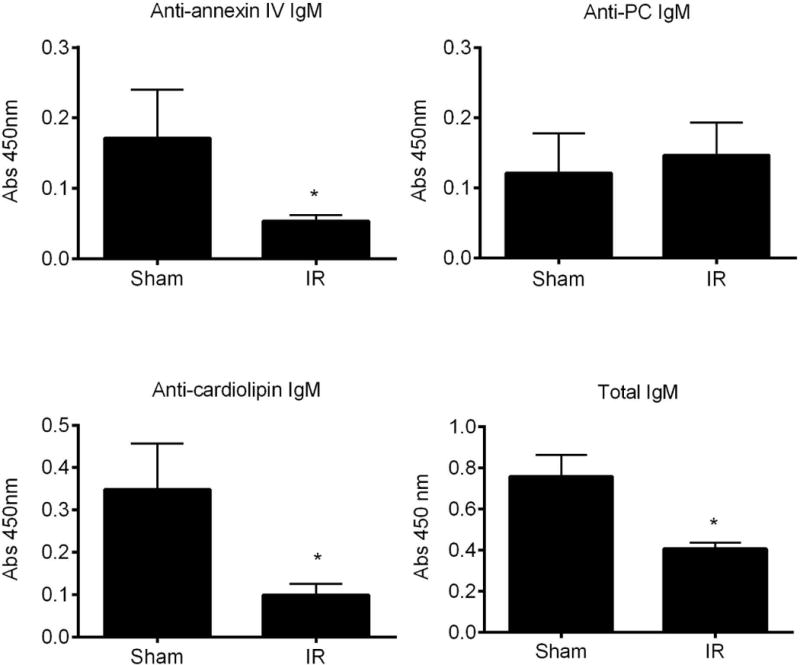
Serum levels of pathogenic natural IgM antibodies decrease following murine hepatic ischemia and reperfusion. Serum levels of IgM antibodies against annexin IV, phosphatidylcholine (PC), cardiolipin, and total IgM were measured by ELISA in mice that underwent IR. Samples were harvested at 6 h post reperfusion. Data is presented as fold change over sham. Mean +/− SEM, n = 7–12, *p < 0.05.
Discussion
It has been shown that complement activation products are essential for eliciting hepatic IRI and also for initiating liver regeneration following PHx or toxic injury (11, 12, 21). Seminal studies demonstrated that natural IgM antibodies activate complement following IR of the intestine (1) and skeletal muscle (3, 4). Here we demonstrate that IgM antibodies are also responsible for activating complement following hepatic IRI as well as PHx. The IgM mAbs used in this study, B4 and C2, recognize antigens uniquely expressed on stressed or damaged cells (1, 7). However, although natural antibodies in general are known to play an important role in innate immunity to infection as well as homeostasis, B4 and C2 have only been investigated in the context of sterile inflammation, and only a pathogenic role for these antibodies has been described. Here we demonstrate a pathogenic role for these mAbs in liver injury, but additionally demonstrate that antigens recognized by these mAbs are also expressed following liver resection and are involved in the recovery process.
With regard to IRI, we found that both B4 and C2 mAbs activate complement and induce hepatic IRI in otherwise protected Rag1−/− mice, respectively implicating annexin IV and a subset of phospholipids as DAMPS expressed in the post-ischemic liver. In addition, we demonstrated that B4 scFv protected against hepatic IRI in WT mice that contained a full natural Ab repertoire. It is interesting that although a diverse set of neoepitopes are exposed following IRI, blocking a single specificity is sufficient to prevent injury. Although this phenomenon has been described in other models of IRI, the physiology is not understood. It has been speculated by Kulik et al. (1) that there may be a sequential exposure of antigens that are recognized by IgM and activate complement, and that blockade of one antigen disrupts this order and prevents maximal complement activation. Alternatively, antigens recognized by pathogenic IgM Abs may be specific to a certain cell type and multiple cell types needed to be involved for IRI to occur, and therefore blocking one antigen could prevent injury to a specific subset of cells and subsequently ameliorate IRI. These theories suggest that recognition of multiple antigen specificities needs to occur in some sort of organized fashion in order to induce IRI. However, studies done in Rag1−/− mice would imply that this is unlikely since a single monoclonal antibody can induce IRI in these mice. Another possible explanation for why a single antibody specificity can be sufficient for inducing IRI may be related to the redundant nature of the innate immune response. Natural IgM Abs are effectors of the innate immune response and as such are part of the DAMP recognition system. Many other effectors molecules of the innate immune system similarly recognize DAMPs, some of which can also directly activate complement. For example, phospholipids and apoptotic cells recognized by B4 and C2 mAbs are also recognized by toll-like receptor 4 (22), C-reactive protein (23), and MBL (24). Thus, it is possible that blocking one antigen limits multiple pathways in the innate DAMP recognition system, leading to a more robust inhibitory effect. Additionally the inflammatory cascade does not progress linearly but rather gets exponentially amplified as more components become involved, and blocking a small subset of antigens early in the injury process may have amplified effects on limiting subsequent damage.
