Abstract
Purpose
Intervertebral disc with Propionibacterium acnes (P. acnes) is suggested to be an etiology of Modic type I changes in the adjacent bone marrow. However it is unknown if disc cells can respond to P. acnes and if bone marrow cells respond to bacterial and disc metabolites draining from infected discs.
Methods
Human disc cells (n=10) were co-cultured with 10- and 100-fold excess of P. acnes over disc cells for 3h and 24h. Lipopolysaccharide was used as positive control. Expression of IL1, IL6, IL8, and CCL2 by disc cells was quantified by quantitative PCR. Lipase activity was measured in culture supernatants (n=6). Human vertebral bone marrow mononuclear cells (BMNCs) (n=2) were cultured in conditioned media from disc cell / P. acnes co-cultures and expression of IL1, IL6, IL8, and CCL2 was measured after 24h.
Results
All disc cells responded to lipopolysaccharide but only 6/10 responded to P. acnes with increased cytokine expression. Cytokine increase was time- but not P. acnes concentration-dependent. Disc cell responsiveness was associated with the presence of lumbar Modic changes in the donor. Lipase activity was increased independent of disc cell responsiveness. BMNCs responded with inflammatory activity only when cultured in supernatants from responsive disc cell lines.
Conclusion
Disc cell responsiveness to P. acnes associates with the presence of lumbar Modic changes. Furthermore, bone marrow cells had an inflammatory response to the cocktail of disc cytokines and P. acnes metabolites. These data indicate that low virulent P. acnes infection of the disc is a potential exacerbating factor to Modic changes.
Keywords: Modic change, P.acnes, immune response, co-culture, bone marrow, inflammation
INTRODUCTION
Low virulent infection of the intervertebral disc with the anaerobic aerotolerant bacteria Propionibacterium acnes (P. acnes) is suggested as the etiology of one phenotype of Modic type I changes (MC1) [26]. P. acnes has been isolated from discs adjacent to MC1, and the presence of P. acnes was prognostic for the development of MC1 [1–5]. P. acnes is assumed to home to structurally damaged discs through hematogenous spread from a distant infection, or through the blood after innocuous skin lesions, e.g. tooth brushing [6, 7]. Once in the disc, the low oxygen tension and low pH in the disc favor the proliferation of P. acnes [8]. P. acnes secrete lipase, a virulence factor that associates with the severity of acne vulgaris pathogenesis [9]. Lipase hydrolyzes triacylglycerides, which are abundant in the bone marrow, into glycerol and free fatty acids. Free fatty acids, in turn, are highly pro-inflammatory [9]. It is unknown if disc cells sense P. acnes or its metabolites, yet disc cells express toll-like receptors (TLR), which bind bacterial cell wall compounds [10]. TLR ligation leads to the activation of inflammatory cascades and the secretion of pro-inflammatory cytokines. Disc cytokines and bacterial metabolites can drain easily into the adjacent bone marrow because endplate damage is present in MC1 [11–13]. Endplate damage also increases convective flow between the disc and the bone marrow and increases the biological cross-talk with the adjacent vertebra. In the bone marrow, pro-inflammatory cytokines and free fatty acids cause hematopoietic changes that are consistent with MC1 [14, 15]. Despite these compelling linkages between P. acnes, disc inflammation, and MC1, it is unknown if disc cells respond directly to P. acnes, and whether bone marrow cells respond to the cocktail of disc cytokines and P. acnes metabolites. Therefore, we co-cultured human disc cells with P. acnes isolated from a clinical disc sample collected at the level of MC1, and subsequently cultured vertebral bone marrow-derived mononuclear cells (BMNCs) in the conditioned media from the disc cell / P. acnes co-culture. We hypothesized that P. acnes induces inflammatory behaviors in disc cells, and that BMNCs have an inflammatory response to conditioned media.
MATERIAL AND METHODS
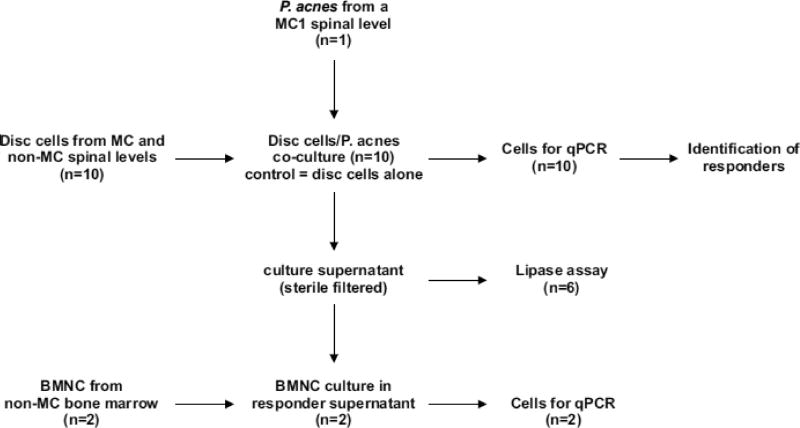
The study was approved by the Institutional Research Board of the University of California San Francisco (13-12489, 13-10863, and 14-13246). The study workflow is provided in Fig. 1.
Fig. 1.
Flow chart of the workflow.
P. acnes strain isolation
P.acnes was aseptically isolated from surgical waste tissue removed from a human L4/5 disc as previously described [3]. The patient (33 years, female, BMI 25.3) underwent discectomy and decompression because of chronic LBP with pain and numbness in both legs. Prior to surgery, serial epidural steroid injections relieved pain for 1–2 months each. Disc tissue was minced using a sterile scalpel and cultured aerobically and anaerobically. P. acnes bacteria were cultured on Brucella agar and cryopreserved at −80°C in 25% glycerol. Subculture colonies of this P.acnes strain were suspended in phosphate buffered saline (PBS) for rat injection. This patient had MC1 at L4-5 level for more than one year prior to surgery. The isolated strain was identified as type II, and was capable to provoke Modic type I-like changes after injection into rat-tail discs [3]. P. acnes was stored in a 25% glycerol stock in PBS at −80°C. The same stock was used for all co-cultures.
P. acnes / disc cell co-culture
Ten lumbar discs from eight patients undergoing anterior, transforaminal, or extreme lateral lumbar interbody fusion for degenerative conditions (five for degenerative disc disease, three for spondylolisthesis) were aseptically collected (Table 1). Disc tissue was minced into few cubic millimeter sized pieces. Tissue pieces with identifiable organized fiber structures, indicative of AF, and bloody tissue pieces were discarded. Disc tissue pieces were washed three times with phosphate buffered saline supplemented with antibiotic antimycotic solution (A/A) (Sigma-Aldrich, St. Louis, MO, USA) and cultured in disc media (DMEM/F12, 10 % fetal calf serum, A/A) for about 10 days until cells migrated out of the tissue and formed colonies. Media supplementation with A/A guarantees that no bacteria from isolated discs were cultured. These culture conditions favor disc cell over macrophage or stromal cell expansion. Qualitative microscopic observation of expanding colonies verified that no macrophage colonies were growing. After the second passage cells were cultured in 12-well plates without A/A at low oxygen tension (2%) and assigned to a treatment (1:10, 1:100, negative control, lipopolysaccharide (LPS)) and a time point (3h or 24h). All cultures were done in triplicates. For groups 1:10 and 1:100, P. acnes were taken from the frozen glycerol stock, diluted in PBS and added at a disc cell:P. acnes ratio of 1:10 or 1:100 at around 50% confluency of disc cells. P. acnes concentration was calculated based on the approximation that OD600 = 0.3 equals 100 Mio/ml P. acnes [16]. Disc cell cultured without P. acnes served as negative control. Supplementation with LPS (1 μg/ml) served as positive control. After 3h and 24h cultures were harvested. Media was sterile filtered and frozen at −80°C. Disc cells were lyzed with Qiagen RNeasy RLT buffer (Qiagen, Valencia, CA, USA) according to manufacturer protocol.
Table 1.
Disc cell donor statistics. P-value for the comparison of responders and non-responders are indicated.