While a role for IgM in IRI of various organs has been described, the role of IgM in liver recovery following resection has not been directly addressed. Recent studies suggested a role for IgM in liver regeneration in a positive feedback loop involving complement activation and IL-4 upregulation (5), but there was no direct evidence of complement activation by IgM following PHx, and whether IgM is necessary for regeneration was not investigated. We show that following PHx, IgM and C3d deposit in the remnant liver in an overlapping sinusoidal pattern in both WT mice and B4 mAb reconstituted Rag1−/− mice, and that C3 deposition is absent in Rag1−/− mice, strongly suggesting IgM is activating complement in the setting of PHx. We also report that Rag1−/− mice have increased injury and diminished regenerative response following PHx, and reconstitution with either B4 or C2 mAb (but not a control IgM mAb) is sufficient to restore regeneration to levels in WT mice. Our finding of increased injury and impaired regeneration after PHx in Rag1−/− mice is contradictory to an earlier report that showed Rag1−/− had similar injury and regeneration as WT mice following PHx (6). We have no explanation for this difference since the mice used and model are the same. On the other hand, a study done by Tumanov et al. (14) showed similar results to ours in that Rag1−/− had increased injury and decreased regeneration, although they found that reconstitution of T cell derived lymphotoxin restored regeneration in these mice, implicating a critical role for T cells in regeneration.
Overlapping roles for T and B cells have been shown in various models of IRI. For example, T and B cell-deficient (Rag1−/− and/or SCID) mice are also protected from intestinal and myocardial IRI, and injury can be restored by independent reconstitution of either T cells or IgM (reviewed in (19)), indicating compensatory mechanisms can contribute to IRI. A more recent study reported that hepatic IRI is independent of B cells and IgM (17). It was shown that while Rag1−/− mice were protected from hepatic IRI, serum transfer did not restore injury, and furthermore that T cell–mice, but not B cell-deficient mice, were protected from hepatic IRI. These data appear to be in contradiction to the current findings. A potential explanation is that B cells can have both protective and pathogenic activities, and in this context it has been shown that specific depletion of peritoneal B-1 cells did not alter circulating levels of IgM, but reduced renal IgM binding and was protective in a model of renal IRI. On the other hand, renal IRI was increased in B cell-deficient mice compered to WT mice (25). The authors attributed this apparent dual role of B cells to the production of B-1 B cell derived pathogenic natural IgM and its binding to post-ischemic mesangium vs. the production of protective IL-10 by mature B cells. Regardless, anti-annexin IV IgM Abs exist in both mice and humans (1, 7), and an anti-annexin IV IgM mAb that specifically targeted the post-ischemic liver restored hepatic IRI in otherwise protected Rag1−/− mice. To further address some of the confounding issues that may be associated with Rag1−/− mice, we used a B4 scFv that we have shown blocks IgM binding in WT mice that have a full natural Ab repertoire (9), and found that B4scFv was protective against hepatic IRI. Thus, in contrast to the earlier report that hepatic IRI is IgM independent, these data imply that targeting IgM and the IgM injury sensing mechanism represents a potential therapeutic avenue for reducing hepatic IRI. Certainly further investigation is needed to fully understand the relative contributions of T and B cells to hepatic IRI.
Although B4scFv inhibited hepatic IRI, it is interesting that it had no effect on liver injury or regeneration after PHx, even at high dose. These data imply that anti-annexin IV antibodies are sufficient, but not necessary for the regenerative response in a WT mouse. So what is the cause of this discrepancy between hepatic IRI and regeneration after PHx? One possibility is that although the B4 antigen is expressed following PHx, it is not the dominant neoepitope and one or more other IgM specificities are more relevant in recognizing cellular alterations in the resected remnant liver. Compared to WT mice, B4 mAb may be able to compensate for the absence of other more relevant IgM Abs in Rag1−/− mice. Further to this, it is also noteworthy that there was a significantly lower uptake of 125I-B4scFv per gram of liver following PHx compared to after IR. This could be due to different relative levels of insult between the two models resulting in differential or just lower levels of neoepitope expression, and consequently lower levels of B4scFv binding accounting for lack of effect of B4scFv on regeneration. In this context, immunohistochemical quantification of IgM revealed less IgM binding following PHx compared to after IR (Fig 3). Another possibility is that there is a compensatory mechanism in the regenerative process and molecules other than IgM are able to activate complement and stimulate regeneration. In support of this, Clark et al. (26) showed that inhibition of all traditional complement activation pathways in mice with a combined deficiency of C4 and fB still resulted in C3 cleavage and normal regeneration following PHx.