| patient | gender | age | weight | pain | batch | level | MC | DD grade |
Pro- cedure |
Res- ponder |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 69 | 68.0 | 9 | 1 | L4/5 | MC1 | 4 | ALIF | Yes |
| 2 | female | 83 | 79.4 | 5 | 2 | L4/L5 | - | 4 | XLIF | Yes |
| 3 | L5/S1 | MC1 | 4 | TLIF | Yes | |||||
| 3 | female | 41 | 99.8 | 5 | 4 | L4/L5 | MC2 | 5 | ALIF | Yes |
| 5 | L5/S1 | MC2 | 5 | ALIF | Yes | |||||
| 4 | female | 60 | 63.5 | 5 | 6 | L4/5 | - | 3 | TLIF | Yes |
| 5 | male | 68 | 86.6 | 0 | 7 | L5/S1 | - | 4 | TLIF | No |
| 6 | female | 63 | 88.5 | 4 | 8 | L5/S1 | - | 4 | ALIF | No |
| 7 | male | 69 | 83.9 | 7 | 9 | L4/5 | - | 4 | ALIF | No |
| 8 | male | 63 | 113 | 3 | 10 | L4/5 | - | 4 | TLIF | No |
|
| ||||||||||
| p-value | 0.49 | 0.79 | 0.20 | 0.20 | <0.05 | 0.68 | ||||
Abbreviations: Modic changes (MC), disc degeneration (DD).
Lipase assay
Lipase activity was measured in cell-free culture supernatants from batch 1–6 (without LPS groups)(Table 1) using Lipase Activity Assay Kit II (MAK047, Sigma-Aldrich, St. Louis, MO, USA) according to manufacturer protocol. Activity was normalized to disc cells cultured without P. acnes and significant differences were calculated with a crossed ANOVA.
Isolation and culture of bone marrow mononuclear cells (BMNC)
BMNC were aspirated from lumbar vertebrae from two patients undergoing spinal fusion as previously described [14]. Briefly, 2 ml bone marrow was aspirated from the vertebral body with a Jamshidi needle placed into the screw trajectory. Aspirates were immediately transferred into sterile K2-EDTA tubes. BMNCs were isolated with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA), washed twice with Iscove's Modified Dulbecco's Media (IMDM), 2% FCS and cultured at 1 Mio/ml in the sterile filtered culture supernatants (without LPS groups) mixed 1:1 with IMDM, 10% FCS. Polymyxin B, a LPS scavenger was added to all washing steps and culture media. BMNC from donor 1 were cultured in disc cell:P. acnes supernatants from batch 1 and BMNC from donor 2 in disc cell:P. acnes supernatants from batch 5. Batch 1 and batch 5 were both from responders (Table 1). After 24h, BMNC were collected and lyzed with RLT buffer. BMNCs cultured in conditioned media from disc cells alone served as negative control.
Gene transcription analysis
RNA from disc cells and BMNCs were isolated with Qiagen RNeasy mini columns and on-column DNase digestion (Qiagen, Valencia, CA, USA). cDNA was synthesized (BioRad iScript cDNA Synthesis Kit) and the gene expression of ribosomal protein L30 (RPL30, LifeTechnologies Hs00265497_m1), interleukin-1β (IL-1β, Hs01555410_m1), IL-6 (Hs00985639_m1), IL-8 (Hs00174103_m1), and chemokine (C-C motif) ligand 2 (CCL2, Hs00234140_m1) were quantified with TaqMan probes. Data were normalized to RPL30 and to negative control and evaluated with the comparative Cq method using the software R (version 2.15.1).
Statistics
Significant differences were detected by comparing ΔCq values of P. acnes and LPS cultures to negative control using t-tests. If gene expression in disc cells of more than one group was significantly increased, the disc cell line was considered as responder. Age, weight, pain, and degree of disc degeneration (Pfirrmann grade) were compared between responders and non-responders using t-tests. Gender and presence of Modic changes (type 1 or type 2) were compared between responders and non-responders using Fisher’s exact test.
RESULTS
Lipase activity
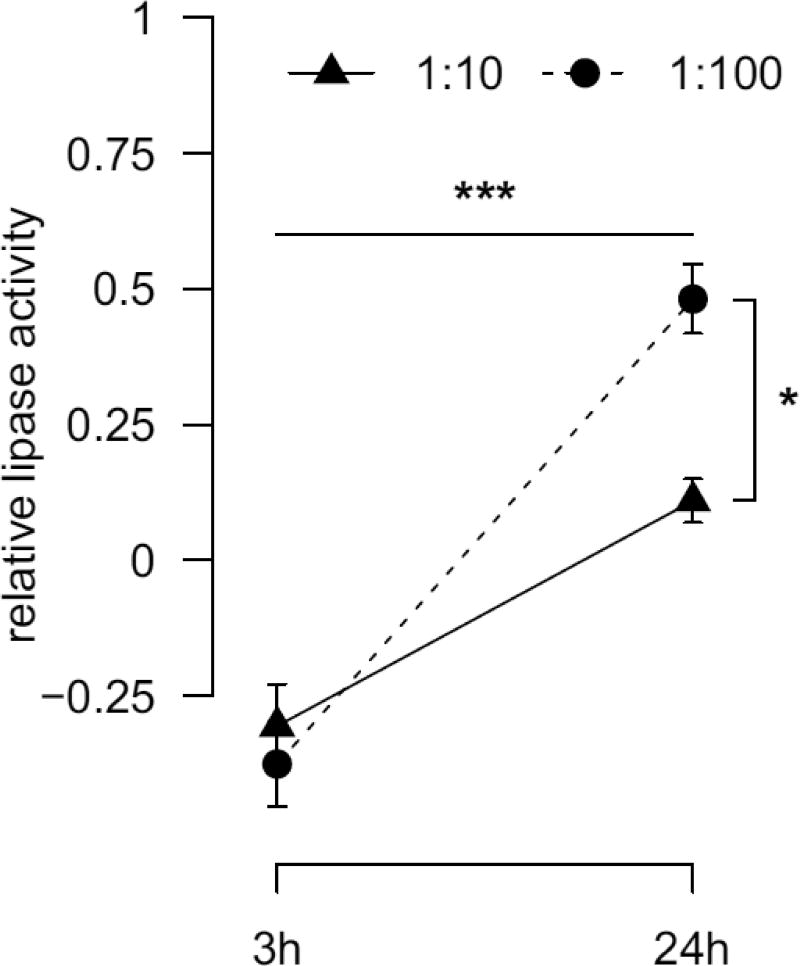
Lipase activity after 3h of co-culture were similar in the 1:10 and 1:100 group (p=0.87; Fig. 2). From 3h to 24h lipase activity increased in both groups (both p<0.001) with a stronger increase in the 1:100 group (+0.79 U/ml vs. +0.42 U/ml, p<0.001). This resulted in a time (p<0.001) and P. acnes concentration (p<0.05) dependent lipase activity. There was no disc cell donor variation (p=0.10).
Fig. 2.
Relative lipase activity after 3h and 24h of 1:10 and 1:100 disc cell / P.acnes co-cultures normalized to disc cells cultured without P. acnes. Significant main group effects are indicated: time effect (horizontal line), concentration effect (vertical line). * p<0.05, *** p<0.001
Response of disc cells to co-cultured P.acnes
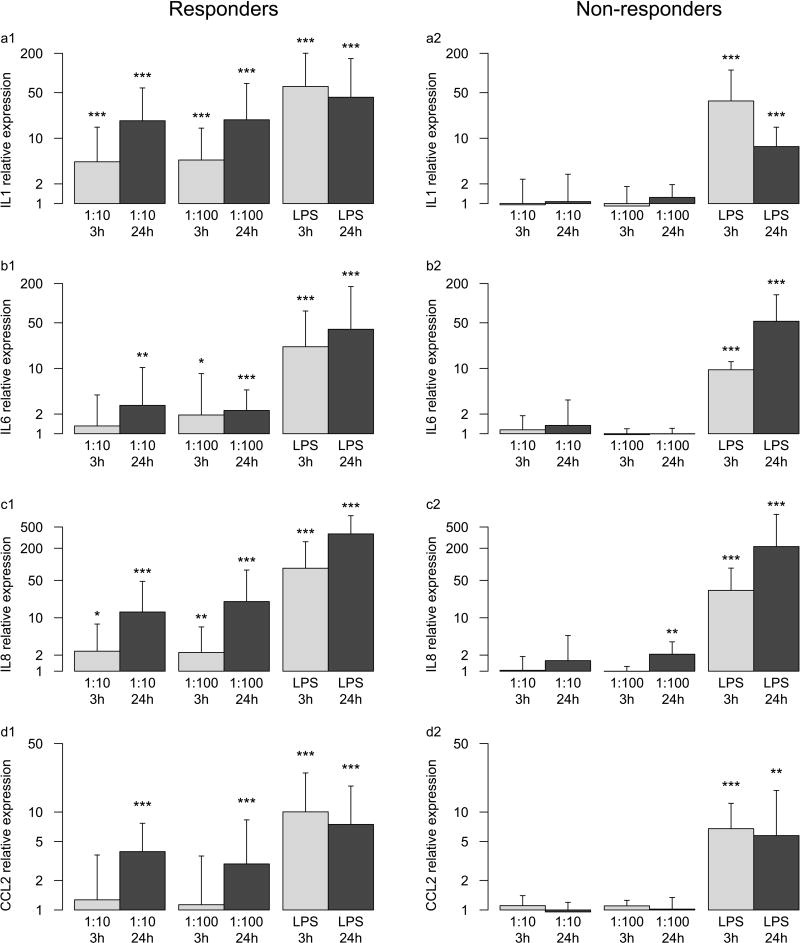
Disc cells from all donors responded to LPS (Fig. 3). This verified disc cell viability and the ability to respond to a bacterial TLR agonist [10, 17]. However, only six of ten disc cell lines responded to P. acnes with up-regulation of IL1, IL6, IL8, and CCL2 gene transcription. Four of these six cell lines were isolated from discs adjacent to Modic changes, one cell line from a disc one level separated from Modic changes, and one cell line from a patient without lumbar Modic changes (Table 1). The four non-responding cell lines were all from patients without lumbar Modic changes. The presence of lumbar Modic changes at the same or adjacent level from where disc cells were isolated associated significantly with disc cell responsiveness to P.acnes (p<0.05). Gender, age, weight, degree of DD, and level of pre-operative pain did not associate with disc cell responsiveness.