Together, the above data indicate that although complement plays a key role in both hepatic IRI and regeneration, there are some potential differences in the initiating activation event or neoepitope expression. The fact that B4 scFv limits IRI but does not prevent regeneration makes it a potential therapeutic candidate for use in the clinical setting of liver resection or transplantation, where the goal of therapy would be to limit IRI without hindering regeneration. In this regard, we show that B4 mAb binds to post-ischemic endothelium in human liver biopsy samples, and that IgM of B4 and C2 specificity is depleted from human serum of liver transplant recipients. In summary, our data indicate that self-reactive IgM activates complement following both hepatic IRI and PHx, and plays an important role in both liver injury and regeneration. We also present evidence indicating that annexin IV represents a post-ischemic neoepitope expressed in both mouse and humans following hepatic IR. These data indicating a similar cross-species injury-specific recognition system highlights the potential for development of translational therapies.
Supplementary Material
Acknowledgments
The authors thank Farris Langley, Wenxue Wang, Hong Yu and Xian Zhang for technical assistance. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (RO1DK102912), and the National Natural Science Foundation of China (No. 81430014, 31370917).
List of Abbreviations
- IR
ischemia reperfusion
- IRI
ischemia reperfusion injury
- PHx
hepatectomy
- WT
wild type
- mAb
monoclonal antibody
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- FFP
fresh frozen plasma
- DAMPs
damage-associated molecular patterns
Footnotes
Authorship
S.T., K.M. and S.H. planned the research and analysed data; S.T. and K.M. wrote the manuscript; K.M., S.H., J.J., A.A., F.Q, and C.A, performed the research, and K.M, S.H., A.A. and C.A. additionally analysed the data. K.D.C. analysed the data. S.T. and C.A. have a patent pending for natural antibody-targeted complement inhibitors.
Contributor Information
Keely Marshall, Email: Keely.Morris@gmail.com.
Junfei Jin, Email: jin@musc.edu.
Carl Atkinson, Email: atkinsoc@musc.edu.
Ali Alawieh, Email: alawieh@musc.edu.
Fei Qiao, Email: qiaofei99@yahoo.com.
Kenneth D. Chavin, Email: ChavinKd@musc.edu.
Songqing He, Email: hsqhaoren@aliyun.com.
Stephen Tomlinson, Email: tomlinss@musc.edu.
References
- 1.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, et al. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeAngelis RA, Markiewski MM, Kourtzelis I, Rafail S, Syriga M, Sandor A, Maurya MR, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188:641–648. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graubardt N, Fahrner R, Trochsler M, Keogh A, Breu K, Furer C, Stroka D, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology. 2013;57:1969–1979. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 7.Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, et al. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87:618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, Goddard M, et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131:1171–1180. doi: 10.1161/CIRCULATIONAHA.114.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigala F, Kostopanagiotou G, Andreadou I, Kavatzas N, Felekouras E, Sigalas P, Bastounis E, et al. Histological and lipid peroxidation changes after administration of 2-acetylaminofluorene in a rat liver injury model following selective periportal and pericentral damage. Toxicology. 2004;196:155–163. doi: 10.1016/j.tox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119:2304–2316. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694–704 e694. doi: 10.1053/j.gastro.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2006;41:62–67. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Diepenhorst GM, de Graaf W, Niessen HW, van Vliet AK, Hack CE, van Gulik TM. Immunoglobulin M, C-reactive protein and complement activation in rat hepatic ischemia-reperfusion injury. Eur Surg Res. 2014;52:50–62. doi: 10.1159/000360474. [DOI] [PubMed] [Google Scholar]
- 17.Richards JA, Bucsaiova M, Hesketh EE, Ventre C, Henderson NC, Simpson K, Bellamy CO, et al. Acute Liver Injury Is Independent of B Cells or Immunoglobulin M. PLoS One. 2015;10:e0138688. doi: 10.1371/journal.pone.0138688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng M, Li G, Qian X, Fan Y, Huang X, Zhang F, Lu L. IL-17A-producing NK cells were implicated in liver injury induced by ischemia and reperfusion. Int Immunopharmacol. 2012;13:135–140. doi: 10.1016/j.intimp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci U S A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 25.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, Takahashi K, et al. B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol. 2010;185:4393–4400. doi: 10.4049/jimmunol.0903239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark A, Weymann A, Hartman E, Turmelle Y, Carroll M, Thurman JM, Holers VM, et al. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol Immunol. 2008;45:3125–3132. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