Fig. 3.
Gene expression of disc cells after 3h and 24h of co-culture with a 10-fold and 100-fold excess of P. acnes relative to disc cells alone. Lipopolysaccaride (LPS) was used as positive control. a1, b1, c1, d1: relative gene expression of IL1, IL6, IL8, and CCL2 of responders, respectively; a2, b2, c2, d2: relative gene expression of non-responders. Significant differences to disc cells alone are indicated: * p<0.05, ** p <0.01, *** p<0.001.
Only considering responsive cell lines, IL1, IL6, IL8, and CCL2 gene transcriptions were increased under most conditions and increased over time (p<0.001, except for IL6: p=0.48) but were independent of the amount of P. acnes used in the co-culture (p=0.83, p=0.73, p=0.49, p=0.42). In non-responding cell lines, only IL8 after 24h in the 1:100 group was slightly increased (2.1-fold, p<0.01).
Response of BMNCs in conditioned media from P.acnes / disc cell cultures
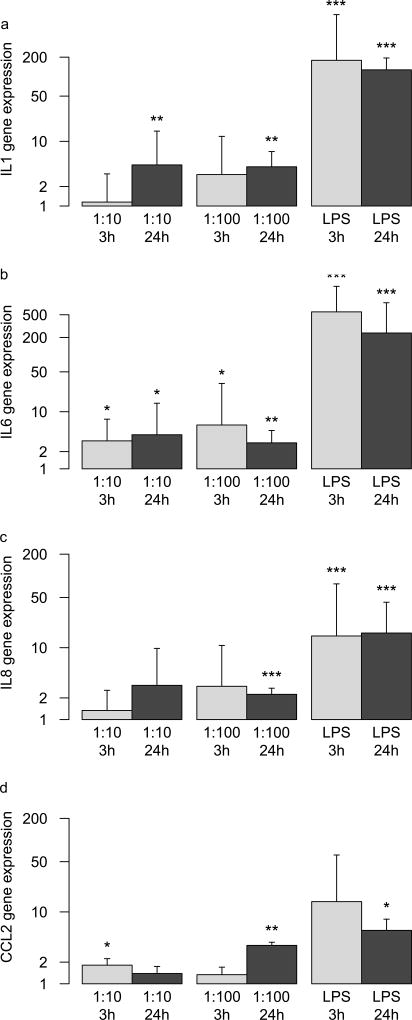
BMNC responded to LPS by increasing IL1, IL6, IL8, and CCL2 expression (Fig. 4) in the supernatants of the selected responder cell lines. IL6 expression was increased in all P. acnes-conditioned media, irrespective of the amount of P. acnes (1:10, 1:100) and conditioning time (3h, 24h). IL1 expression was only increased in media conditioned for 24 h but irrespective of P. acnes concentration. IL8 and CCL2 expression were increased in 24 h conditioned media from high P. acnes concentration. In addition, CCL2 was also increased in 3h conditioned media from low P. acnes concentration.
Fig. 4.
Gene expression of BMNC after 24h in conditioned media from disc cell / P. acnes co-cultures. Time points (3h, 24h) and condition (1:10, 1:100, LPS) relate to the conditioned media. a, b, c, d: relative gene expression of IL1, IL6, IL8, and CCL2, respectively. Significant differences to BMNCs cultured in conditioned media from disc cells alone are indicated: * p<0.05, ** p <0.01, *** p<0.001.
DISCUSSION
We hypothesized that disc cells have an inflammatory response to P. acnes, and that BMNC respond with inflammation to conditioned media from disc cells co-cultured with P. acnes. We found that only disc cells from patients with lumbar Modic changes respond to P. acnes by increasing expression of IL1, IL6, IL8, and CCL2. The activity of the P. acnes virulence factor lipase was increased in P. acnes co-cultures but independent from the transcriptional response of disc cells. BMNCs increased expression of IL1, IL6, IL8, and CCL2 when cultured in transcriptional responding cell media. These findings show that the presence of P. acnes bacteria can activate disc cells, and that the cocktail of disc cytokines and bacterial metabolites can activate BMNCs. Therefore, low virulent disc infection with P. acnes is a potential etiology of MC1.
Responder versus non-responder disc cell lines
Disc cells are not considered part of the immune system since they are normally sequestered from the blood supply. Therefore, it cannot be expected a priori that they respond to pathogens. However, the recent identification of TLR expression by disc cells grants them this capability because TLRs bind bacterial cell wall proteins [10]. This also explains the responsiveness of disc cells to LPS [17]. LPS is a substance derived from bacterial cell walls and is often used as TLR agonist. Here we showed that cells from all analyzed discs, i.e. responder and non-responder, react to LPS while only 6 of 10 react to P. acnes. All responsive discs cell lines were taken from donors with lumbar Modic changes but not necessarily from the disc at the level of Modic changes. In contrast, 4 of 5 disc cell lines from donors without Modic changes did not respond to P. acnes. This resulted in a significant association of disc cell responsiveness with the presence of lumbar Modic changes. Consequently, lumbar Modic changes are highly sensitive but only moderately specific for disc cell responsiveness to P. acnes. This suggests that disc cell presensitization occurs in-vivo and that Modic changes are a sequela that may be exacerbated by P. acnes infection. Structural (micro)-damage of the endplates are characteristic for Modic changes and may be an important factor for triggering disc cell responsiveness to P. acnes because it violates the discs’ immune privilege [11–13, 18]. How disc cells sense P. acnes remains unknown. Since non-responder cell lines also react to LPS, P. acnes may engage other receptors than TLR2 and TLR4, which bind LPS. The disc cell reaction may also be caused by virulence factors not investigated here and not be directly sensing bacterial cell wall components.
If disc cells react to P. acnes, they are highly sensitive to the presence of P. acnes bacteria. Since the elevated cytokine expression of responsive cell lines was independent from the P. acnes concentration, the sensitivity threshold to react to P. acnes appears to be even lower than the ten P. acnes per disc cells applied in this study.
The P. acnes load in discs from levels with MC is unknown yet P. acnes concentration in non-MC discs is 12–21,000 CFU [27]. Therefore, this is as physiological or non-pathological P. acnes concentration [28]. Assuming a nucleus pulposus volume of 6.6 cm3 with 950,000 cells [29, 30], the used P. acnes/disc cell ratio is 1450-times higher in the 1:100 culture than in a non-MC disc with a high bacterial load. However, there are reasons to assume that the true P.acnes load in MC discs is much higher. Assuming MC1 can be caused by P. acnes, MC1 is a clinical sign of an infected disc and bacterial load in the disc is likely much higher than the reported CFU of non-MC discs. Furthermore, CFU measurements are generally lower than true CFU for methodological reasons [27].
Similar to MC1, Modic type II changes (MC2) associate with endplate damage and represent fatty marrow conversion [12, 13, 19]. In this study disc cells from two out of two patients with MC2 were also responsive to P. acnes, which suggests that the responsiveness is independent of the bone marrow composition and that it is rather the violation of the discs immune privilege by endplate damage that triggers the responsiveness. This is not surprising since MC1 and MC2 are interconvertible and responsiveness to P. acnes is likely a permanently acquired capability [20]. In order to test this hypothesis, the responsiveness of more discs from MC2 levels need to be analyzed. The responsiveness also does not appear to be a consequence of the primary P. acnes virulence factor lipase because lipase activity was the same in responder and non-responder cell lines. However, other P. acnes virulence factors such as propionic acid could also contribute to the disc cell response.
Response of the bone marrow
The bone marrow of Modic changes shows a dysregulated myelopoiesis [14]. Similar dysmyelopoiesis was described in mice after exposure to IL-1 and LPS [15, 21, 22]. It is likely that the disc at the level of Modic changes is the trigger of the hematopoietic changes because Modic changes typically occur symmetric cephaled and caudad to a particular disc [23], because the disc at the level of Modic changes releases higher amounts of pro-inflammatory cytokines [24], and because Modic changes stand in inflammatory cross-talk with the adjacent disc [14]. With this current study we showed that healthy BMNCs respond to the cocktail that is released by co-localized P. acnes and disc cells. This cocktail not only contains the pro-inflammatory cytokines released by disc cells but also P. acnes virulence factors and its catabolites. GehA lipase is one of the most important P. acnes virulence factors and associates with the pathogenesis of acnes vulgaris [9]. Lipase hydrolysis triacylglycerides into glycerol and free fatty acids. Glycerol is a P. acnes nutrient and free fatty acids are chemotactic and highly pro-inflammatory [9]. GehA lipase has an optimal stability and activity at pH 6.8 and is stable as low as pH 5 [9]. The disc tissue provides the optimal environment for lipase activity and the generation of highly pro-inflammatory free fatty acids because even in healthy discs glycosaminoglycan cause a drop in pH by about 0.5 pH compared to surrounding tissue and in degenerated discs accumulation of lactic acid cause pH as low as 6 [8]. Therefore, it is likely that free fatty acids add to the inflammatory milieu in Modic changes. In addition, free fatty acids also skew lineage choice of myeloid progenitor cells toward the osteoclastic lineage and of bone marrow stromal cells towards adipocytes [13]. High bone turn-over is characteristic for Modic change type I and abundant adipocytes are a hallmarks of MC2 [12, 13, 19, 25]. Further experiments will be necessary to determine the effect of bacterially-induced free fatty acids on bone marrow response in a Modic change environment. Furthermore, investigating the response of BMNC to non-transcriptional responding disc cells would show if the BMNC response is a direct response to virulence factors or to disc cytokines.
In summary, this study supports a previously suggested model for the P. acnes infection phenotype of MC where disc damage allows the introduction of P. acnes into the disc (Fig. 5) [13, 26]. In the disc, P. acnes proliferate and abundant disc cytokines, P. acnes virulence factors, and presumably free fatty acids are produced. All these factors can drain into the bone marrow, where they cause MC. While we observed statistically-significant relationships between P. acnes reactivity and donor MC status, these findings are based on a small sample size and need to be replicated, in particular using P. acnes isolated from another MC disc. Furthermore, the response should be stratified for the type of MC.
Fig. 5.
Suggested model for the P. acnes infection phenotype of Modic changes. Disc damage is a prerequisite for P. acnes to invade the disc. Inside the disc, P. acnes proliferate and release virulence factors. Disc cells release cytokines as response to P. acnes. Free fatty acids are the consequence of lipase activity and are highly pro-inflammatory. This cocktail drains into the bone marrow, triggers a pro-inflammatory response in bone marrow cells, and may eventually cause Modic changes.
In conclusion, disc cell responsiveness to P. acnes associates with the presence of lumbar Modic changes. Furthermore, bone marrow cells yield an inflammatory transcriptional response to the cocktail of disc cytokines and P. acnes metabolites. Therefore, low virulent P. acnes infection of the disc is a potential exacerbating factor to Modic changes, following an initiating event such as endplate damage or disc herniation.
Acknowledgments
This study was supported by the Swiss National Science Foundation Grant 145961, 158792, and 164726 as well as the National Institutes of Health Grant AR063705
Footnotes
ETHICAL APPROVAL: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.”
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
References
- 1.Aghazadeh J, Salehpour F, Ziaeii E, et al. Modic changes in the adjacent vertebrae due to disc material infection with Propionibacterium acnes in patients with lumbar disc herniation. Eur Spine J. 2016 doi: 10.1007/s00586-016-4887-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Albert HB, Lambert P, Rollason J, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013;22:690–6. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudli S, Liebenberg E, Magnitsky S, et al. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J Orthop Res. 2016;34:1447–55. doi: 10.1002/jor.23265. [DOI] [PubMed] [Google Scholar]
- 4.Capoor MN, Ruzicka F, Machackova T, et al. Prevalence of Propionibacterium acnes in Intervertebral Discs of Patients Undergoing Lumbar Microdiscectomy: A Prospective Cross-Sectional Study. PLoS One. 2016;11:e0161676. doi: 10.1371/journal.pone.0161676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollason J, McDowell A, Albert HB, et al. Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int. 2013;2013:530382. doi: 10.1155/2013/530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritzell P, Bergström T, Welinder-Olsson C. Detection of bacterial DNA in painful degenerated spinal discs in patients without signs of clinical infection. Eur Spine J. 2004;13:702–6. doi: 10.1007/s00586-004-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urquhart DM, Zheng Y, Cheng AC, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 2015;13:13. doi: 10.1186/s12916-015-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban JPG. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30:858–863. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 9.Falcocchio S, Ruiz C, Pastor FIJ, et al. Propionibacterium acnes GehA lipase, an enzyme involved in acne development, can be successfully inhibited by defined natural substances. J Mol Catal B Enzym. 2006;40:132–137. [Google Scholar]
- 10.Klawitter M, Hakozaki M, Kobayashi H, et al. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J. 2014;23:1878–91. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- 11.Weiner BK, Vilendecic M, Ledic D, et al. Endplate changes following discectomy: natural history and associations between imaging and clinical data. Eur Spine J. 2015;24:2449–57. doi: 10.1007/s00586-014-3734-8. [DOI] [PubMed] [Google Scholar]
- 12.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–9. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 13.Dudli S, Fields AJ, Samartzis D, et al. Pathobiology of Modic changes. Eur Spine J. 2016;25:3723–3734. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudli S, Sing DC, Hu SS, et al. Intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J. 2017 doi: 10.1007/s00586-017-4955-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirantes C, Passegué E, Pietras EM. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res. 2014;329:248–54. doi: 10.1016/j.yexcr.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D. Molecular Detection of Human Bacterial Pathogens. 1. CRC Press; Boca Raton: 2011. p. 147. [Google Scholar]
- 17.Burke JG, G Watson RW, Conhyea D, et al. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine (Phila Pa 1976) 2003;28:2685–93. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z-H, Sun Z, Wang H-Q, et al. FasL Expression on Human Nucleus Pulposus Cells Contributes to the Immune Privilege of Intervertebral Disc by Interacting with Immunocytes. Int J Med Sci. 2013;10:1053–1060. doi: 10.7150/ijms.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Glob spine J. 2013;3:153–64. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen TS, Bendix T, Sorensen JS, et al. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler P, Boettcher S, Takizawa H, et al. LPS-stimulated human bone marrow stroma cells support myeloid cell development and progenitor cell maintenance. Ann Hematol. 2016;95:173–8. doi: 10.1007/s00277-015-2550-5. [DOI] [PubMed] [Google Scholar]
- 22.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuisma M, Karppinen J, Niinimäki J, et al. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine (Phila Pa 1976) 2006;31:1714–8. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 24.Burke J, Watson R, McCormack D, et al. Endplate changes are associated with increased disc inflammatory mediator production. Proc. North Am. Spine Soc. Annu. Meet. 2001:3–4. [Google Scholar]
- 25.Perilli E, Parkinson IH, Truong L-H, et al. Modic (endplate) changes in the lumbar spine: bone micro-architecture and remodelling. Eur Spine J. 2015;24:1926–34. doi: 10.1007/s00586-014-3455-z. [DOI] [PubMed] [Google Scholar]
- 26.Albert HB, Kjaer P, Jensen TS, et al. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–8. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Capoor MN, Ruzicka F, Schmitz JE, et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS One. 2017;12:e0174518. doi: 10.1371/journal.pone.0174518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajasekaran S, Tangavel C, Aiyer SN, et al. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur Spine J. 2017;26:1384–1400. doi: 10.1007/s00586-017-4972-3. [DOI] [PubMed] [Google Scholar]
- 29.Liebscher T, Haefeli M, Wuertz K, et al. Age-related variation in cell density of human lumbar intervertebral disc. Spine. 2011;36:153–9. doi: 10.1097/BRS.0b013e3181cd588c. [DOI] [PubMed] [Google Scholar]
- 30.Showalter BL, Beckstein JC, Martin JT, et al. Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine. 2012;37:E900–7. doi: 10.1097/BRS.0b013e31824d911c. [DOI] [PMC free article] [PubMed] [Google Scholar]